Abstract
In most organs, the bulk of cholesterol is unesterified, although nearly all possess a varying capability of esterifying cholesterol through the action of either sterol O-acyltransferase (SOAT) 1 or, in the case of hepatocytes and enterocytes, SOAT2. Esterified cholesterol (EC) carried in plasma lipoproteins is hydrolyzed by lysosomal acid lipase (LAL) when they are cleared from the circulation. Loss-of-function mutations in LIPA, the gene that encodes LAL, result in Wolman disease or cholesteryl ester storage disease (CESD). Hepatomegaly and a massive increase in tissue EC levels are hallmark features of both disorders. While these conditions can be corrected with enzyme replacement therapy, the question arose as to whether pharmacological inhibition of SOAT2 might reduce tissue EC accretion in CESD. When weaned at 21 days, Lal−/− mice, of either gender, had a whole liver cholesterol content that was 12- to 13-fold more than that of matching Lal+/+ littermates (23 versus 1.8 mg, respectively). In Lal−/− males given the selective SOAT2 inhibitor PRD125 1,11-O-o-methylbenzylidene-7-O-p-cyanobenzoyl-1,7,11-trideacetylpyripyropene A in their diet (∼10 mg/day per kg body weight) from 21 to 53 days, whole liver cholesterol content was 48.6 versus 153.7 mg in untreated 53-day-old Lal−/− mice. This difference reflected a 59% reduction in hepatic EC concentration (mg/g), combined with a 28% fall in liver mass. The treated mice also showed a 63% reduction in plasma alanine aminotransferase activity, in parallel with decisive falls in hepatic mRNA expression levels for multiple proteins that reflect macrophage presence and inflammation. These data implicate SOAT2 as a potential target in CESD management.
Introduction
In healthy young adult mammals ranging from mice to primates, the pool of cholesterol in the whole animal averages about 2200 mg/kg body weight, with this sterol originating in varying degrees from endogenous and exogenous sources (Grundy, 1983; Turley and Dietschy, 1988; Dietschy and Turley, 2004). Ordinarily, in nearly all the major organ systems, the bulk of this cholesterol is unesterified, (d’ Hollander and Chevallier, 1969). Exceptions include the adrenal glands and plasma (Goodman, 1965; d’ Hollander and Chevallier, 1969). In some organs, most notably the liver, the esterified cholesterol (EC) content can vary over a wide range in response to shifts in the dietary intake of cholesterol or specific fatty acids (Rudel et al., 1997; Xie et al., 2002; Turley et al., 2010).
Most dietary cholesterol is unesterified (Tso, 1994). Even if this was not the case, none of the esterified cholesterol present in tissues originates from external sources because pancreatic cholesterol esterase hydrolyzes cholesteryl esters present in foodstuff (Tso, 1994). Moreover, esterified cholesterol is poorly absorbed (Goodman, 1965). Several organs are capable of generating esterified cholesterol through the action of either sterol O-acyltransferase (SOAT) 1 (also known as acyl-coenzyme A cholesterol O-acyltransferase 1), which is present in steroidogenic tissues, kidneys, sebaceous glands, and macrophages, or SOAT2 (acyl-coenzyme A cholesterol O-acyltransferase 2), which is expressed in hepatocytes and enterocytes (Cases et al., 1998; Lee et al., 2000; Parini et al., 2004). In the plasma compartment, cholesterol esterification is facilitated by lecithin cholesterol acyltransferase (Rousset et al., 2011). The roles that both SOAT1 and SOAT2 play in generating cholesteryl esters, and therefore in the pathogenesis of atherosclerosis, have made these enzymes, particularly SOAT2, attractive targets for pharmacological intervention (Lada et al., 2004; Rudel et al., 2005; Chang et al., 2006; Farese, 2006). This has led to the identification of a new group of compounds that are selective inhibitors of SOAT2 (Ohshiro et al., 2011; Ohtawa et al., 2013). Prior to the discovery that there are two sterol O-acyltransferases, decades of research into the development of various classes of SOAT inhibitors yielded a number of compounds that markedly suppressed cholesterol esterification in vitro and in animal models. Clinical trials were carried out to evaluate the efficacy of two of these compounds, avasimibe and pactimibe, in slowing the progression of coronary atherosclerosis. In neither case was the outcome favorable (Tardif et al., 2004; Nissen et al., 2006).
Although the major focus on EC accumulation in disease centers on its involvement in arterial plaque formation (Peng et al., 2000), there are a number of rare disorders that are characterized by elevated tissue EC content (Goodman, 1965). These include Tangier disease, Wolman disease (WD), and cholesteryl ester storage disease (CESD) (Remaley et al., 1997; Grabowski et al., 2015). WD and CESD result from mutations in LIPA, the gene that encodes lysosomal acid lipase (LAL), which hydrolyzes EC and triglycerides contained in low density lipoproteins (LDLs) and related particles that are cleared from the circulation by receptor-mediated and bulk-phase endocytosis (Goldstein et al., 1975). An enzyme replacement therapy for WD and CESD is currently being evaluated (Balwani et al., 2013). The study of these two diseases has been aided by the development of animal models. In 1990, the phenotype of a rat model for WD was described (Kuriyama et al., 1990). Subsequently, a mouse model for CESD was developed (Du et al., 2001). A detailed characterization of multiple parameters of cholesterol metabolism in a CESD mouse model was recently reported (Aqul et al., 2014).
The massive accumulation of EC in tissues caused by LAL deficiency raised the question of what effect the loss of SOAT2 function in Lal−/− mice might have on the severity of their disease. Our initial observations in the LAL:SOAT2 double-knockout mice showed a decisive beneficial effect, with substantial reductions in the degree of hepatomegaly, liver EC concentrations, and plasma transaminase activities (Lopez et al., 2014). These findings prompted us to now investigate the efficacy of blocking SOAT2 activity pharmacologically in Lal−/− mice, starting from the time of weaning. This was done by adding a new SOAT2-selective inhibitor [1,11-O-o-methylbenzylidene-7-O-p-cyanobenzoyl-1,7,11-trideacetylpyripyropene A (PRD125)], a more potent derivative of another compound called pyripyropene A, to their diet (Ohshiro et al., 2011; Ohtawa et al., 2013). The data reported here show that in the treated Lal−/− mice, hepatic EC accumulation was substantially reduced, as were the levels of expression of mRNA markers for macrophage presence and inflammation and plasma transaminase activities. Together, these findings suggest that suppression of SOAT2 activity in CESD may have a therapeutic benefit.
Materials and Methods
Animals and Diets.
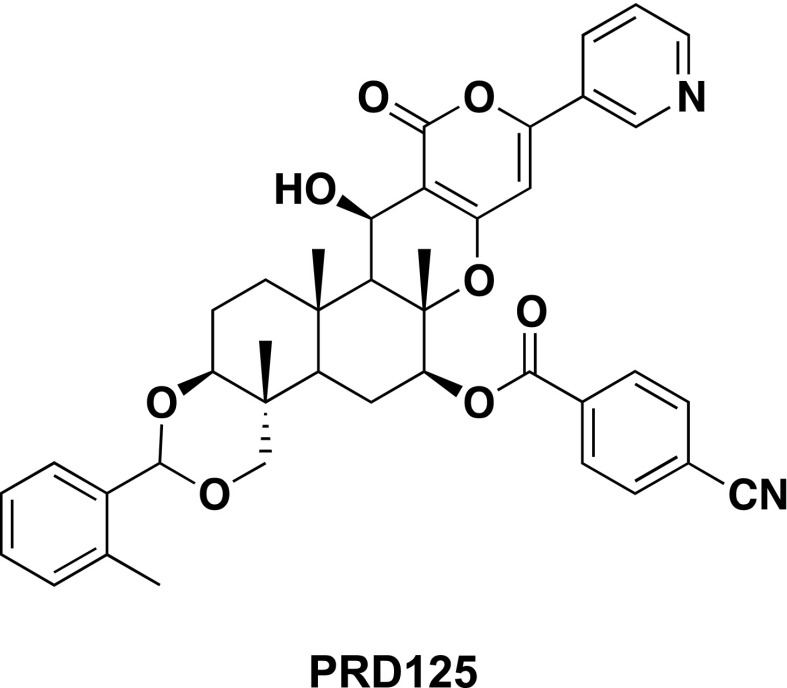
The Lal+/− breeding stock used to generate the Lal+/+ and Lal−/− mice originated from the laboratory of Drs. Grabowski and Du at the Children’s Hospital Research Foundation in Cincinnati, OH (Du et al., 1998, 2001). All mice were of the Friend leukemia virus B/N strain (FVB/N). Litters were genotyped at 18–20 days and weaned at 21 days. For all studies, the diet used was a low fat rodent chow formulation (Teklad No. 7001; Harlan Laboratories, Madison, WI), with an inherent cholesterol content of 0.02% w/w (0.2 mg cholesterol/g diet). The development of PRD125 was carried out at the Graduate School of Pharmaceutical Sciences, Kitasato University, Tokyo, Japan. Its structure is shown in Fig. 1. PRD125 is a derivative of pyripyropene A (hence the prefix PRD), the first compound to exhibit strong and selective inhibitory action toward SOAT2 (Ohshiro et al., 2011). Its inhibitory activity toward SOAT1 and SOAT2 and isozyme selectivity were reported before the name PRD125 was applied. Initially, it was called compound 7q (Ohtawa et al., 2013) and was reported to have a selectivity index toward SOAT2 versus SOAT1 that was >6161. PRD125 was added directly to the meal (powder) form of this diet in the proportion of 62.5 mg of compound per 1000 g of chow. Initially, the compound was incorporated into a small amount of chow using a mortar and pestle. The resulting premix was then dispersed thoroughly in the remaining chow using a food mixer (Hobart Corp., Troy, OH). This formulation provided the mice with an approximate dose of PRD125 of 10 mg/day per kg body weight (bw) based on a daily food intake of about 160 g/day per kg bw. This figure was obtained from measurements of the food intake of three Lal+/+ and three Lal−/− mice, all housed individually, over 4 consecutive days, starting when they were 40 days old. An estimated daily spillage of 10% was used in calculating their daily intake, which did not vary as a function of genotype.
Fig. 1.
Structure of PRD125. The chemical name for PRD125 is 1,11-O-o-methylbenzylidene-7-O-p-cyanobenzoyl-1,7,11-trideacetylpyripyropene A.
Although the PRD125 feeding experiments were carried out in male mice only, the decisions regarding the age of when to start treatment and also the length of the treatment period were based to a considerable degree on metabolic data obtained from female Lal−/− mice at different stages of disease progression (21, 49–51, and 140–142 days of age). In all cases, those mice had been fed only the basal chow diet from the time of weaning to the day of study. For the PRD125 experiment, it was decided that treatment would start on the day of weaning (21 days) and continue until the mice were 52 or 53 days of age. Matching groups of Lal+/+ and Lal−/− mice were given the chow diet alone, whereas corresponding groups were fed the diet containing PRD125. These mice were group housed up until about 1 week before study. They were then housed individually to facilitate stool collection over a 4-day period. In both studies, all animals had unrestricted access to their respective diet and water and were in the fed state at the time of study. All studies were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center.
Organ Resection and Processing.
The mice were anesthetized with isoflurane [2-chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane] and exsanguinated from the vena cava into a heparinized syringe. Plasma was obtained immediately thereafter and used for measurements of transaminase activity and total cholesterol concentration. The liver and whole small intestine were excised, and the latter was cut into eight segments that were perfused with saline from a syringe fitted with a blunt 16 G needle. After blotting on filter paper, the weight of all sections combined was recorded. The liver was also rinsed with saline and blotted before being weighed. The whole small intestine and several aliquots of liver were each added to about 30 ml of chloroform/methanol (2:1 v/v). The residual carcass was weighed and added to 200 ml of ethanolic potassium hydroxide.
Hepatic, Intestinal, Whole Body, and Plasma Cholesterol Measurements.
For the liver and small intestine, the concentrations of unesterified and esterified cholesterol were measured using a combination of column and gas chromatography as described (Turley et al., 2010). These data were expressed as mg/g wet weight of tissue. The unesterified and esterified concentrations were summed to give the total cholesterol concentration (mg/g), which in turn was multiplied by the respective liver or small intestine weight to yield the whole organ cholesterol content (mg/organ). The total cholesterol concentration of the residual carcass (mg/g) was multiplied by its entire weight to obtain the whole carcass cholesterol content. The summation of these values for the liver, small intestine, and carcass yielded the whole body cholesterol content, expressed as mg/animal. Aliquots of plasma were saponified in 5 ml of ethanolic KOH, and the total cholesterol concentration (mg/dl plasma) was determined using the same method as for the tissue cholesterol content. Plasma alanine aminotransaminase (ALT) and aspartate aminotransferase (AST) activities were measured by a commercial laboratory.
Hepatic Triacylglycerol Measurement.
Hepatic total triacylglycerol concentrations (mg/g) were measured using a method that combines column chromatography and an enzymatic colorimeteric assay (Beltroy et al., 2005). The initial extracts of liver tissue contained [14C]triolein (American Radiolabeled Chemicals, Inc, St. Louis, MO) to correct for the procedural losses. These values and the liver weight were used to determine whole liver triacylglycerol content (mg/organ).
Fecal Neutral Sterol Excretion.
Rates of fecal neutral sterol excretion were based on a 4-day stool collection. Additional details for the neutral sterol assay using gas chromatography were described earlier (Schwarz et al., 1998). The dominant neutral sterol was unmetabolized cholesterol, together with small amounts of coporostanol, epicoprostanol, and cholestanone. The rate of neutral sterol excretion was expressed as the equivalent mass of cholesterol appearing as neutral sterols per animal per day per 100 g bw.
Relative mRNA Expression Analysis.
Aliquots of liver were quickly frozen in liquid nitrogen. mRNA levels were measured using a Bio-Rad CFX384 real-time polymerase chain reaction detection system (Hercules, CA). The primer sequences used to measure RNA levels for six genes are given in an earlier publication (Taylor et al., 2012). All analyses were determined by the comparative cycle number at the threshold method with cyclophilin as the internal control (Valasek and Repa, 2005). The mRNA levels were normalized to cyclophilin, and the values for each mouse were then expressed relative to those obtained for their matching Lal+/+ controls, which, in each case, were arbitrarily set at 1.0.
Analysis of Data.
Values are mean ± S.E.M. for the specified number of animals. GraphPad Prism software, version 6.02 (San Diego, CA), was used to perform all statistical analyses. Depending on the design of each experiment, differences between mean values were tested for statistical significance (P < 0.05) by an unpaired two-tailed Student’s t test or a two-way analysis of variance, with genotype and treatment as the variables.
Results
Lal−/− Mice Exhibit a Marked Increase in Hepatic Esterified Cholesterol Concentrations at an Early Age.
The data in Table 1 demonstrate multiple striking differences between Lal−/− mice and their Lal+/+ littermates, starting at the time of weaning (21 days) and at early (49–51 days) and midadulthood (140–142 days). Genotypic differences in liver weights, already apparent at weaning, became highly pronounced as the mice aged. A very distinctive feature of the Lal−/− mice was their dramatic increase in hepatic EC concentration (mg/g), even at the time of weaning. In contrast, hepatic unesterified cholesterol (UC) concentrations in the mutants were elevated only moderately. The increases in both liver mass and hepatic EC concentration resulted in a whole liver cholesterol content (mg/organ) in the Lal−/− mice that was 12.2-, 53.1-, and 99-fold more than in their matching Lal+/+ controls at 21, 49–51, and 140–142 days, respectively. The genotypic differences in liver triacylglycerol (TAG) concentrations and contents generally followed those for esterified cholesterol, but were not as pronounced. The profound increases in hepatic TAG and especially EC levels in this mouse model are reflective of what has been described for the livers in a limited number of CESD patients (Sloan and Fredrickson, 1972; Hoeg et al., 1984; Todoroki et al., 2000). In the 49–51 and 140–142 day old Lal−/− mice, plasma ALT activities were elevated 8.1- and 22-fold, respectively. Based on the data in Table 1, it was decided to start PRD125 treatment on the day the mice were weaned (21 days) and to continue it for 29–32 days.
TABLE 1.
Characteristics of lysosomal acid lipase-deficient female mice at different stages of disease progression.
Data are presented as mean ± S.E.M. of values in four mice per group. All mice were weaned at 21 days of age and thereafter fed a basal rodent chow diet ad libitum. They were studied in the fed state.
| Age |
||||||
|---|---|---|---|---|---|---|
| 21 days |
49–51 days |
140–142 days |
||||
| Lal+/+ | Lal−/− | Lal+/+ | Lal−/− | Lal+/+ | Lal−/− | |
| Body weight (g) | 10.6 ± 0.6 | 10.3 ± 0.4 | 20.8 ± 0.3 | 20.8 ± 0.5 | 26.1 ± 0.5 | 24.2 ± 1.1 |
| Liver weight (g) | 0.53 ± 0.04 | 0.64 ± 0.02 | 1.07 ± 0.04 | 2.32 ± 0.10* | 1.08 ± 0.04 | 4.93 ± 0.26* |
| Hepatic esterified cholesterol concentration (mg/g) | 1.0 ± 0.3 | 30.0 ± 0.6* | 0.4 ± 0.1 | 56.3 ± 3.8* | 0.8 ± 0.1 | 53.7 ± 2.3* |
| Hepatic unesterified cholesterol concentration (mg/g) | 2.1 ± 0.1 | 2.7 ± 0.1* | 2.0 ± 0.1 | 3.2 ± 0.1* | 2.0 ± 0.1 | 6.8 ± 0.5* |
| Whole-liver cholesterol content (mg/organ) | 1.7 ± 0.2 | 20.8 ± 1.0* | 2.6 ± 0.1 | 138.1 ± 11.3* | 3.0 ± 0.2 | 297 ± 10.7* |
| Hepatic triacylglycerol concentration (mg/g) | 7.2 ± 2.0 | 10.2 ± 1.8 | 6.0 ± 0.7 | 14.6 ± 0.6* | 12.6 ± 1.5 | 19.4 ± 0.8* |
| Whole-liver triacylglycerol content (mg/organ) | 3.9 ± 1.1 | 6.5 ± 1.3 | 6.5 ± 0.8 | 40.0 ± 2.8* | 13.6 ± 1.9 | 97.4 ± 10.6* |
| Plasma ALT activity (units/l) | 32 ± 2 | 39 ± 5 | 33 ± 2 | 268 ± 37* | 28 ± 4 | 617 ± 97* |
P < 0.05 compared with corresponding Lal+/+ mice of the same age.
PRD125 Treatment Markedly Lowered the Esterified Cholesterol Concentration in the Small Intestine of Lal−/− Mice.
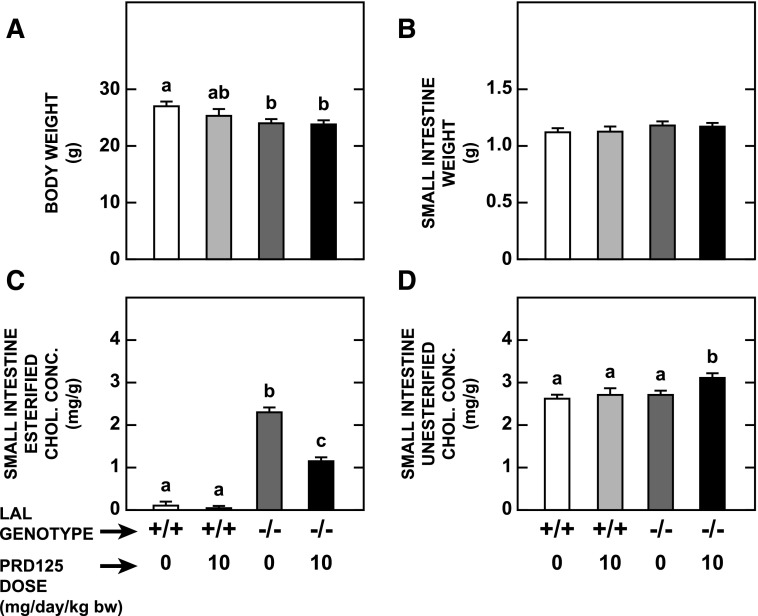
The final body weights of the Lal+/+ and Lal−/− mice given PRD125 were not different than those of the littermates of the same genotype that were fed the basal diet alone (Fig. 2A). Small intestine weights did not vary as a function of either genotype or treatment (Fig. 2B). Consistent with previous findings, the concentration of EC in the small intestine of the Lal+/+ mice was very low, representing only about 4% of the total cholesterol concentration. There was a trend (P > 0.05) to an even lower level when these mice were given PRD125 (Fig. 2C). In marked contrast, the intestinal EC level in the Lal−/− mice on chow only was elevated nearly 22-fold. Treatment with PRD125 blunted this increase by 50% (Fig. 2C). Apart from a marginal increase in the treated Lal−/− mice, the unesterified cholesterol concentrations were about the same in the four groups of mice (Fig. 2D).
Fig. 2.
Weight of small intestine and intestinal concentration of esterified and unesterified cholesterol in Lal+/+ and Lal−/− mice given PRD125. As described in Materials and Methods male Lal−/− mice were fed ad libitum a rodent chow diet either alone or containing PRD125 at a level (0.00625% w/w) that provided an approximate dose of 10 mg/day per kg bw. Matching groups of Lal+/+ mice were fed chow alone or containing PRD125. All mice were given their respective diets from when they were weaned at 21 days until they were 50–53 days old. The concentrations of EC and UC were measured and expressed as mg/g wet weight of tissue. Values are the mean ± S.E.M. of data from five animals in each group. Different letters denote statistically significant (P < 0.05) differences between values, as determined by two-way analysis of variance, with genotype and treatment as the variables.
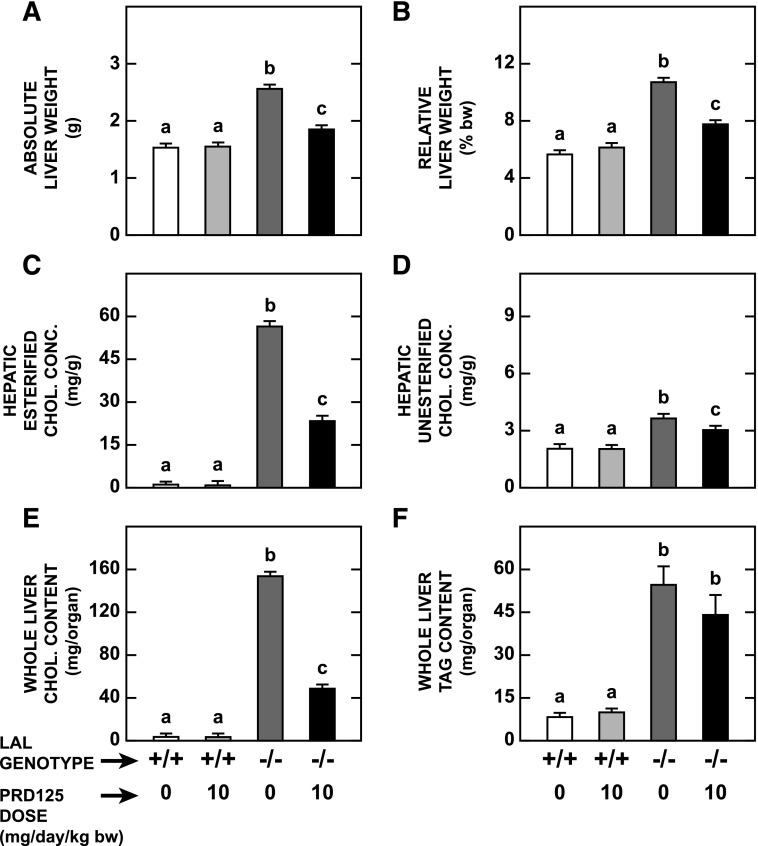
PRD125 Treatment Decreased Liver Mass and Effected a Pronounced Reduction in Hepatic Esterified Cholesterol Concentration in Lal−/− Mice.
The data for multiple parameters relating to the liver in the same groups of mice that the small intestines were taken from are shown in Fig. 3. In the treated Lal−/− mice, the absolute and relative liver weights were each 28% lower than those in their matching untreated Lal−/− littermates (Fig. 3, A and B, respectively). The contraction in liver mass was accompanied by a 59% reduction in the EC concentration (Fig. 3C), with only a marginal fall seen in the UC concentration (Fig. 3D). The parameter that changed most in the treated mutants was whole liver cholesterol content, which contracted by 68% (Fig. 3E). Hepatic TAG concentrations were unchanged by PRD125 treatment in mice of either genotype. In the case of the mutants, these values were 21.1 ± 1.9 mg/g without treatment and 23.7 ± 3.5 mg/g with treatment. Hence, the modest reduction (P > 0.05) in whole liver TAG content in the treated mutants (Fig. 3F) was accounted for fully by the contraction in liver mass in these mice (Fig. 3A).
Fig. 3.
Weight of liver, hepatic concentrations of esterified and unesterified cholesterol, and whole liver contents of cholesterol and triacylglycerol in Lal+/+ and Lal−/− mice given PRD125. The livers used for these measurements were derived from the same mice used in the experiment described in the legend of Fig. 2. Values are the mean ± S.E.M. of data from five animals in each group. Different letters denote statistically significant (P < 0.05) differences between values, as determined by two-way analysis of variance, with genotype and treatment as the variables.
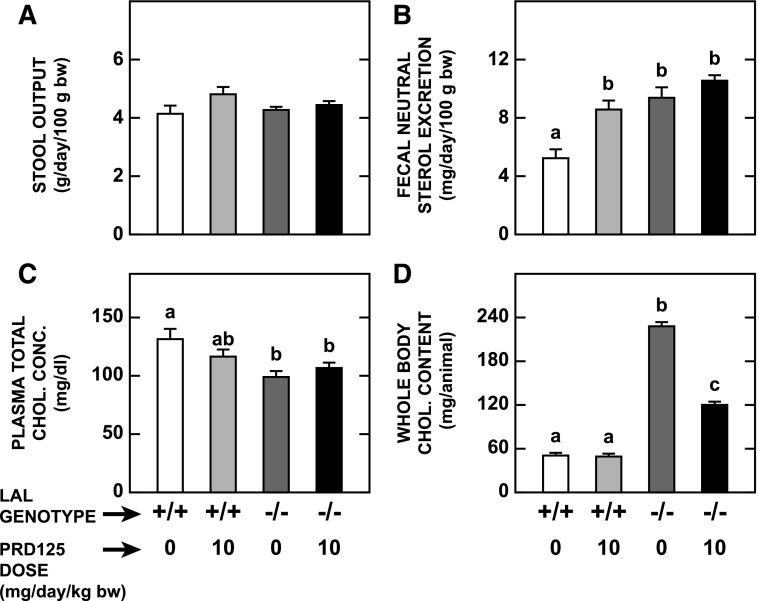
Actions of PRD125 in the Liver and Small Intestine Resulted in a Marked Reduction in Whole Body Cholesterol Content in Lal−/− Mice.
The next set of data broadly defines cholesterol metabolism in the animal as a whole. A key parameter in determining cholesterol turnover is the rate of fecal neutral excretion, which is a function of stool output and the concentration of neutral sterols per gram of stool. No genotypic or treatment-related changes in stool output were evident (Fig. 4A). A documented feature of LAL-deficient mice is an increased rate of fecal neutral sterol excretion (Aqul et al., 2014). This was evident from a comparison of the data for the chow-fed mutants versus wild-types in the current studies (Fig. 4B). PRD125 treatment clearly raised fecal sterol loss in the Lal+/+ mice. A similar trend (P > 0.05) was evident in the treated Lal−/− mice. Plasma total cholesterol concentrations did not change significantly with PRD125 treatment in mice of either genotype (Fig. 4C). Whole body cholesterol content was unchanged in the treated Lal+/+ mice, whereas in their Lal−/− counterparts, it fell dramatically, largely because of the marked reduction in liver cholesterol content (Fig. 4D).
Fig. 4.
Stool output, rate of fecal neutral sterol excretion, plasma total cholesterol concentration, and whole animal cholesterol content in Lal+/+ and Lal−/− mice given PRD125. These data were derived from the same mice used for the measurements described in Figs. 2 and 3. Starting 4 days before the study, stools were collected from each mouse for the measurement of the rate of fecal neutral sterol excretion. Whole body cholesterol contents were determined by summing the quantity of cholesterol within the liver and small intestine with those present in all of the other organs combined. Values are the mean ± S.E.M. of data from five animals in each group. Different letters denote statistically significant (P < 0.05) differences between values, as determined by two-way analysis of variance, with genotype and treatment as the variables.
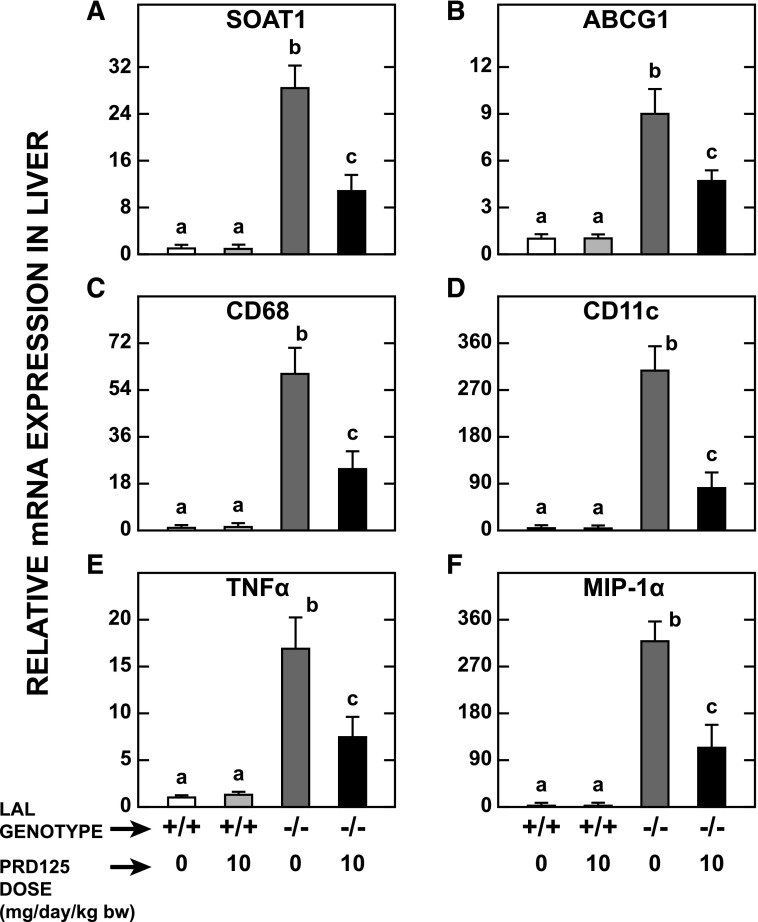
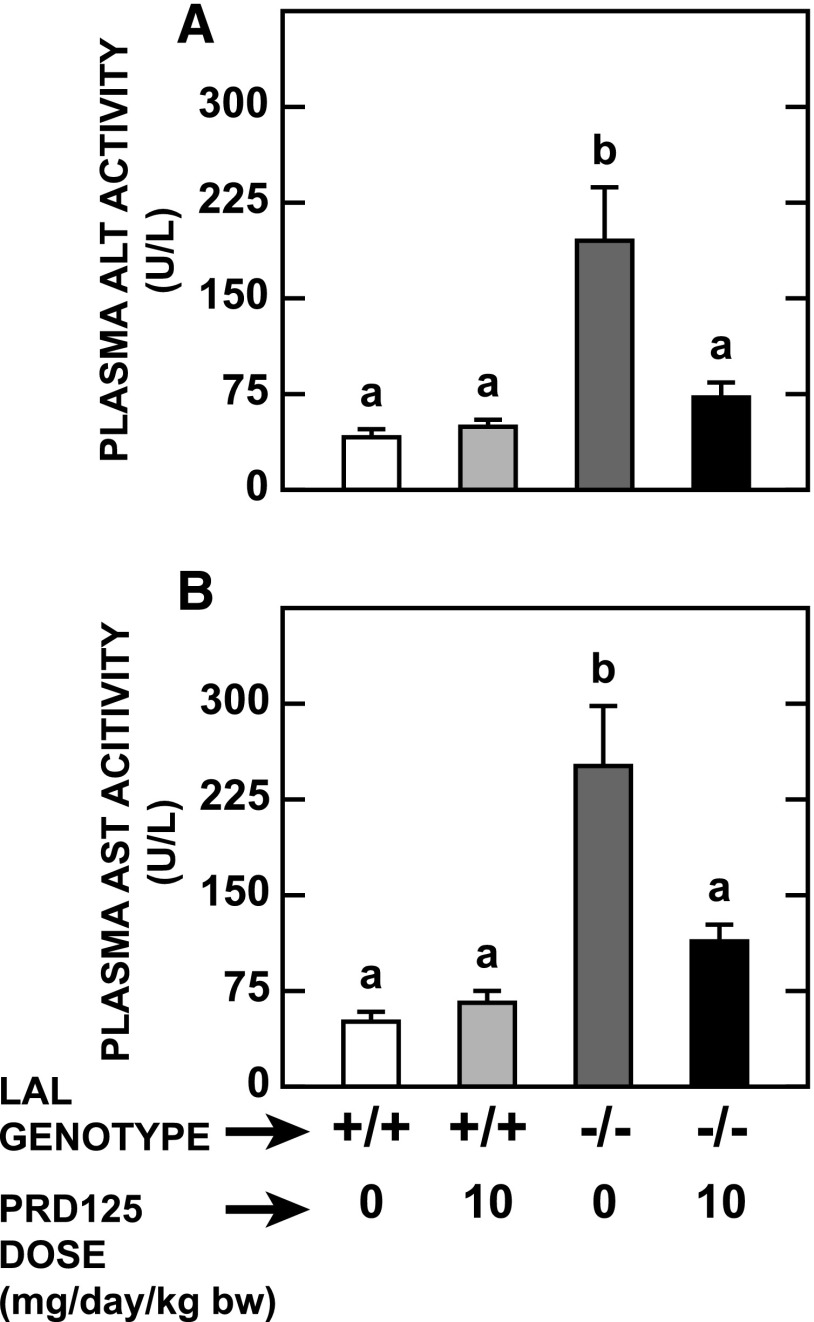
Lal−/− Mice Given PRD125 Showed Substantial Reductions in Liver Inflammation and Plasma Transaminase Activities.
One of the most striking features of livers from Lal−/− mice, apart from their size and EC content, is their pronounced degree of inflammation and macrophage infiltration (Du et al., 2001; Aqul et al., 2014). The data in Fig. 5 illustrate the dramatic reduction in the expression levels of mRNA for six genes that together reflect parameters, such as inflammation and macrophage presence, in the LAL-deficient mice given PRD125. Accompanying these positive changes were decisive reductions in the plasma activities of ALT (Fig. 6A) and aspartate aminotransferase (Fig. 6B). It is noteworthy that the remarkable improvements in these indices of liver health occurred despite there being no reduction in hepatic TAG concentrations.
Fig. 5.
Relative expression levels of mRNA for various genes in the livers of Lal+/+and Lal−/− mice given PRD125. The livers for these measurements were derived from the same mice used in the experiment described in the legend of Fig. 2. The mRNA levels were normalized to cyclophilin, and the values for each mouse were then expressed relative to those obtained for their matching untreated Lal+/+ controls, which, in each case, were arbitrarily set at 1.0. ABCG1, ATP-binding cassette G1; CD68, CD68 antigen; CD11c, integrin alpha x; TNFα, tumor necrosis factor α; MIP-1α, chemokine (c-c motif) ligand 3. Values are the mean ± S.E.M. of data from five animals in each group. Different letters denote statistically significant (P < 0.05) differences between values, as determined by two-way analysis of variance, with genotype and treatment as the variables.
Fig. 6.
Indices of liver function in Lal+/+and Lal−/− mice given PRD125. Plasma activities of ALT and aspartate aminotransferase (AST) were determined in the same mice used in the experiment described in the legend to Fig. 2. Values are the mean ± S.E.M. of data from five animals in each group. Different letters denote statistically significant (P < 0.05) differences between values, as determined by two-way analysis of variance, with genotype and treatment as the variables.
Discussion
The efficacy of PRD125 in blunting the continual expansion of the intestinal and hepatic pools of EC in Lal−/− mice is best illustrated by comparing the data for the treated mutants with those previously reported for Lal−/−:Soat2−/− mice (Lopez et al., 2014). In those studies, the hepatic EC concentrations in the Lal−/−:Soat2−/− mice versus their Lal−/−:Soat2+/+ littermates, all at 52 days of age, were 15.2 versus 54.3 mg/g, respectively. The corresponding values for LAL mutants given PRD125 versus their untreated Lal−/− mice controls were 23.4 and 56.5 mg/g, respectively (Fig. 3C). In the Lal−/− mice given PRD125, liver mass was 28% less than that in the untreated Lal−/− mice (Fig. 3A). This marked contraction in liver weight was comparable to that manifested in the Lal−/−:Soat2−/− mice (34%) (Lopez et al., 2014). From these sets of data, it can be calculated that the percentage reduction in whole liver cholesterol content (mg/organ) in the PRD125-treated Lal−/− mice (68%) (Fig. 3E) was pronounced, although not as dramatic as that seen in the Lal−/−:Soat2−/− mice (80%). It is not yet known whether a higher dose of PRD125 would reduce hepatic EC content even further.
The mechanism(s) through which the remarkable cholesterol-lowering effects of SOAT2 deficiency or suppression are articulated in this CESD mouse model relate to the key role that this enzyme plays in determining the EC content of specific types of lipoprotein particles generated within the small intestine and liver. When SOAT2 in the small intestine is either absent or suppressed, this would not only contribute to a diminution of the EC concentration in the small intestine as a whole, but would also likely reduce the EC content of chylomicrons that are assembled in the intestine and ultimately cleared by the liver (Cooper, 1997). Such a reduced delivery of intestinal cholesterol to the liver would mimic the actions of the selective intestinal cholesterol absorption inhibitor ezetimibe in the Lal−/− mouse (Chuang et al., 2014). Thus, to some extent, the pronounced fall in EC levels in the livers of the Lal−/−:Soat2−/− mice and PRD125-treated Lal−/− mice is the product of a reduction in net cholesterol delivery from the small intestine to the liver. Clearance by the liver of chylomicron remnants with a reduced EC content would culminate in less EC becoming sequestered in the lysosomal compartment of hepatocytes and other cell types in the liver.
Ultimately, however, it is likely that the beneficial effect of suppressing or deleting SOAT2 activity arises primarily from the role that this enzyme plays in the formation of EC in the liver, and its subsequent incorporation into nascent very low density lipoproteins (VLDLs). This conclusion stems from a plethora of studies in various in vitro systems and animal models, particularly mouse models with liver- or intestine-specific deletion of SOAT2 (Zhang et al., 2012). The secretion from the liver of VLDL with a reduced EC content would potentially lower the amount of EC carried in mature LDL particles and VLDL remnants that are subsequently cleared from the circulation primarily by the liver, but also by the small intestine and other organs (Osono et al., 1995). In the case of the liver, the clearance, not only of LDL and related particles with a reduced EC content, but also, as already noted, of chylomicron remnants containing diminished amounts of EC would result in a marked fall in the mass of EC sequestered in the lysosomes. Although tissue EC and UC levels were not determined in organs other than the liver and small intestine in either the PRD125-treated Lal−/− mice or previously in the Lal−/−:Soat2−/− mice, one might anticipate a reduction in EC content given that most extrahepatic organs take up some LDL from the circulation (Osono et al., 1995).
Several additional points relating to the data for the small intestine and also the rates of fecal neutral sterol excretion warrant discussion. Although hepatomegaly is pronounced in Lal−/− mice by early adulthood, the mass of their small intestine has not changed significantly at that time. However, there are already marked histologic changes and increased levels of EC, both of which become very pronounced as disease progresses (Du et al., 2001; Aqul et al., 2014). The fall in intestinal EC concentration in the LAL mutants given PRD125 (Fig. 2) was comparable to that manifested in the Lal−/−:Soat2−/− mice (Lopez et al., 2014). In interpreting data for intestinal cholesterol levels, one must take into account the fact that the mucosal surface is constantly undergoing renewal. In mice, the lifespan of the absorptive cells is only 2–3 days (Lipkin, 1981). If this turnover did not occur, the intestinal EC levels found in Lal−/− mice would likely be even more elevated than shown here and in previous publications (Du et al., 2001; Aqul et al., 2014). Nevertheless, based on the present findings, the EC levels in either Lal−/−:Soat2−/− mice or PRD125-treated LAL mutants would still be expected to be much lower than in unmanipulated Lal−/− mice of the same age.
In the Lal+/+ mice given PRD125, there was a clear elevation in the rate of fecal neutral sterol excretion. This suggests either a marked inhibition of cholesterol absorption and/or an increased delivery of biliary cholesterol into the lumen as a consequence of SOAT2 inhibition in hepatocytes. As found previously, untreated Lal−/− mice manifest higher rates of sterol excretion (Aqul et al., 2014). In this case, the increase in fecal sterol loss might reflect either a higher cholesterol content of sloughed mucosal cells and/or an increase in biliary cholesterol delivery into the lumen because of the elevated rate of hepatic cholesterol synthesis (Aqul et al., 2014). A comparison of the sterol excretion data for the Lal−/− mice given PRD125 versus chow only (Fig. 4B) implies a marginal inhibition of cholesterol absorption in the treated mutants.
In contrast to the potency of PRD125 in blunting the rise in intestinal and hepatic EC levels, there was no discernible impact on the entrapment of TAG, at least in the liver. This was also true in Lal−/−:Soat2−/− mice (Lopez et al., 2014). In that model and in Lal−/− mice given PRD125, hepatic TAG concentrations (mg/g) were unchanged in the face of decisive falls in EC concentrations. Whole liver TAG contents (mg/organ) were reduced only marginally because of the decrease in liver mass. This result was not surprising given that a lowering of the EC content of nascent VLDL or chylomicrons because of the absence or suppression of SOAT2 activity should not cause a commensurate fall in the TAG content of these particles. In Lal−/− mice given repeated injections of recombinant LAL, marked reductions in tissue cholesterol and TAG occurred (Sun et al., 2014). It is noteworthy that SOAT2 deficiency prevented cholesterol-associated steatosis in a mouse model by enhancing TAG mobilization (Alger et al., 2010). However, in that model, the excess TAG is contained in lipid droplets outside lysosomes. It is not known whether autophagocytosis of intracellular lipid droplets (Dong and Czaja, 2011) contributes to the buildup of EC and TAG in LAL deficiency, especially in the liver, or the extent to which such a contribution might shift in the face of treatment with a SOAT2 inhibitor.
As is the case with most classes of cholesterol-lowering agents, inhibitors of cholesterol esterification are designed primarily to treat dyslipidemia. Here, an important point should be made about plasma cholesterol levels in LAL-deficient mice. While there are many similarities in mouse models for CESD and humans with this disorder, the type of dyslipidemia seen in human LAL deficiency (Reiner et al., 2014) is not replicated in Lal−/− mice. Although published data are limited, it appears that in Lal−/− mice, there is a shift in lipoprotein composition, but no change in the plasma total cholesterol concentration (Du et al., 1998, 2001). It remains to be determined whether this is the case in all strains of mice with LAL deficiency.
The data from both PRD125-treated Lal−/− mice and Lal−/−:Soat2−/− mice indicate that while cholesterol esterification via SOAT2 is a major factor in driving disease progression in LAL deficiency, the role of SOAT1 in this process remains to be defined in more quantitative terms. It may be appreciable because SOAT1 is found in many cell types, in particular, macrophages, which have an increasing presence throughout the body as disease progresses (Du et al., 2001). Models that might yield clearer insights into the role of SOAT1 in CESD would be either Lal−/−:Soat1−/− mice or Lal−/− mice treated with PRD125, together with moderate doses of the nonspecific SOAT inhibitor F1394 (Rong et al., 2013).
With respect to the therapeutic potential of PRD125 for managing CESD, it will now be necessary to investigate its efficacy in Lal−/− mice that are at a more advanced stage of disease than is manifested in the 21-day-old animal. The data in Table 1 suggest that PRD125 treatment commencing at about 50 days of age and continuing for 3 months might establish whether sustained SOAT2 inhibition could serve as an adjunctive treatment option for enzyme replacement therapy.
Acknowledgments
The gift of Lal heterozygous mice, together with a genotyping protocol, from Drs. Gregory Grabowski and Hong Du is gratefully acknowledged. The authors also thank Dr. Joyce Repa for making the primers used for the quantitative polymerase chain reaction analyses available. Stephen Ostermann and Monti Schneiderman provided excellent assistance with animal care and stool collections.
Abbreviations
- ALT
alanine transaminase
- CESD
cholesteryl ester storage disease
- EC
esterified cholesterol
- LAL
lysosomal acid lipase
- LDL
low density lipoprotein
- PRD125
1,11-O-o-methylbenzylidene-7-O-p-cyanobenzoyl-1,7,11-trideacetylpyripyropene A
- SOAT
sterol O-acyltransferase
- TAG
triacylglycerol
- UC
unesterified cholesterol
- VLDL
very low density lipoprotein
- WD
Wolman disease
Authorship Contributions
Participated in research design: Turley, Lopez, Posey, Rudel, Tomoda, Ohshiro.
Conducted experiments: Lopez, Chuang, Posey, Turley.
Contributed new reagents or analytic tools: Tomoda, Ohshiro.
Performed data analysis: Posey, Lopez, Chuang, Turley.
Wrote or contributed to the writing of the manuscript: Turley, Lopez, Chuang, Rudel, Tomoda, Ohshiro.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01HL009610 to S.D.T.] and [Grant PO1HL049373 to L.L.R]. Dr. Ohshiro was supported by a grant from Wake Forest Innovations as well as a grant-in-aid for scientific research from the Japan Society for the Promotion of Science.
References
- Alger HM, Brown JM, Sawyer JK, Kelley KL, Shah R, Wilson MD, Willingham MC, Rudel LL. (2010) Inhibition of acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2) prevents dietary cholesterol-associated steatosis by enhancing hepatic triglyceride mobilization. J Biol Chem 285:14267–14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqul A, Lopez AM, Posey KS, Taylor AM, Repa JJ, Burns DK, Turley SD. (2014) Hepatic entrapment of esterified cholesterol drives continual expansion of whole body sterol pool in lysosomal acid lipase-deficient mice. Am J Physiol Gastrointest Liver Physiol 307:G836–G847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balwani M, Breen C, Enns GM, Deegan PB, Honzík T, Jones S, Kane JP, Malinova V, Sharma R, Stock EO, et al. (2013) Clinical effect and safety profile of recombinant human lysosomal acid lipase in patients with cholesteryl ester storage disease. Hepatology 58:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltroy EP, Richardson JA, Horton JD, Turley SD, Dietschy JM. (2005) Cholesterol accumulation and liver cell death in mice with Niemann-Pick type C disease. Hepatology 42:886–893. [DOI] [PubMed] [Google Scholar]
- Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, et al. (1998) ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem 273:26755–26764. [DOI] [PubMed] [Google Scholar]
- Chang C, Dong R, Miyazaki A, Sakashita N, Zhang Y, Liu J, Guo M, Li BL, Chang TY. (2006) Human acyl-CoA:cholesterol acyltransferase (ACAT) and its potential as a target for pharmaceutical intervention against atherosclerosis. Acta Biochim Biophys Sin (Shanghai) 38:151–156. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Lopez AM, Posey KS, Turley SD. (2014) Ezetimibe markedly attenuates hepatic cholesterol accumulation and improves liver function in the lysosomal acid lipase-deficient mouse, a model for cholesteryl ester storage disease. Biochem Biophys Res Commun 443:1073–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AD. (1997) Hepatic uptake of chylomicron remnants. J Lipid Res 38:2173–2192. [PubMed] [Google Scholar]
- d’ Hollander F, Chevallier F. (1969) [Qualitative and quantitative estimation of free and esterified sterols in whole rat and in 23 of its tissues and organs]. Biochim Biophys Acta 176:146–162. [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. (2004) Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res 45:1375–1397. [DOI] [PubMed] [Google Scholar]
- Dong H, Czaja MJ. (2011) Regulation of lipid droplets by autophagy. Trends Endocrinol Metab 22:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Duanmu M, Witte D, Grabowski GA. (1998) Targeted disruption of the mouse lysosomal acid lipase gene: long-term survival with massive cholesteryl ester and triglyceride storage. Hum Mol Genet 7:1347–1354. [DOI] [PubMed] [Google Scholar]
- Du H, Heur M, Duanmu M, Grabowski GA, Hui DY, Witte DP, Mishra J. (2001) Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J Lipid Res 42:489–500. [PubMed] [Google Scholar]
- Farese RV., Jr (2006) The nine lives of ACAT inhibitors. Arterioscler Thromb Vasc Biol 26:1684–1686. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Dana SE, Faust JR, Beaudet AL, Brown MS. (1975) Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem 250:8487–8495. [PubMed] [Google Scholar]
- Goodman DS. (1965) Cholesterol ester metabolism. Physiol Rev 45:747–839. [DOI] [PubMed] [Google Scholar]
- Grabowski GA, Du H, Charnas L. (2015) Lysosomal acid lipase deficiencies: the Wolman disease/cholesteryl ester storage disease spectrum, in Scriver’s Online Metabolic and Molecular Bases of Inherited Metabolic Disease (Valle D, Beaudet A, Vogelstein B, Kinzler K, Antonarakis S, Ballabio A. eds) McGraw-Hill; New York. [Google Scholar]
- Grundy SM. (1983) Absorption and metabolism of dietary cholesterol. Annu Rev Nutr 3:71–96. [DOI] [PubMed] [Google Scholar]
- Hoeg JM, Demosky SJ, Jr, Pescovitz OH, Brewer HB., Jr (1984) Cholesteryl ester storage disease and Wolman disease: phenotypic variants of lysosomal acid cholesteryl ester hydrolase deficiency. Am J Hum Genet 36:1190–1203. [PMC free article] [PubMed] [Google Scholar]
- Kuriyama M, Yoshida H, Suzuki M, Fujiyama J, Igata A. (1990) Lysosomal acid lipase deficiency in rats: lipid analyses and lipase activities in liver and spleen. J Lipid Res 31:1605–1612. [PubMed] [Google Scholar]
- Lada AT, Davis M, Kent C, Chapman J, Tomoda H, Omura S, Rudel LL. (2004) Identification of ACAT1- and ACAT2-specific inhibitors using a novel, cell-based fluorescence assay: individual ACAT uniqueness. J Lipid Res 45:378–386. [DOI] [PubMed] [Google Scholar]
- Lee RG, Willingham MC, Davis MA, Skinner KA, Rudel LL. (2000) Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J Lipid Res 41:1991–2001. [PubMed] [Google Scholar]
- Lipkin M. (1981) Proliferation and differentiation of gastrointestinal cells in normal and disease states, in Physiology of the Gastrointestinal Tract (Johnson LR. ed) pp 145–167, Raven Press, New York. [Google Scholar]
- Lopez AM, Posey KS, Turley SD. (2014) Deletion of sterol O-acyltransferase 2 (SOAT2) function in mice deficient in lysosomal acid lipase (LAL) dramatically reduces esterified cholesterol sequestration in the small intestine and liver. Biochem Biophys Res Commun 454:162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Tuzcu EM, Brewer HB, Sipahi I, Nicholls SJ, Ganz P, Schoenhagen P, Waters DD, Pepine CJ, Crowe TD, et al. ACAT Intravascular Atherosclerosis Treatment Evaluation (ACTIVATE) Investigators (2006) Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 354:1253–1263. [DOI] [PubMed] [Google Scholar]
- Ohshiro T, Matsuda D, Sakai K, Degirolamo C, Yagyu H, Rudel LL, Omura S, Ishibashi S, Tomoda H. (2011) Pyripyropene A, an acyl-coenzyme A:cholesterol acyltransferase 2-selective inhibitor, attenuates hypercholesterolemia and atherosclerosis in murine models of hyperlipidemia. Arterioscler Thromb Vasc Biol 31:1108–1115. [DOI] [PubMed] [Google Scholar]
- Ohtawa M, Yamazaki H, Ohte S, Matsuda D, Ohshiro T, Rudel LL, Ōmura S, Tomoda H, Nagamitsu T. (2013) Synthesis and structure-activity relationship of pyripyropene A derivatives as potent and selective acyl-CoA:cholesterol acyltransferase 2 (ACAT2) inhibitors: part 3. Bioorg Med Chem Lett 23:3798–3801. [DOI] [PubMed] [Google Scholar]
- Osono Y, Woollett LA, Herz J, Dietschy JM. (1995) Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J Clin Invest 95:1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parini P, Davis M, Lada AT, Erickson SK, Wright TL, Gustafsson U, Sahlin S, Einarsson C, Eriksson M, Angelin B, et al. (2004) ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation 110:2017–2023. [DOI] [PubMed] [Google Scholar]
- Peng S, Guo W, Morrisett JD, Johnstone MT, Hamilton JA. (2000) Quantification of cholesteryl esters in human and rabbit atherosclerotic plaques by magic-angle spinning (13)C-NMR. Arterioscler Thromb Vasc Biol 20:2682–2688. [DOI] [PubMed] [Google Scholar]
- Reiner Ž, Guardamagna O, Nair D, Soran H, Hovingh K, Bertolini S, Jones S, Ćorić M, Calandra S, Hamilton J, et al. (2014) Lysosomal acid lipase deficiency--an under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis 235:21–30. [DOI] [PubMed] [Google Scholar]
- Remaley AT, Schumacher UK, Stonik JA, Farsi BD, Nazih H, Brewer HB., Jr (1997) Decreased reverse cholesterol transport from Tangier disease fibroblasts. Acceptor specificity and effect of brefeldin on lipid efflux. Arterioscler Thromb Vasc Biol 17:1813–1821. [DOI] [PubMed] [Google Scholar]
- Rong JX, Blachford C, Feig JE, Bander I, Mayne J, Kusunoki J, Miller C, Davis M, Wilson M, Dehn S, et al. (2013) ACAT inhibition reduces the progression of preexisting, advanced atherosclerotic mouse lesions without plaque or systemic toxicity. Arterioscler Thromb Vasc Biol 33:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset X, Shamburek R, Vaisman B, Amar M, Remaley AT. (2011) Lecithin cholesterol acyltransferase: an anti- or pro-atherogenic factor? Curr Atheroscler Rep 13:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel LL, Haines J, Sawyer JK, Shah R, Wilson MS, Carr TP. (1997) Hepatic origin of cholesteryl oleate in coronary artery atherosclerosis in African green monkeys. Enrichment by dietary monounsaturated fat. J Clin Invest 100:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel LL, Lee RG, Parini P. (2005) ACAT2 is a target for treatment of coronary heart disease associated with hypercholesterolemia. Arterioscler Thromb Vasc Biol 25:1112–1118. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Russell DW, Dietschy JM, Turley SD. (1998) Marked reduction in bile acid synthesis in cholesterol 7α-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J Lipid Res 39:1833–1843. [PubMed] [Google Scholar]
- Sloan HR, Fredrickson DS. (1972) Enzyme deficiency in cholesteryl ester storage idisease. J Clin Invest 51:1923–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Xu YH, Du H, Quinn B, Liou B, Stanton L, Inskeep V, Ran H, Jakubowitz P, Grilliot N, et al. (2014) Reversal of advanced disease in lysosomal acid lipase deficient mice: a model for lysosomal acid lipase deficiency disease. Mol Genet Metab 112:229–241. [DOI] [PubMed] [Google Scholar]
- Tardif JC, Grégoire J, L’Allier PL, Anderson TJ, Bertrand O, Reeves F, Title LM, Alfonso F, Schampaert E, Hassan A, et al. Avasimibe and Progression of Lesions on UltraSound (A-PLUS) Investigators (2004) Effects of the acyl coenzyme A:cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation 110:3372–3377. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Liu B, Mari Y, Liu B, Repa JJ. (2012) Cyclodextrin mediates rapid changes in lipid balance in Npc1-/- mice without carrying cholesterol through the bloodstream. J Lipid Res 53:2331–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todoroki T, Matsumoto K, Watanabe K, Tashiro Y, Shimizu M, Okuyama T, Imai K. (2000) Accumulated lipids, aberrant fatty acid composition and defective cholesterol ester hydrolase activity in cholesterol ester storage disease. Ann Clin Biochem 37:187–193. [DOI] [PubMed] [Google Scholar]
- Tso P. (1994) Intestinal lipid absorption, in Physiology of the Gastrointestinal Tract (Johnson LR. ed) pp 1867–1907, Raven Press, New York. [Google Scholar]
- Turley SD, Dietschy JM. (1988) The metabolism and excretion of cholesterol by the liver, in The Liver: Biology and Pathobiology (Arias IM, Jakoby WB, Popper H, Schachter D, Shafritz DA. eds) pp 617–641, Raven Press, New York. [Google Scholar]
- Turley SD, Valasek MA, Repa JJ, Dietschy JM. (2010) Multiple mechanisms limit the accumulation of unesterified cholesterol in the small intestine of mice deficient in both ACAT2 and ABCA1. Am J Physiol Gastrointest Liver Physiol 299:G1012–G1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valasek MA, Repa JJ. (2005) The power of real-time PCR. Adv Physiol Educ 29:151–159. [DOI] [PubMed] [Google Scholar]
- Xie C, Woollett LA, Turley SD, Dietschy JM. (2002) Fatty acids differentially regulate hepatic cholesteryl ester formation and incorporation into lipoproteins in the liver of the mouse. J Lipid Res 43:1508–1519. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kelley KL, Marshall SM, Davis MA, Wilson MD, Sawyer JK, Farese RV, Jr, Brown JM, Rudel LL. (2012) Tissue-specific knockouts of ACAT2 reveal that intestinal depletion is sufficient to prevent diet-induced cholesterol accumulation in the liver and blood. J Lipid Res 53:1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]