Abstract
Pulmonary embolism (PE) poses a challenge to physicians, as it can be difficult to diagnose but results in significant mortality and morbidity in patients. Diagnosing PE requires an integrated approach using clinical findings, electrocardiography (ECG), blood investigations and imaging modalities. Abnormalities in ECG are common among patients with massive acute PE and can serve as a prognostic indicator. In this article, we describe the ECG presentations of two patients diagnosed with PE, and review the literature on the various types of ECG presentations and their role in predicting the prognosis of PE.
Keywords: diagnosis, ECG, management, prognosis, pulmonary embolism
CASE 1 CLINICAL PRESENTATION
A 55-year-old woman with a history of hypertension and a recent haemorrhagic stroke was admitted with an acute onset of shortness of breath for a duration of one day. She also reported an episode of near syncope while engaging in physiotherapy. At the emergency department, her initial vital parameters included blood pressure of 139/97 mmHg, heart rate of 99 beats per minute (bpm) and oxygen saturation of 89% on room air. Other than being tachypnoeic (25 per minute), the patient had normal heart and breath sounds. Electrocardiography (ECG) was performed (Fig. 1).
Fig. 1.
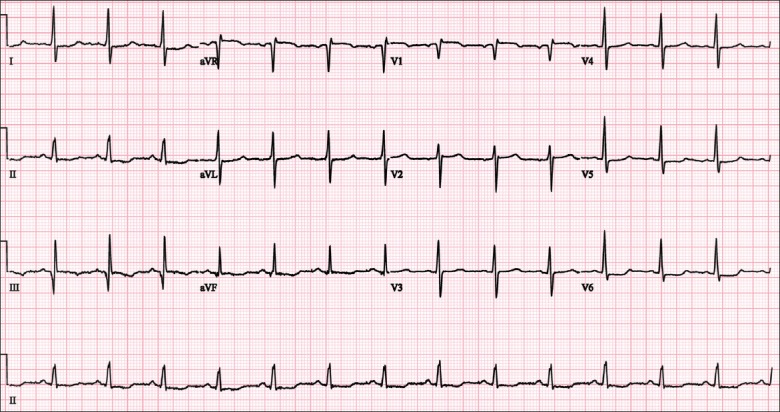
Case 1: ECG shows an S1Q3T3 pattern.
ECG INTERPRETATION
The ECG (Fig. 1) shows sinus rhythm and the presence of an S1Q3T3 pattern. There is an S wave in lead I, a Q wave with T wave inversion in lead III.
CLINICAL COURSE
In view of the clinical presentation, ECG changes and the presence of a risk factor for immobilisation from a recent stroke, the patient underwent computed tomography angiography of the pulmonary arteries (CT-PA), which showed bilateral filling defects extending from the main pulmonary trunk to the peripheries. Her arterial blood gas analysis showed type 1 respiratory impairment with a PaO2 of 73 mmHg on room air. Serum cardiac enzymes were mildly elevated with a troponin I level of 0.193 ug/L. Transthoracic echocardiography (TTE) showed evidence of severe right ventricular systolic dysfunction and right ventricular dilatation (Fig. 2).
Fig. 2.
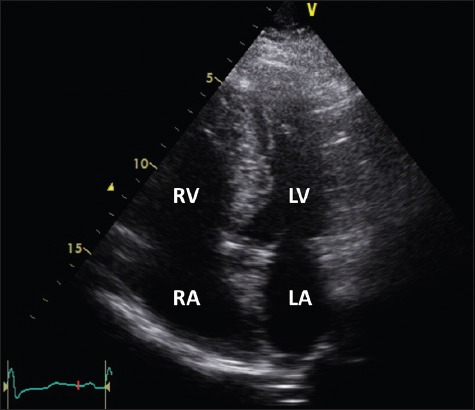
Case 1: Transthoracic echocardiogram of the apical 4-chamber window shows dilatation of the right heart chambers, including the right ventricle (RV). LA: left atrium; LV: left ventricle; RA: right atrium
The patient was diagnosed with submassive pulmonary embolism. However, in view of her recent intracranial bleed, she was not thrombolysed. She was started on subcutaneous low-molecular-weight heparin (i.e. enoxaparin) and supplemental oxygen, and subsequently discharged with three months of oral anticoagulation medication (i.e. warfarin) and an outpatient appointment for follow-up.
CASE 2 CLINICAL PRESENTATION
A 62-year-old man presented with shortness of breath upon exertion. This was associated with pleuritic chest pain of mild intensity and dizziness for several days. His symptoms started after a 16-hour flight. He had a significant history of hypertension, dyslipidaemia, atrial fibrillation on aspirin (CHADSVAS score 1) and previous deep vein thrombosis affecting the right lower limb. At the emergency department, his vital parameters included blood pressure of 108/75 mmHg, heart rate of 78 bpm and oxygen saturation of 94% on room air. Examination findings included normal heart sounds and respiration. His right calf was tender, erythematous and swollen. An ECG (Fig. 3) was performed.
Fig. 3.
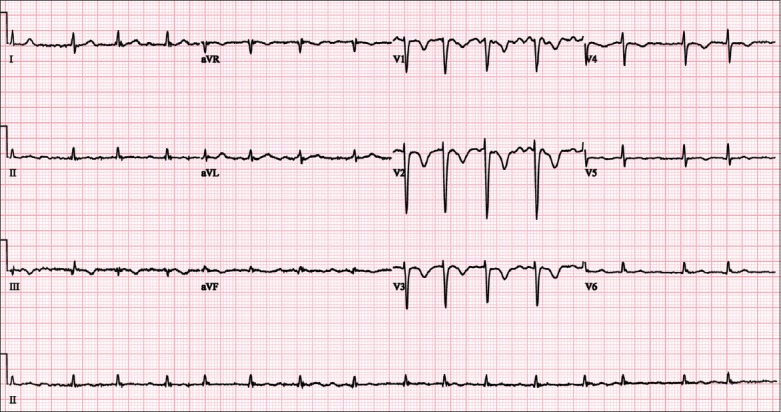
Case 2: ECG shows atrial fibrillation and T wave inversions in V1–4.
ECG INTERPRETATION
The ECG (Fig. 3) shows atrial fibrillation at a rate of 75–115 bpm, with T wave inversion in the right-sided precordial leads V1–4. There are also low voltage QRS complexes in the limb leads.
CLINICAL COURSE
Initial blood investigations revealed an elevated D-dimer level of more than 4.0 μg/L and an elevated troponin I level of 0.118 μg/L. In view of the suggestive history, risk factors and ECG changes, a CT-PA was performed, which showed the presence of a saddle embolus and emboli in the bilateral pulmonary trunks (Fig. 4). The patient was monitored in the intensive care unit and started on intravenous heparin before bridging to warfarin upon discharge, with plans for lifelong oral anticoagulation.
Fig. 4.
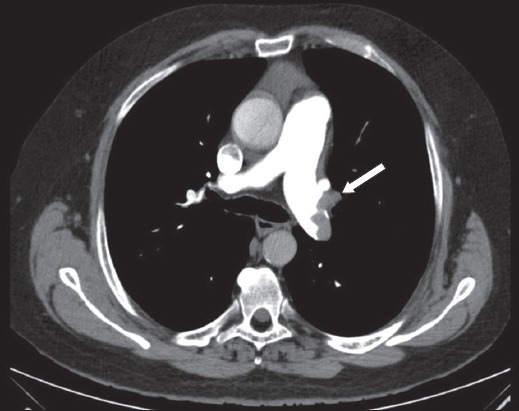
Case 2: CT image shows a filling defect (arrow) in the left main pulmonary trunk of the pulmonary artery.
DISCUSSION
Acute pulmonary embolism (PE) is a potentially fatal disorder with a high mortality rate. According to the International Cooperative Pulmonary Embolism Registry, the mortality rate at three months was 15.3%.(1) Survivors of the acute event may also have to contend with morbidity due to chronic thromboembolic pulmonary hypertension or post-thrombotic syndrome.
In a study of over 42 million deaths occurring in the United States within a 20-year period, almost 600,000 patients (approximately 1.5%) were diagnosed with PE, with PE being the presumed cause of death in 200,000 of the patients.(2) In the local context, a study of three National Healthcare Group hospitals in 2006 estimated the population-based incidence of PE to be 15 per 100,000, with lower rates of venous thromboembolism among Asians compared to Caucasians and Eurasians.(3)
Although it is important to diagnose and treat PE early, the clinical presentation of PE is known to be variable and nonspecific, rendering an accurate diagnosis difficult. Symptoms suggestive of an acute PE include dyspnoea at rest or upon exertion, pleuritic chest pain, cough, orthopnoea, and calf or thigh pain or swelling. Signs include tachypnoea, tachycardia, rales, decreased breath sounds, an accentuated pulmonic component of the second heart sound and jugular venous distention. Massive PE may be accompanied by acute right ventricular failure, manifested as elevated jugular venous pressure, a right-sided third heart sound and a parasternal lift. In addition, signs of lower extremity deep venous thrombosis (DVT) may be present.
It is important, however, to bear in mind that symptoms may be mild or even absent, especially in cases involving only the segmental pulmonary branches. Having a high or intermediate probability on objective clinical assessment prompts the need for further diagnostic study, although a low probability does not exclude the diagnosis. The maintenance of a high level of suspicion is critical in a physician’s approach to the diagnosis of PE.(4)
Abnormalities upon investigation include ECG findings, elevated D-dimer blood test, evidence of right heart strain or visualisation of the embolus on TTE and filling defects representative of PE on CT-PA. Although some ECG changes are common in PE, ECG changes alone are insufficient to make a reliable diagnosis of PE. However, there is increasing interest in the prognostic use of ECG, as well as evidence to suggest that certain ECG findings can play a role in predicting which patients have a more complicated disease or worse prognosis, and hence require more invasive testing and earlier aggressive treatment.
ECG features
There is a wide range of ECG features associated with PE. 10%–25% of patients with PE have a completely normal ECG.(5) The most well-known finding is the S1Q3T3 pattern, as seen in Case 1. The most common presentation is that of sinus tachycardia.(6) This occurs in response to the physiological demand for cardiac output with decreased left-sided stroke volume. There have also been case reports of electrical alternans(7) and ST segment elevation in lead aVR with ST segment depression in leads I and V4–V6.(8)
Some ECG features suggest a more extensive and complicated disease. For example, low QRS voltages, right bundle branch block and ST segment elevation in lead V1 have been shown to be associated with cardiogenic shock.(9) Atrial arrhythmias, complete right bundle branch block, peripheral low voltages, pseudoinfarction patterns (Q waves) in leads III and aVF, and ST segment changes over the left precordial leads were found to be more frequent in patients with a fatal outcome. In a study examining the prognostic relevance of ECG findings in relation to 30-day mortality outcomes, 29% of the patients who exhibited at least one of these abnormalities on admission did not survive to hospital discharge.(10) In comparison, only 11% of the patients without such ECG features did not survive to hospital discharge. An ECG case series also reports that PE is one of the causes of T wave abnormalities.(11) Another study showed that the most common finding in confirmed massive PE was T wave inversions in the precordial leads with an earlier onset in the more severe cases.(12) This was observed in Case 2.
Furthermore, ECG features associated with right ventricular strain have been found to correlate with the degree of pulmonary artery obstruction due to pulmonary embolism, increased pressure and wall tension on the right heart.(13) The presence of the right ventricular strain pattern on the ECG is associated with an increased risk of all-cause death and clinical deterioration, even in patients with normal systemic blood pressure.(14) In patients with right ventricular dysfunction, T wave inversions in leads V1–V3 had greater sensitivity and diagnostic accuracy compared with the S1Q3T3 and right bundle branch block features, which had good specificity but only moderate accuracy.(15)
Several scoring systems have been proposed for predicting which patients have a more complicated disease. A retrospective study that scored patients with PE according to their ECG findings found that a greater score correlated to higher systolic pulmonary artery pressures.(16) Daniel et al developed a 21-point scoring system based on ECG abnormalities associated with right ventricular strain secondary to submassive or massive PE.(17) It was found that although the score only had a marginally increased diagnostic performance, it correlated well with the pulmonary artery pressure in patients with angiographically confirmed PE.(17) The development of such scoring systems suggests that ECG features can play a greater role as a prognostic adjunct in the management of patients with PE.
Acute PE can be classified into massive, submassive or low-risk PE. Massive PE results in hypotension, and should be considered if the patient has hypotension lasting more than 15 minutes or requiring inotropic support, with elevated central venous pressure that has no other attributable cause, or if hypotension is associated with profound bradycardia or pulselessness. Massive PE is a medical emergency that frequently results in acute right ventricular failure and death. In submassive PE, patients do not have hypotension; however, there is evidence of right ventricular strain in the form of right ventricular dilatation, elevation of serum brain natriuretic peptide, ECG changes or myocardial necrosis. This population of patients is at increased risk for adverse short-term outcomes. Patients without the above clinical features of adverse prognosis are considered to have low-risk PE.
Management of patients with PE depends on the severity of the disease. After resuscitation and stabilisation of the patient, anticoagulation therapy should be started for all eligible patients. Patients with massive or submassive PE should be considered for surgical embolectomy or fibrinolysis unless contraindicated. Catheter embolectomy is also an alternative.
Anticoagulation options include intravenous heparin or subcutaneous unfractionated heparin, with subsequent conversion to vitamin K antagonists (VKA) or novel oral anticoagulants. If anticoagulation is contraindicated or if patients have recurrent acute PE despite therapeutic anticoagulation, alternative strategies such as inferior vena cava filters may be considered.(18) In recent years, novel anticoagulants have been introduced as an alternative to VKA. For instance, rivaroxaban has been approved by the United Stated Food and Drug Administration for use in treating PE. The use of rivaroxaban in patients diagnosed with PE was shown to be non-inferior to the standard therapy of low-molecular-weight heparin followed by oral VKA.(19) The duration of anticoagulation therapy is determined after weighing the risk of recurrent thromboembolism against the risk for bleeding. In general, it is recommended that patients who have their first episode of PE with reversible risk factor undergo three months of anticoagulation therapy. After weighing the risk of recurrence against the risk of bleeding, patients who have had their first episode of acute unprovoked PE or recurrent PE should receive anticoagulant therapy for three months before being reassessed for the need for prolonged anticoagulation therapy.(17)
REFERENCES
- 1.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–9. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 2.Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–7. doi: 10.1001/archinte.163.14.1711. [DOI] [PubMed] [Google Scholar]
- 3.Molina JA, Jiang ZG, Heng BH, Ong BK. Venous thromboembolism at the National Healthcare Group, Singapore. Ann Acad Med Singapore. 2009;38:470–8. [PubMed] [Google Scholar]
- 4.Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120:871–9. doi: 10.1016/j.amjmed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubloue I, Schoors D, Diltoer M, Van Tussenbroek F, de Wilde PP. Early electrocardiographic signs in acute massive pulmonary embolism. Eur J Emerg Med. 1996;3:199–204. doi: 10.1097/00063110-199609000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Petruzzelli S, Palla A, Pieraccini F, Donnamaria V, Giuntini C. Routine electrocardiography in screening for pulmonary embolism. Respiration. 1986;50:233–43. doi: 10.1159/000194933. [DOI] [PubMed] [Google Scholar]
- 7.Nichols KB, Littman L. Electrical alternans as a manifestation of pulmonary embolism. Am J Emerg Med. 2011;29:1236.e5–7. doi: 10.1016/j.ajem.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Zhong-Qun Z, Chong-Quan W, Nikus KC, Sclarovsky S, Chao-Rong H. A new electrocardiogram finding for massive pulmonary embolism: ST elevation in lead aVR with ST depression in leads I and V(4) to V(6) Am J Emerg Med. 2013;31:456.e5–8. doi: 10.1016/j.ajem.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Kukla P, McIntyre WF, Fijorek K, et al. Electrocardiographic abnormalities in patients with acute pulmonary embolism complicated by cardiogenic shock. Am J Emerg Med. 2014;32:507–10. doi: 10.1016/j.ajem.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 10.Geibel A, Zehender M, Kasper W, et al. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J. 2005;25:843–8. doi: 10.1183/09031936.05.00119704. [DOI] [PubMed] [Google Scholar]
- 11.Lin W, Teo SG, Poh KK. Electrocardiography series. Electrocardiographic T wave abnormalities. Singapore Med J. 2013;54:606–10. [PubMed] [Google Scholar]
- 12.Ferrari E, Imbert A, Chevalier T, et al. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads--80 case reports. Chest. 1997;111:537–43. doi: 10.1378/chest.111.3.537. [DOI] [PubMed] [Google Scholar]
- 13.Ullman E, Brady WJ, Perron AD, Chan T, Mattu A. Electrocardiographic manifestations of pulmonary embolism. Am J Emerg Med. 2001;19:514–9. doi: 10.1053/ajem.2001.27172. [DOI] [PubMed] [Google Scholar]
- 14.Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med. 2009;122:257–64. doi: 10.1016/j.amjmed.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Punukollu G, Gowda RM, Vasavada BC, Khan IA. Role of electrocardiography in identifying right ventricular dysfunction in acute pulmonary embolism. Am J Cardiol. 2005;96:450–2. doi: 10.1016/j.amjcard.2005.03.099. [DOI] [PubMed] [Google Scholar]
- 16.Iles S, Le Heron CJ, Davies G, Turner JG, Beckert LE. ECG score predicts those with the greatest percentage of perfusion defects due to acute pulmonary thromboembolic disease. Chest. 2004;125:1651–6. doi: 10.1378/chest.125.5.1651. [DOI] [PubMed] [Google Scholar]
- 17.Daniel KR, Courtney DM, Kline JA. Assessment of cardiac stress from massive pulmonary embolism with 12-lead ECG. Chest. 2001;120:474–81. doi: 10.1378/chest.120.2.474. [DOI] [PubMed] [Google Scholar]
- 18.Kearon C, Akl EA, Comerota AJ, et al. American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EINSTEIN-PE Investigators. Büller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]


