Abstract
Detection of drug-drug interactions is essential during the early stages of drug discovery and development, and the understanding of drug-botanical interactions is important for the safe use of botanical dietary supplements. Among the different forms of drug interactions that are known, inhibition of cytochrome P450 (P450) enzymes is the most common cause of drug-drug or drug-botanical interactions. Therefore, a rapid and comprehensive mass spectrometry–based in vitro high-throughput P450 cocktail inhibition assay was developed that uses 10 substrates simultaneously against nine CYP isoforms. Including probe substrates for CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and two probes targeting different binding sites of CYP3A4/5, this cocktail simultaneously assesses at least as many P450 enzymes as previous assays while remaining among the fastest due to short incubation times and rapid analysis using ultrahigh pressure liquid chromatography–tandem mass spectrometry. The method was validated using known inhibitors of each P450 enzyme and then shown to be useful not only for single-compound testing but also for the evaluation of potential drug-botanical interactions using the botanical dietary supplement licorice (Glycyrrhiza glabra) as an example.
Introduction
Drugs and botanical dietary supplements can interact with some therapeutic agents by inhibiting or inducing drug-metabolizing enzymes or drug transporters. Inhibition of these enzymes and transporters can result in longer half-lives and higher and possibly toxic concentrations of therapeutic agents, whereas induction can have the opposite effect. The most common form of drug-drug or drug-botanical interaction is inhibition of cytochrome P450 (P450) enzymes. Examples include inhibition of CYP2D6 by paroxetine (Bertelsen et al., 2003) and inhibition of CYP3A4 by goldenseal (Hydrastis canadensis) (Chatterjee and Franklin, 2003; Gurley et al., 2005).
In vitro P450 inhibition assays are widely used to study potential drug-drug and drug-botanical interactions. Although these assays typically evaluate inhibition of one P450 isoform at a time, the U.S. Food and Drug Administration (FDA) recommends that at least seven P450 isoforms should be investigated for possible inhibition by each new drug entity under development (Food and Drug Administration, 2012). To expedite these assays, several cocktail approaches, also known as n-in-one assays, have been developed to test for inhibition of several P450 isoforms simultaneously. Most of these assays test for inhibition of five to eight P450 isoforms and use a wide variety of experimental conditions and probe substrates (Dierks et al., 2001; Testino and Patonay, 2003; He et al., 2007; Li et al., 2007; Smith et al., 2007; Workman and Raynaud, 2007; Zientek et al., 2008; Alden et al., 2010; Otten et al., 2011; Yao et al., 2012; Kozakai et al., 2012; Lee and Kim, 2013; Qiao et al., 2014; Qin et al., 2014; Liu et al., 2015). Although a few approaches have claimed using 9 or 10 substrates to evaluate nine isoforms, they actually carry out separate incubations of subsets of probe substrates to avoid P450 interactions before pooling the mixtures immediately prior to a quantitative analysis step, use P450 substrates which are not recommended by the FDA, and/or use long incubation times of up to 60 minutes (Kim et al., 2005; Turpeinen et al., 2005; Tolonen et al., 2007; Dinger et al., 2014).
To address these assay limitations for the investigation of drug-drug interactions while including drug-botanical interactions which are important to our laboratory, we developed and validated a high-throughput P450 cocktail inhibition assay using 10 substrates against nine P450 enzymes. Simultaneously assessing the inhibition of nine P450 isoforms can significantly reduce the cost and time needed for the evaluation of drug-drug and drug-botanical interactions. Our in vitro high-throughput cocktail approach optimized enzyme protein concentration, minimized probe substrate interactions, minimized solvent effects, and used a fast and sensitive ultrahigh pressure liquid chromatography (UHPLC)–tandem mass spectrometry (MS/MS) quantitative assay (Chauret et al., 1998; Busby et al., 1999; Yuan et al., 2002; Turpeinen et al., 2005; Jia and Liu, 2007; Smith et al., 2007; Otten et al., 2011; Kozakai et al., 2012; Lee and Kim, 2013; Spaggiari et al., 2014). After validating the new assay using nine known P450 inhibitors, an extract of the botanical dietary supplement licorice (Glycyrrhiza glabra) was evaluated for P450 inhibition.
Materials and Methods
Materials and Chemicals
Phenacetin was purchased from Tokyo Chemical Industry (Tokyo, Japan). Acetaminophen, coumarin, bupropion hydrochloride, tolbutamide, dextrorphan tartrate, chlorzoxazone, 6β-hydroxytestosterone, furafylline, ticlopidine hydrochloride, quercetin, sulfaphenazole, quinidine, ketoconazole, ammonium diethyldithiocarbamate, NADPH, formic acid, potassium phosphate monobasic, and potassium phosphate dibasic were purchased from Sigma-Aldrich (St. Louis, MO). 1′-Hydroxymidazolam and 6-hydroxychlorzoxazone were purchased from Cayman Chemical (Ann Arbor, MI). Dextromethorphan hydrobromide was obtained from MP Biomedicals (Santa Ana, CA). 7-hydroxycoumarin was purchased from Indofine Chemical (Hillsborough, NJ). Hydroxybupropion and hydroxybupropion-d6 were purchased from Santa Cruz Biotechnology (Dallas, TX). Midazolam and testosterone were obtained from Cerilliant Corporation (Round Rock, TX). [d5]-7-Hydroxycoumarin, [d7]-6β-hydroxytestosterone, and [13C3]-1′-hydroxymidazolam were purchased from BD Gentest (Woburn, MA). Amodiaquine, N-desethylamodiaquine hydrochloride, [d5]-N-desethylamodiaquine, (S)-mephenytoin, [d4]-acetaminophen, [d2]-6-hydroxychlorzoxazone, hydroxytolbutamide, [d9]-4-hydroxytolbutamide, (±)-4′-hydroxymephenytoin, [d3]-(±)-4′-hydroxymephenytoin, and [d3]-dextrorphan tartrate were obtained from Toronto Research Chemicals (Toronto, Canada).
Pooled human liver microsomes from 200 donors were purchased from XenoTech (Lenexa, KS). Liquid chromatography/mass spectrometry–grade acetonitrile and methanol were purchased from Thermo Fisher (Fair Lawn, NJ). Water was prepared using an Elga Purelab Ultra water purification system (Siemens Water Technologies, Woodridge, IL). An extract of licorice roots (G. glabra) was prepared from botanically authenticated plant material provided by the University of Illinois at Chicago/National Institutes of Health Center for Botanical Dietary Supplements Research (Chicago, IL). The licorice root was extracted with 90% ethanol, 5% isopropanol, 5% water (v/v/v) [weight root powder (grams)/volume solvent (milliliters): 1:15].
Microsomal Incubations
Potassium phosphate buffer (100 µl, 0.1 M, pH 7.4) containing 1.3 mM NADPH, 0.2 mg/ml human liver microsomes, and a cocktail of 10 probe substrates [phenacetin for CYP1A2, coumarin for CYP2A6, bupropion for CYP2B6, amodiaquine for CYP2C8, tolbutamide for CYP2C9, (S)-mephenytoin for CYP2C19, dextromethorphan for CYP2D6, chlorzoxazone for CYP2E1, and midazolam and testosterone for CYP3A4/5] (Table 1) or a single substrate (around Km) was incubated at 37°C for 10 minutes. Methanol was used to dissolve the substrate cocktail or individual substrate and comprised <0.3% (v/v) of the total incubation mixture. The reactions were terminated by adding 20 µl of a stop solution consisting of water/acetonitrile/formic acid (92:5:3, v/v/v) containing stable isotope-labeled surrogate standards (Table 1). The samples were then centrifuged at 13,000 × g at 4°C for 15 minutes prior to analysis using UHPLC-MS/MS.
TABLE 1.
Km values and concentrations of P450-specific probe substrates, and the MS/MS selected reaction monitoring (SRM) transitions and collision energies (CEs) for substrate metabolites and stable isotope-labeled internal standards used in the cocktail assays
| Enzyme | Substrate | Km | Concentration | Metabolite | SRM Transition (Polarity) | CE | Internal Standard | SRM Transition (Polarity) | CE |
|---|---|---|---|---|---|---|---|---|---|
| µM | µM | V | V | ||||||
| CYP1A2 | Phenacetin | 112.7 ± 10.9 | 100 | Acetaminophen | 152.2 > 110.0 (+) | −17 | [d4]-Acetaminophen | 156.2 > 114.1 (+) | −19 |
| CYP2A6 | Coumarin | 1.5 ± 0.2 | 1.5 | 7-Hydroxycoumarin | 161.1 > 133.1 (−) | 23 | [d5]-7-Hydroxycoumarin | 166.1 > 138.1 (−) | 22 |
| CYP2B6 | Bupropion | 125.2 ± 14.0 | 12 | Hydroxybupropion | 256.2 > 139.1 (+) | −26 | [d6]-Hydroxybupropion | 262.2 > 139.0 (+) | −26 |
| CYP2C8 | Amodiaquine | 1.0 ± 0.1 | 1 | N-desethylamodiaquine | 328.1 > 283.0 (+) | −21 | [d5]-N-desethylamodiaquine | 333.1 > 283.1 (+) | −29 |
| CYP2C9 | Tolbutamide | 110.7 ± 11.6 | 100 | Hydroxytolbutamide | 285.1 > 186.0 (−) | 18 | [d9]-Hydroxytolbutamide | 294.2 > 186.0 (−) | 18 |
| CYP2C19 | (S)-mephenytoin | 52.5 ± 10.6 | 50 | (±)-4′-Hydroxymephenytoin | 235.1 > 133.1 (+) | −18 | [d3]-(±)-4′-Hydroxymephenytoin | 238.1 > 133.1 (+) | −22 |
| CYP2D6 | Dextromethorphan | 2.9 ± 0.5 | 2.5 | Dextrorphan | 258.1 > 157.0 (+) | −37 | [d3]-Dextrorphan | 261.2 > 157.1 (+) | −40 |
| CYP2E1 | Chlorzoxazone | 149.8 ± 12.6 | 15 | 6-Hydroxychlorzoxazone | 184.0 > 120.0 (−) | 18 | [d2]-6-Hydroxychlorzoxazone | 186.2 > 122.0 (−) | 18 |
| CYP3A4/5 | Midazolam | 2.7 ± 0.1 | 2.5 | 1′-Hydroxymidazolam | 342.1 > 324.0 (+) | −21 | [13C3]-1′-hydroxymidazolam | 347.2 > 329.0 (+) | −22 |
| Testosterone | 50.5 ± 5.6 | 50 | 6β-Hydroxytestosterone | 305.2 > 269.2 (+) | −18 | [d7]-6β-Hydroxytestosterone | 312.2 > 276.2 (+) | −16 |
Michaelis Constant Determination.
Human liver microsomes (0.05–0.20 mg/ml) were incubated at 37°C for 5–20 minutes with each P450 substrate at 8–10 different concentrations. After quantitative analysis using UHPLC-MS/MS, Km values were calculated.
Linearity of Metabolite Formation in the Cocktail Assay.
Each cocktail reaction mixture was incubated at 37°C for 2.5, 5, 10, 15, or 20 minutes. After quenching and quantitative analysis, the linearity of metabolite formation was evaluated.
Comparison of Cocktail and Single-Substrate Assays Using Known P450 Inhibitors and Licorice Root Extract.
Known P450 inhibitors recommended by the FDA were used at 10 different concentrations as follows: 0.01–200 µM furafylline for CYP1A2, 0.005–100 µM methoxsalen for CYP2A6, 0.05–500 µM ticlopidine for CYP2B6 and CYP2C19, 0.01–200 µM quercetin for CYP2C8, 0.05–500 µM sulfaphenazole for CYP2C9, 0.005–100 µM quinidine for CYP2D6, 0.1–2000 µM diethyldithiocarbamate for CYP2E1, and 0.005–100 µM ketoconazole for CYP3A4/5. Licorice root extract at 11 concentrations from 0.005 to 250 µg/ml was also evaluated for inhibition. After incubation with probe substrates as described earlier and quantitative analysis of metabolites as described later, IC50 values were calculated.
UHPLC-MS/MS
All metabolites and surrogate standards were analyzed in a single run using UHPLC-MS/MS on a Shimadzu Nexera UHPLC and LCMS-8050 triple quadrupole mass spectrometer (Kyoto, Japan). The 10 metabolites of the probe substrates and their corresponding isotope-labeled internal standards were separated on a Waters ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm) (Milford, MA) using a 3-minute gradient from 20 to 75% acetonitrile in water containing 0.1% formic acid. The flow rate was 0.5 ml/min, and the column oven temperature was 40°C. Detection was carried out using electrospray with polarity switching, collision-induced dissociation, and selected reaction monitoring (Table 1).
Data Analysis
Quantitative UHPLC-MS/MS data were analyzed using Shimadzu LabSolutions software. The Km and IC50 values were determined using the Enzyme Kinetics module of SigmaPlot (Systat Software, San Jose, CA). The percentage of control activity, linearity of metabolite formation, and other calculations were carried out using Excel (Microsoft, Seattle, WA).
Results
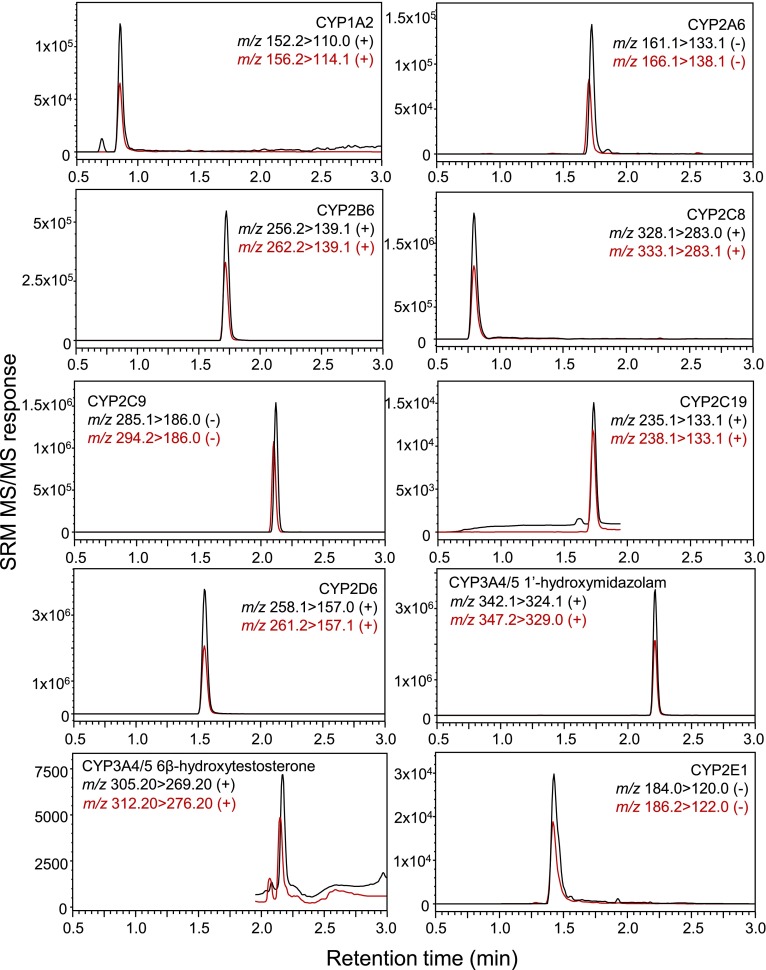
The 10 probe substrates specific to nine P450 isoforms (Table 1) were selected based on U.S. FDA recommendation (http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm), specificity of the enzymatic reaction, sensitivity of analytical detection, and availability of the stable isotope-labeled surrogate standard of the corresponding metabolites. The initial concentration of each substrate was determined based on its Km value (Table 1) and systematic evaluation of probe interactions. The selected reaction monitoring transitions for all 10 metabolites and their corresponding isotope-labeled internal standards were selected based on the most abundant fragment ions of each protonated or deprotonated molecule and are summarized in Table 1. The elution profiles of all metabolites and internal standards detected during UHPLC-MS/MS are shown in Fig. 1. Note that all compounds eluted within 3 minutes.
Fig. 1.
UHPLC-MS/MS chromatograms of probe substrate metabolites (100 nM) and their corresponding stable isotope-labeled internal standards. SRM, selected reaction monitoring.
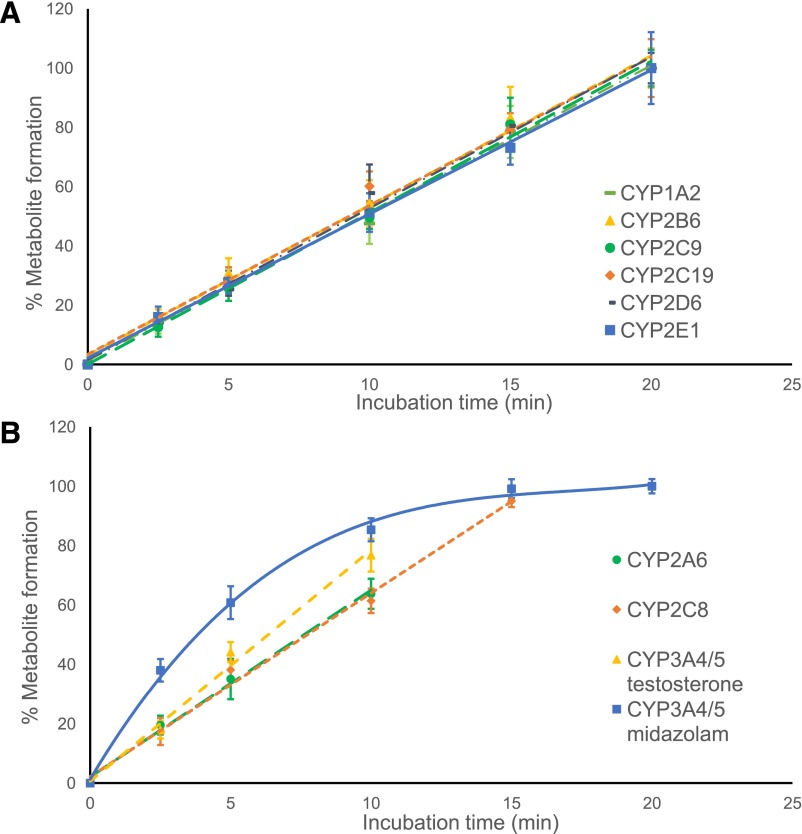
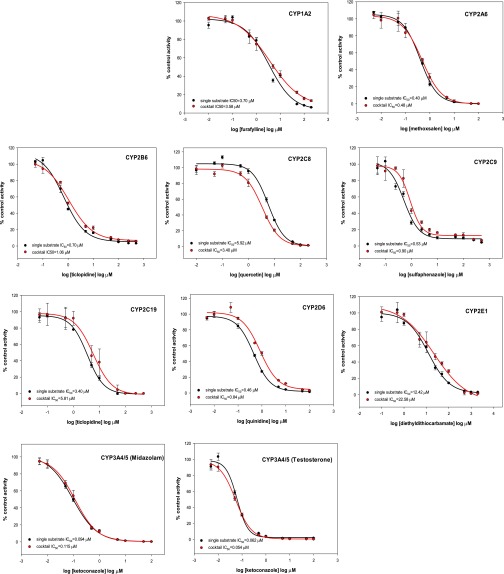
Although the metabolic transformation of most probe substrates in the cocktail was linear for at least 20 minutes, the rates of metabolism of coumarin by CYP2A6, of amodiaquine by CYP2C8, and of testosterone by CYP3A4/5 showed linearity for up to only 10–15 minutes. The formation of 1′-hydroxymidazolam was not linear under any incubation conditions but was most linear during the first 10 minutes (Fig. 2). Therefore, an incubation time of 10 minutes was selected for the entire cocktail assay based on the linearity of formation of most metabolites during this period. Using known inhibitors of each enzymatic reaction, validation of the cocktail assay was carried out by comparing the IC50 values obtained using the cocktail approach with those obtained using only single substrates. Inhibition curves and IC50 values (Fig. 3; Table 2) showed good accordance between the cocktail assay and the single-substrate method.
Fig. 2.
Linearity of substrate metabolite formation during the cocktail assay. Metabolite formation is expressed as a percentage of the amount of metabolite at 20 minutes. A) P450 enzymes CYP1A2, CYP2B6, CYP2C9, CYP2CI9, CYP2D6, and CYP2E1. B) P450 enzymes CYP2A6, CYP2C8, CYP3A4/5 (testosterone substrate), and CYP3A4/5 (midazolam substrate).
Fig. 3.
Inhibition curves of known inhibitors obtained using single substrates and substrate cocktails. Each inhibitor was incubated in separate experiments with single substrates or the substrate cocktail. The activity is expressed as a percentage of remaining activity compared with the control containing no inhibitor. Experiments were carried out three times.
TABLE 2.
Comparison of IC50 values of known inhibitors obtained using the single-substrate approach, the new cocktail approach, and literature values
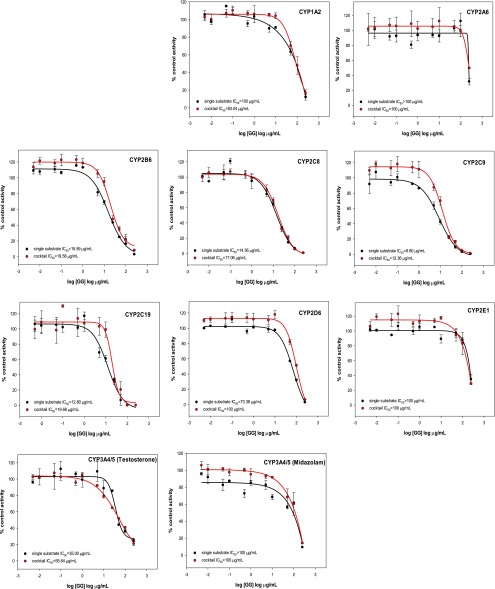
The new cocktail assay was then used to assess the potential for drug-botanical interactions of a licorice root extract from G. glabra (Fig. 4). The licorice root extract inhibited CYP2B6 and the CYP2C family of enzymes (CYP2C8, CYP2C9, and CYP2C19), with IC50 values <20 μg/ml. Note that good agreement was observed between the cocktail and single-substrate approaches as indicated by the data in Table 3.
Fig. 4.
Inhibition curves of a licorice root extract obtained using single substrates or the substrate cocktail. Each inhibitor was incubated in separate experiments with a single substrate or the substrate cocktail. The activity is shown as a percentage of remaining activity compared with the control when no inhibitor was added. Experiments were carried out three times. GG = Glycyrrhiza glabra extract.
TABLE 3.
IC50 values for inhibition of P450 enzymes by a licorice extract (G. glabra) obtained using the single-substrate approach or the new cocktail approach and comparison with literature values
| Enzyme | Substrate | Single-Substrate IC50 | Cocktail IC50 |
|---|---|---|---|
| µg/ml | µg/ml | ||
| CYP1A2 | Phenacetin | >100 | 83.04 ± 16.96 |
| CYP2A6 | Coumarin | >100 | >100 |
| CYP2B6 | Bupropion | 15.59 ± 1.79 | 19.58 ± 1.80 |
| CYP2C8 | Amodiaquine | 14.36 ± 2.62 | 17.06 ± 1.54 |
| CYP2C9 | Tolbutamide | 8.80 ± 1.20 | 12.36 ± 1.14 |
| CYP2C19 | (S)-mephenytoin | 12.80 ± 2.34 | 19.68 ± 2.70 |
| CYP2D6 | Dextromethorphan | 73.38 ± 11.72 | >100 |
| CYP3A4/5 | Midazolam | >100 | >100 |
| Testosterone | 35.00 ± 4.81 | 55.64 ± 18.49 | |
| CYP2E1 | Chlorzoxazone | >100 | >100 |
Discussion
Probe Substrate Selection and Mass Spectrometry.
Phenacetin O-deethylation by CYP1A2 was selected for the cocktail assay and is often used in other cocktails (Yuan et al., 2002; Spaggiari et al., 2014) because of its superior specificity compared with other FDA-recommended substrates. Among several possible probes for CYP2A6, CYP2B6, and CYP2E1, coumarin-7-hydroxylation, bupropion-hydroxylation, and chlorzoxazone 6-hydroxylation were selected based on the commercial availability of the corresponding stable isotope-labeled metabolites for use as surrogate standards during UHPLC-MS/MS (Fig. 1). For CYP2C8, taxol and amodiaquine are frequently used as probe substrates; however, the higher solubility of amodiaquine makes it superior for cocktail applications (Spaggiari et al., 2014). Therefore, amodiaquine N-deethylation by CYP2C8 was measured instead of taxol hydroxylation.
Tolbutamide and diclofenac are frequently used as probe substrates for CYP2C9, and bufuralol and dextromethorphan are often used as substrates of CYP2D6. Although either probe substrate of each pair could have been used in our assay, tolbutamide methyl-hydroxylation and dextromethorphan O-demethylation were used to measure interactions with CYP2C9 and CYP2D6, respectively. As a probe for CYP2C19, (S)-mephenytoin 4′-hydroxylation is highly specific; however, cocktail assays typically use the less-specific omeprazole due to the sensitivity limitations of most detection methods (Yuan et al., 2002; Testino and Patonay, 2003; Spaggiari et al., 2014). Here, we were able to use the preferred probe substrate, (S)-mephenytoin, due to the high sensitivity of UHPLC-MS/MS (Fig. 1). For evaluation of CYP3A4/5 inhibition, the use of two structurally unrelated substrates is recommended (Yuan et al., 2002; http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm). Therefore, midazolam 1-hydroxlyation and testosterone 6β-hydroxylation were used to probe inhibition of CYP3A4/5 as recommended by the FDA. For [13C3]-1′-hydroxymidazolam, we monitored the second most abundant protonated ion m/z 247 instead of the most abundant protonated ion m/z 245 due to the isotopic interference from 1′-hydroxymidazolam.
7-Hydroxycoumarin, hydroxytolbutamide, and 6-hydroxychlorzoxazone were measured using negative electrospray, whereas the other seven metabolites were measured in positive ion mode (Fig. 1). Poor ionization efficiency of 6-hydroxychlorzoxazone during positive ion electrospray and the inability of some mass spectrometers to carry out rapid polarity switching have been cited as reasons for excluding CYP2E1 from some previous cocktail assays (He et al., 2007). Chlorzoxazone was included in our cocktail assay as a probe for CYP2E1 (Fig. 1), because the fast polarity switching and high sensitivity of this generation triple quadrupole mass spectrometer enabled the measurement of all 10 probes including 6-hydroxychlorzoxazone and their surrogate standards in a single analysis.
Optimization of Probe Substrate Concentrations and Incubation Conditions.
The probe substrate concentrations in cocktail assays should be less than or equal to the Km of the corresponding cytochrome P450 enzymes. Although Km values from the literature have been used in the design of most existing cocktail assays, the reported values can span a wide range (Turpeinen et al., 2005; Liu et al., 2015). For example, the Km values reported for phenacetin O-deethylation range from 1.7 to 152 µM, and those for tolbutamide methyl-hydroxylation range from 67 to 838 µM (http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm). The variability of Km values between laboratories is primarily caused by differences in experimental procedures and genetic variations in the enzymes being probed. To be certain that appropriate concentrations of each probe substrate were used in our application, we determined the Km values for each probe substrate using the same experimental procedures, and the same batch of pooled human liver microsomes was used for all subsequent inhibition experiments (Table 1).
Possible interactions between the probe substrates of the P450 enzymes were evaluated, and substrate concentrations for the cocktail assay were adjusted to minimize these interactions. Phenacetin weakly inhibited CYP2B6 and CYP3A4/5 (midazolam); bupropion strongly inhibited the activities of CYP2C19 and CYP2D6 and weakly inhibited CYP3A4/5 (midazolam). Chlorzoxazone inhibited CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, and CYP3A4/5 (midazolam and testosterone), and testosterone moderately inhibited CYP2B6 and CYP3A4/5 (midazolam) while weakly inhibiting CYP2C9.
Lowering substrate concentrations was an effective strategy to decrease interactions in the cocktail assay. For example, because bupropion and chlorzoxazone strongly inhibited multiple P450 isoforms, 10 substrate mixtures containing varying concentrations (0.05Km, 0.1Km, 0.2Km, 0.4Km, and 0.8Km) of bupropion and chlorzoxazone were tested. (S)-mephenytoin is a low-turnover substrate of CYP2C19 (Yao et al., 2012), and to ensure analytical detection of its metabolite 4′-hydroxymephenytoin, both bupropion and chlorzoxazone concentrations needed to be ≤0.1Km (data not shown). Therefore, the concentrations of bupropion and chlorzoxazone were set to 0.1Km in the cocktail assay.
After optimizing the substrate concentrations for the cocktail assay (Table 1), the linearity of metabolite formation was investigated. All 10 substrates were evaluated: six of them showed linearity up to 20 minutes, whereas four substrates were linear for 15 minutes or less (Fig. 2). Although a 10-minute incubation time was not optimal for midazolam, it was the minimum required to ensure sufficient metabolite formation for the low-turnover substrates. Under these conditions, the inhibition potency of moderate inhibitors for midazolam 1-hydroxlyation might be underestimated (Ogilvie et al., 2008). Therefore, further studies on any compounds/extracts that show moderate/weak inhibition of CYP3A4/5 (midazolam) would be recommended.
Validation and Application of the Cocktail Assay.
Ideally, the cocktail cytochrome P450 enzyme inhibition assay should yield the same results as would be obtained if each substrate were assayed separately. As quantitative measures of the potencies of enzyme inhibitors, the IC50 values for inhibitors tested individually or in the cocktail assay should also be comparable (Sittampalam et al., 2004; Davis and Ward, 2014). The ratios of the IC50 values obtained using both approaches (Table 2) were compared and ranged from 1.03 to 1.82. Because these values were within a 2-fold range of each other, they were in good agreement. As additional validation of the cocktail method, the measured IC50 values were consistent with values in the literature (Table 2) (Eagling et al., 1998; Shader et al., 1999; Dierks et al., 2001; Giancarlo et al., 2001; Patki et al., 2003; Testino and Patonay, 2003; Walsky and Obach, 2004; Kim et al., 2005; Turpeinen et al., 2005; Workman and Raynaud, 2007; Zientek et al., 2008; Otten et al., 2011; Kozakai et al., 2012; Yao et al., 2012; Qiao et al., 2014; Qin et al., 2014; Liu et al., 2015).
IC50 values are extrinsic constants and depend on experimental conditions, but Ki values are intrinsic constants. Therefore, researchers sometimes estimate Ki from IC50 values (Otten et al., 2011). Assuming competitive inhibition, Ki values can be calculated using the Cheng-Prusoff equation (Ki = IC50/2 when substrate concentration is ≈Km; and Ki = IC50/1.1 when the substrate concentration is Km/10). Using the IC50 values in Table 2 and the Cheng-Prusoff equation, the calculated cocktail Ki values are in good accordance with those obtained for single-substrate assays, except for ticlopidine (CYP2B6) and diethyldithiocarbamate (CYP2E1), for which the calculated Ki values for the cocktail assays (0.96 and 20.53 µM) were ∼3-fold higher than those for the single-substrate assays (0.35 and 6.21 µM). One possible explanation might be that ticlopidine and diethyldithiocarbamate are mechanism-based inhibitors of CYP2B6 and CYP2E1, respectively, and their IC50 values are susceptible to changes of microsomal conditions (Turpeinen et al., 2004; Ogilvie et al., 2008; Pratt-Hyatt et al., 2010).
Kent et al. (2002) reported that an alcoholic extract of licorice root (G. glabra) moderately inhibited CYP3A4, and its flavonoid glabridin inhibited the activities of CYP2B6, CYP2C9, and CYP3A4. Our G. glabra extract showed moderate inhibition of CYP2B6, CYP2C9, CYP2C8, and CYP2C19 (which were not tested by Kent et al., 2000) but only weak inhibition of CYP3A4/5. The slight difference between inhibition potencies of CYP3A4/5 for these two assays is probably due to the different preparations of licorice extracts used or minor assay variations.
In conclusion, an in vitro high-throughput cytochrome P450 cocktail inhibition assay containing 10 substrates for nine P450 isoforms was developed using UHPLC-MS/MS and validated using known inhibitors of each P450 enzyme. This assay includes all seven cytochrome P450 enzymes recommended for testing by the U.S. FDA as well as two additional isoforms, CYP2A6 and CYP2E1, which are also important in the metabolism of xenobiotic compounds such as nicotine (CYP2A6) and ethanol (CYP2E1) (Pelkonen et al., 2000; Dey, 2013). Besides the obvious time efficiency and resource-saving advantages of combining 10 substrates into a single assay, each incubation was only 10 minutes compared with up to 60 minutes in some previous assays, and each UHPLC-MS/MS analysis required less than 5 minutes per sample, which is up to 5-fold faster than comparable HPLC-MS/MS approaches. The assay was applied to the prediction of drug-botanical interactions for a licorice root dietary supplement. As a complement to drug-drug interaction studies, drug-botanical interactions are understudied and yet can cause drug toxicity and therapeutic failure.
Acknowledgments
The authors thank Dr. Charlotte Simmler and Dr. Guido F. Pauli of the University of Illinois at Chicago/National Institutes of Health Center for Botanical Dietary Supplements for providing extracts of G. glabra. The authors also thank Shimadzu Instruments for providing the UHPLC-MS/MS system used during this investigation.
Abbreviations
- FDA
Food and Drug Administration
- MS/MS
tandem mass spectrometry
- P450
cytochrome P450
- UHPLC
ultrahigh pressure liquid chromatography
Authorship Contributions
Participated in research design: Li, Huang, Nikolic, van Breemen.
Conducted experiments: Li.
Performed data analysis: Li.
Wrote or contributed to the writing of the manuscript: Li, van Breemen.
Footnotes
This work was supported by the National Institutes of Health Office of Dietary Supplements [Grant P50 AT000155] and the National Center for Complementary and Integrative Health [Grants P50 AT000155 and R01 AT007659].
References
- Alden PG, Plumb RS, Jones MD, Rainville PD, Shave D. (2010) A rapid ultra-performance liquid chromatography/tandem mass spectrometric methodology for the in vitro analysis of Pooled and Cocktail cytochrome P450 assays. Rapid Commun Mass Spectrom 24:147–154. [DOI] [PubMed] [Google Scholar]
- Bertelsen KM, Venkatakrishnan K, Von Moltke LL, Obach RS, Greenblatt DJ. (2003) Apparent mechanism-based inhibition of human CYP2D6 in vitro by paroxetine: comparison with fluoxetine and quinidine. Drug Metab Dispos 31:289–293. [DOI] [PubMed] [Google Scholar]
- Busby WF, Jr, Ackermann JM, Crespi CL. (1999) Effect of methanol, ethanol, dimethyl sulfoxide, and acetonitrile on in vitro activities of cDNA-expressed human cytochromes P-450. Drug Metab Dispos 27:246–249. [PubMed] [Google Scholar]
- Chatterjee P, Franklin MR. (2003) Human cytochrome p450 inhibition and metabolic-intermediate complex formation by goldenseal extract and its methylenedioxyphenyl components. Drug Metab Dispos 31:1391–1397. [DOI] [PubMed] [Google Scholar]
- Chauret N, Gauthier A, Nicoll-Griffith DA. (1998) Effect of common organic solvents on in vitro cytochrome P450-mediated metabolic activities in human liver microsomes. Drug Metab Dispos 26:1–4. [PubMed] [Google Scholar]
- Davis A, Ward SE. (2014) The Handbook of Medicinal Chemistry: Principles and Practice, Royal Society of Chemistry, Cambridge, UK. [Google Scholar]
- Dey A(2013) Cytochrome P450 2E1: Its Role in Disease and Drug Metabolism, Springer., Dordrecht, The Netherlands. [Google Scholar]
- Dierks EA, Stams KR, Lim HK, Cornelius G, Zhang H, Ball SE. (2001) A method for the simultaneous evaluation of the activities of seven major human drug-metabolizing cytochrome P450s using an in vitro cocktail of probe substrates and fast gradient liquid chromatography tandem mass spectrometry. Drug Metab Dispos 29:23–29. [PubMed] [Google Scholar]
- Dinger J, Meyer MR, Maurer HH. (2014) Development of an in vitro cytochrome P450 cocktail inhibition assay for assessing the inhibition risk of drugs of abuse. Toxicol Lett 230:28–35 Elsevier Ireland Ltd. [DOI] [PubMed] [Google Scholar]
- Eagling VA, Tjia JF, Back DJ. (1998) Differential selectivity of cytochrome P450 inhibitors against probe substrates in human and rat liver microsomes. Br J Clin Pharmacol 45:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (2012). Guidance for Industry: Drug Interaction Studies-Study Design, Data Analysis, Implications for Dosing and Labeling Recommendations (Draft). http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm292362.pdf.
- Giancarlo GM, Venkatakrishnan K, Granda BW, von Moltke LL, Greenblatt DJ. (2001) Relative contributions of CYP2C9 and 2C19 to phenytoin 4-hydroxylation in vitro: inhibition by sulfaphenazole, omeprazole, and ticlopidine. Eur J Clin Pharmacol 57:31–36. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Khan IA, Shah A. (2005) In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clin Pharmacol Ther 77:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Bi HC, Xie ZY, Zuo Z, Li JK, Li X, Zhao LZ, Chen X, Huang M. (2007) Rapid determination of six metabolites from multiple cytochrome P450 probe substrates in human liver microsome by liquid chromatography/mass spectrometry: application to high-throughput inhibition screening of terpenoids. Rapid Commun Mass Spectrom 21:635–643. [DOI] [PubMed] [Google Scholar]
- Jia L, Liu X. (2007) The conduct of drug metabolism studies considered good practice (II): in vitro experiments. Curr Drug Metab 8:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent UM, Aviram M, Rosenblat M, Hollenberg PF. (2002) The licorice root derived isoflavan glabridin inhibits the activities of human cytochrome P450S 3A4, 2B6, and 2C9. Drug Metab Dispos 30:709–715. [DOI] [PubMed] [Google Scholar]
- Kim M-J, Kim H, Cha I-J, Park J-S, Shon J-H, Liu K-H, Shin J-G. (2005) High-throughput screening of inhibitory potential of nine cytochrome P450 enzymes in vitro using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 19:2651–2658. [DOI] [PubMed] [Google Scholar]
- Kozakai K, Yamada Y, Oshikata M, Kawase T, Suzuki E, Haramaki Y, Taniguchi H. (2012) Reliable high-throughput method for inhibition assay of 8 cytochrome P450 isoforms using cocktail of probe substrates and stable isotope-labeled internal standards. Drug Metab Pharmacokinet 27:520–529. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kim SK. (2013) Direct and metabolism-dependent cytochrome P450 inhibition assays for evaluating drug-drug interactions. J Appl Toxicol 33:100–108. [DOI] [PubMed] [Google Scholar]
- Li X, Chen X, Li Q, Wang L, Zhong D. (2007) Validated method for rapid inhibition screening of six cytochrome P450 enzymes by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 852:128–137. [DOI] [PubMed] [Google Scholar]
- Liu L, Han Y, Zhu J, Yu Q, Yang Q. (2015) A sensitive and high-throughput LC-MS/MS method for inhibition assay of seven major cytochrome P450s in human liver microsomes using an in vitro cocktail of probe substrates. Biomed Chromatogr 29:437–444. [DOI] [PubMed] [Google Scholar]
- Ogilvie BW, Usuki E, Yerino P, Parkinson A. (2008) In Vitro Approaches for Studying the Inhibition of Drug-Metabolizing Enzymes and Identifying the Drug-Metabolizing Enzymes Responsible for the Metabolism of Drugs (Reaction Phenotyping) with Emphasis on Cytochrome, in Drug-Drug Interactions (Rodrigues DA. ed) p 450, Informa Healthcare, New York. [Google Scholar]
- Otten JN, Hingorani GP, Hartley DP, Kragerud SD, Franklin RB. (2011) An in vitro, high throughput, seven CYP cocktail inhibition assay for the evaluation of new chemical entities using LC-MS/MS. Drug Metab Lett 5:17–24. [DOI] [PubMed] [Google Scholar]
- Patki KC, Von Moltke LL, Greenblatt DJ. (2003) In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes p450: role of cyp3a4 and cyp3a5. Drug Metab Dispos 31:938–944. [DOI] [PubMed] [Google Scholar]
- Pelkonen O, Rautio A, Raunio H, Pasanen M. (2000) CYP2A6: a human coumarin 7-hydroxylase. Toxicology 144:139–147. [DOI] [PubMed] [Google Scholar]
- Pratt-Hyatt M, Lin HL, Hollenberg PF. (2010) Mechanism-based inactivation of human CYP2E1 by diethyldithocarbamate. Drug Metab Dispos 38:2286–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X, Ji S, Yu S-W, Lin X-H, Jin H-W, Duan Y-K, Zhang L-R, Guo D-A, Ye M. (2014) Identification of key licorice constituents which interact with cytochrome P450: evaluation by LC/MS/MS cocktail assay and metabolic profiling. AAPS J 16:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C-Z, Ren X, Tan Z-R, Chen Y, Yin J-Y, Yu J, Qu J, Zhou H-H, Liu Z-Q. (2014) A high-throughput inhibition screening of major human cytochrome P450 enzymes using an in vitro cocktail and liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 28:197–203. [DOI] [PubMed] [Google Scholar]
- Shader RI, Granda BW, von Moltke LL, Giancarlo GM, Greenblatt DJ. (1999) Inhibition of human cytochrome P450 isoforms in vitro by zafirlukast. Biopharm Drug Dispos 20:385–388. [DOI] [PubMed] [Google Scholar]
- Sittampalam GS, Gal-Edd N, Arkin M, Sittampalam GS, Coussens NP, Nelson H, Arkin M, Auld D, Austin C, Bejcek B, et al. (2004) Assay Guidance Manual National Center for Biotechnology Information, Bethesda, MD. [Google Scholar]
- Smith D, Sadagopan N, Zientek M, Reddy A, Cohen L. (2007) Analytical approaches to determine cytochrome P450 inhibitory potential of new chemical entities in drug discovery. J Chromatogr B Analyt Technol Biomed Life Sci 850:455–463. [DOI] [PubMed] [Google Scholar]
- Spaggiari D, Geiser L, Daali Y, Rudaz S. (2014) A cocktail approach for assessing the in vitro activity of human cytochrome P450s: an overview of current methodologies. J Pharm Biomed Anal 101:221–237. [DOI] [PubMed] [Google Scholar]
- Testino SA, Jr, Patonay G. (2003) High-throughput inhibition screening of major human cytochrome P450 enzymes using an in vitro cocktail and liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 30:1459–1467. [DOI] [PubMed] [Google Scholar]
- Tolonen A, Petsalo A, Turpeinen M, Uusitalo J, Pelkonen O. (2007) In vitro interaction cocktail assay for nine major cytochrome P450 enzymes with 13 probe reactions and a single LC/MSMS run: analytical validation and testing with monoclonal anti-CYP antibodies. J Mass Spectrom 42:960–966. [DOI] [PubMed] [Google Scholar]
- Turpeinen M, Nieminen R, Juntunen T, Taavitsainen P, Raunio H, Pelkonen O. (2004) Selective inhibition of CYP2B6-catalyzed bupropion hydroxylation in human liver microsomes in vitro. Drug Metab Dispos 32:626–631. [DOI] [PubMed] [Google Scholar]
- Turpeinen M, Uusitalo J, Jalonen J, Pelkonen O. (2005) Multiple P450 substrates in a single run: rapid and comprehensive in vitro interaction assay. Eur J Pharm Sci 24:123–132. [DOI] [PubMed] [Google Scholar]
- Walsky RL, Obach RS. (2004) Validated assays for human cytochrome P450 activities. Drug Metab Dispos 32:647–660. [DOI] [PubMed] [Google Scholar]
- Workman P, Raynaud FI. (2007) Analysis of potential drug-drug interactions for anti-cancer agents in human liver microsomes by high throughput liquid chromatography/mass spectrometry assay. Austral-Asian J Cancer 6:55–69. [Google Scholar]
- Yao M, Cai H, Zhu M. (2012) Fast and reliable cyp inhibition assays, in ADME-Enabling Technologies in Drug Design and Development (Zhang D, Surapaneni S. ed) pp 213–232, John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- Yuan R, Madani S, Wei X-X, Reynolds K, Huang S-M. (2002) Evaluation of cytochrome P450 probe substrates commonly used by the pharmaceutical industry to study in vitro drug interactions. Drug Metab Dispos 30:1311–1319. [DOI] [PubMed] [Google Scholar]
- Zientek M, Miller H, Smith D, Dunklee MB, Heinle L, Thurston A, Lee C, Hyland R, Fahmi O, Burdette D. (2008) Development of an in vitro drug-drug interaction assay to simultaneously monitor five cytochrome P450 isoforms and performance assessment using drug library compounds. J Pharmacol Toxicol Methods 58:206–214. [DOI] [PubMed] [Google Scholar]