Abstract
An increasing number of therapies have proven effective at reversing hyperglycemia in the nonobese diabetic (NOD) mouse model of type 1 diabetes (T1D), yet situations of successful translation to human T1D are limited. This may be partly due to evaluating the effect of treating immediately at diagnosis in mice, which may not be reflective of the advanced disease state in humans at disease onset. In this study, we treated NOD mice with new-onset as well as established disease using various combinations of four drugs: antithymocyte globulin (ATG), granulocyte-colony stimulating factor (G-CSF), a dipeptidyl peptidase IV inhibitor (DPP-4i), and a proton pump inhibitor (PPI). Therapy with all four drugs induced remission in 83% of new-onset mice and, remarkably, in 50% of NOD mice with established disease. Also noteworthy, disease remission occurred irrespective of initial blood glucose values and mechanistically was characterized by enhanced immunoregulation involving alterations in CD4+ T cells, CD8+ T cells, and natural killer cells. This combination therapy also allowed for effective treatment at reduced drug doses (compared with effective monotherapy), thereby minimizing potential adverse effects while retaining efficacy. This combination of approved drugs demonstrates a novel ability to reverse T1D, thereby warranting translational consideration.
Introduction
Type 1 diabetes (T1D) is an autoimmune disorder long thought amenable to disease prevention and perhaps even reversal (1). Indeed, a rich history of agents has shown the capacity to prevent T1D in NOD mice (2), with an increasing number of reports positing the capability to reverse hyperglycemia in animals at disease diagnosis (3). Although numerous human trials have used therapies shown effective in NOD mice, few translational success stories exist (e.g., anti-CD3, CTLA-4 Ig, anti-CD20), and even those have proven heterogeneous in their efficacy in new-onset populations (4).
To improve this situation, we considered the regulatory approval status of potential drugs, their mechanism of action, and the notion that any such effort would most likely require a combination approach to maximize efficacy. We selected drugs having prior U.S. Food and Drug Administration approval and proven effectiveness in settings of autoimmunity, transplantation, and/or β-cell dysfunction in NOD mice or related clinical settings. Specifically, we previously noted the synergism of antithymocyte globulin (ATG) with granulocyte-colony stimulating factor (G-CSF) in reversing T1D in NOD mice (5). This was mechanistically tied to enhanced immunoregulation involving an increase in regulatory T-cell (Treg) activities and alterations in dendritic cell phenotype and function (6). Translation of this therapy from studies of mice to man also proved remarkably successful. Specifically, we recently reported the results from a pilot clinical trial assessing the effectiveness of this combination therapy (i.e., ATG plus G-CSF) in humans. That effort, which used not recent-onset patients but those with somewhat “established” disease (6 to 24 months after onset), noted the ability for this combination of agents to preserve C-peptide (7).
We have long been interested in combination therapies for T1D, and as part of this, we also demonstrated that a combination of a dipeptidyl peptidase IV inhibitor (DPP-4i) and a proton pump inhibitor (PPI) synergize to reverse T1D in NOD mice (8). The DPP-4i allows for a longer half-life of glucagon-like petide-1 (GLP-1) that has been shown to have favorable effects on β-cell regeneration and survival (8–10). The PPI class of drugs raises circulating levels of gastrin in vivo, which also allows for enhanced β-cell function and survival (11–14).
From these observations, we hypothesized that a combination of ATG, G-CSF, DPP-4i, and PPI (AGDP) would not only allow for reversal of new-onset T1D in NOD mice but also perhaps in animals with established disease. This approach represented quite a challenge to existing dogma regarding reversal of T1D in NOD mice, where essentially all agents noted for efficacy require administration at the earliest signs of hyperglycemia or glycosuria (i.e., within hours to 1 or 2 days) and when blood glucose levels are modestly elevated (e.g., as low as 180 mg/dL) (3). Indeed, reports to date suggest reversal of established disease in NOD mice requires administration of an alternate source of insulin production (i.e., transplanted islet cells).
Research Design and Methods
Mice
The studies described here were approved by the University of Florida Institutional Animal Care and Use Committee. Eight-week-old female NOD/LtJ mice were purchased from The Jackson Laboratory and monitored twice weekly for diabetes by tail bleed. Diabetes was defined as blood glucose ≥240 mg/dL on 2 consecutive days, measured by a hand-held glucometer. Diabetic mice were randomized into the new-onset or established-disease treatment groups. Nondiabetic mice analyzed in this study were age-matched female NOD/LtJ mice that had neither developed diabetes nor received treatment. These control mice were killed in parallel with treated euglycemic mice at the study end point (120 days posttreatment).
Reversal Studies
Upon diabetes determination, all mice were implanted with a LinBit subcutaneous insulin pellet (LinShin Canada, Inc.). Mice in the new-onset groups began therapy that day (new-onset day 0), whereas mice in the established groups began therapy 15 days later (established day 0). Therapy groups consisted of insulin pellet alone, ATG+G-CSF therapy, DPP-4i+PPI therapy, AGDP therapy, or control treatment. Murine ATG (Genzyme, Cambridge, MA) was administered intraperitoneally at 250 µg/dose on days 0 and 3. Human G-CSF (Neulasta; Amgen) was administered intraperitoneally at 120 µg/dose on days 0 and 15. The selective DPP-4i 1-{[(3-hydroxy-1-adamantyl)amino]acetyl}-2-cyano-(S)-pyrrolidine (Dalton Chemical Laboratories, Toronto, ON, Canada) was administered orally at 200 µg daily for 84 days. PPI (pantoprazole; Nycomed, Oakville, ON, Canada) was given subcutaneously at 600 µg twice daily for 84 days. Control mice received control treatments for all four drugs on their respective schedules: recombinant IgG (Jackson ImmunoResearch Laboratories, Inc.) for ATG, 5% dextrose for G-CSF, 5 mg/mL methylcellulose for DPP-4i, and saline for PPI. A group given the insulin pellet alone, without further manipulation, was also analyzed.
Mechanistic Studies
Mechanistic studies were conducted similarly to reversal studies (described above), with the exception that treatment was initiated 10 days post-T1D onset in established groups, and tissues were harvested on day 30. The batch of LinBit insulin pellets being used was observed to be less effective, resulting in an earlier return to hyperglycemia than expected; thus, treatment was initiated at day 10 rather than day 14, which theoretically still allowed for appreciable continued β-cell loss.
Histology
For reversal studies, necropsy was performed at day 120, the pancreas fixed in 10% neutral buffered formalin overnight, and embedded in paraffin. Sections were stained with hematoxylin and eosin and scanned to create digital images using a GS ScanScope (Aperio Technologies, Inc., Vista, CA). Each section was reviewed for islets and insulitis scored according to the following: 0, no insulitis; 1, peri-islet only; 2, <50% infiltrates within islet; 3, >50% infiltrates within islets. Total numbers of islets within each scoring category were tabulated by animal, and the average islet number per score within each treatment group was compared with age-matched female nondiabetic NOD mice.
For mechanistic studies, necropsy was performed at day 30. The pancreata from four mice per treatment group were fixed and embedded as described above. Sections were stained for insulin and Ki-67 via immunohistochemistry (IHC) and scanned to create digital images using an Aperio GS ScanScope. Each section was reviewed for the number of insulin-positive and -negative islets. Fractional insulin area was determined using CytoNuclear IHC Quantification software (Indica Laboratories, Albuquerque, NM). An ImageScope plug-in tool (Aperio Technologies) was used to calibrate the red, green, and blue optical density values of the underlying tissues and set the input parameters. Serial pancreatic sections from mice killed at day 30 were also stained for CD4 and CD8 via IHC, scanned, and analyzed using the Aperio GS ScanScope with the CytoNuclear IHC Quantification software for absolute number and percentage of islet cells staining positive for CD4 and CD8.
ELISA: Serum
For mechanistic studies, necropsy was performed at day 30. Maximum attainable blood volume was collected, and serum C-peptide levels were measured via ELISA (ALPCO Diagnostics, Salem, NH).
ELISA: Pancreas
For mechanistic studies, necropsy was performed at day 30. The pancreata for two to three mice per treatment group were processed for total protein by acid ethanol extraction. Briefly, pancreata were weighed and incubated overnight at −20°C in 5 mL 1.5% HCl in 70% ethanol before homogenization. Homogenates were again incubated overnight at −20°C and pelleted by centrifugation. The supernatant solution was brought to neutral pH with 1 mol/L Tris (pH 7.5) and tested by ELISA for insulin, proinsulin, and C-peptide (ALPCO Diagnostics). Results were normalized against pancreatic total protein as quantified by a Bradford assay.
Flow Cytometry
Spleens were harvested and passed through a 100-µm filter to obtain a cell suspension, and 1 × 106 cells were stained for flow cytometric analysis. For reversal studies, Tregs were stained with anti-mouse FoxP3-phycoerythrin (PE) (clone FJK-16s), CD8a-fluorescein isothiocyanate (FITC) (53-6.7), CD25-allophycocyanin (APC) (PC61.5), and CD4-PE-Cy7 (RM4-5) using standard procedures. Naïve and memory T cells were determined by staining with anti-mouse CD44-FITC (IM7), CD8b-PE (H35-17.2), CD62L-APC (MEL-14), and CD4-PE-Cy7 (RM4-5). Samples were run on an Accuri flow cytometer (BD Accuri Cytometers, Ann Arbor, MI). For mechanistic studies, Tregs were stained with anti-mouse FoxP3-PE (clone FJK-16s), CD8a-eFluor450 (53–6.7), CD25-APC (PC61.5), CD4-PE-Cy7 (GK1.5), CD62L-FITC (MEL-14) and CD44-PerCP-Cy5.5 (IM7). Natural killer (NK) cells were stained with anti-mouse CD49b-FITC (HMa2), CD122-PE (TM-b1), CD11b-PE-Cy7 (M1/70), and CD3-APC (17A2). Samples were run on a LSR Fortessa flow cytometer (BD Biosciences, San Jose, CA). Briefly, cells underwent surface staining for 30 min, followed by fixation for 2 h. Samples were washed in permeabilization buffer and blocked (anti-mouse CD16/CD32) for 15 min, followed by intracellular staining for 30 min (all antibodies and buffers from eBioscience, San Diego, CA). Results were analyzed in FCS Express 4 software (De Novo Software, Los Angeles, CA).
Statistics
All data were analyzed on GraphPad Prism 5.1 software (GraphPad Software, Inc., La Jolla, CA). Survival curves were analyzed using a Kaplan-Meier test. Comparisons between multiple data sets were analyzed by ANOVA (nonparametric Kruskal-Wallis test) with the Dunn multiple comparison posttest. The Student t test was used for comparison of remitted versus failed mice.
Results
AGDP Combination Therapy Induces Durable Diabetes Remission in NOD Mice With New-Onset and Established Diabetes
On the day of diabetes onset, NOD mice received a slow-release subcutaneous insulin pellet and were randomized into the following treatment groups: murine ATG and human G-CSF, DPP-4i and PPI, AGDP therapy, or control treatments. Treatment was initiated in these cohorts at disease onset (new-onset groups) or after a 2-week delay (established groups). In new-onset mice, ATG+G-CSF therapy resulted in durable remission (i.e., euglycemic for 120 days) in 6 of 12 mice (50%, P = 0.0010 vs. control), whereas 7 of 12 mice (54%, P < 0.0001) treated with DPP-4i+PPI therapy at onset showed durable remission (Fig. 1A). Treating new-onset mice with AGDP increased the remission rate to 11 of 13 mice (83%, P < 0.0001 vs. control). However, compared with ATG+G-CSF or DPP-4i+PPI, AGDP therapy did not significantly increase remission rates in mice with established diabetes. In established mice, 4 of 12 (33%, P = 0.0223 vs. control) treated with ATG+G-CSF, 5 of 12 (42%, P = 0.0962) treated with DPP-4i+PPI, and strikingly, 6 of 12 (50%, P = 0.0013) treated with AGDP therapy exhibited durable remission (Fig. 1B). Nonfasting morning blood glucose curves for each of these treatment groups (Fig. 1C–K) demonstrate that remitted mice maintained stable blood glucose levels for 120 days post-T1D onset.
Figure 1.
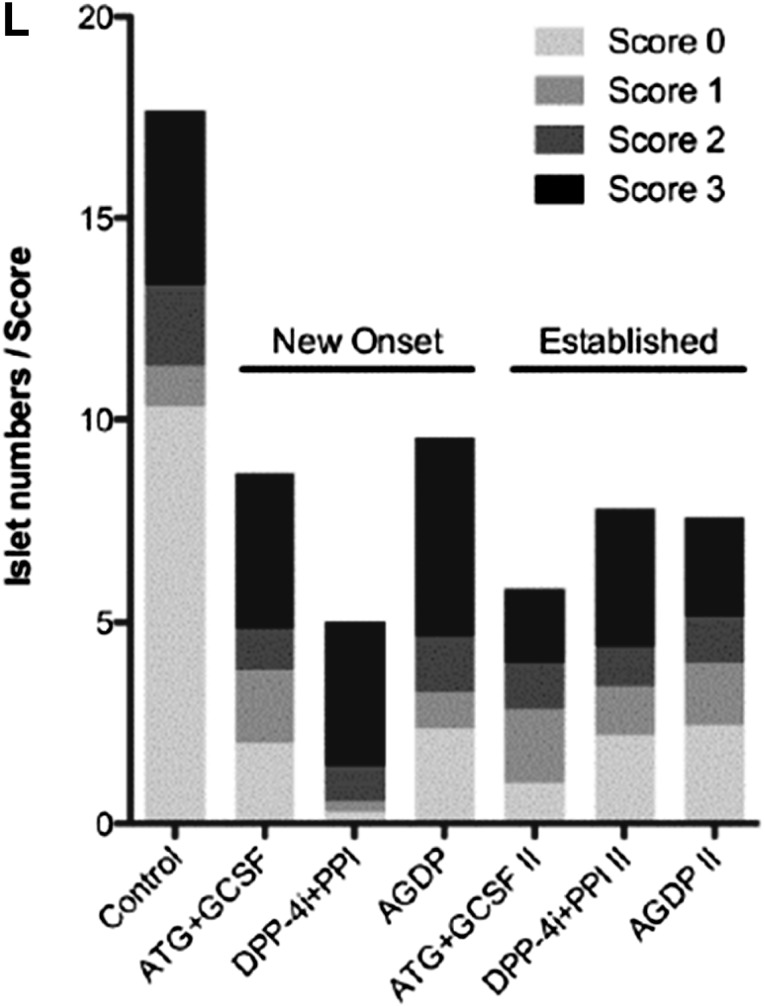
AGDP combination therapy induces long-term euglycemia in NOD mice with new-onset and established T1D. A: At diabetes onset, mice were implanted with an insulin pellet and treatment was initiated (n = 11–13 per group). AGDP therapy induced durable remission in 83% of mice (P < 0.0001 vs. control, Kaplan-Meier survival test), compared with 54% of mice treated with DPP-4i+PPI and 50% of mice treated with ATG+G-CSF. B: Mice were implanted with an insulin pellet at onset, with treatment delayed 2 weeks (n = 11–13 per group). AGDP therapy induced remission in 50% of mice (P < 0.0013 vs. control) compared with 42% of mice treated with DPP-4i+PPI and 33% of mice treated with ATG+G-CSF. C–K: Nonfasting blood glucose curves are shown for individual mice in each treatment group. The day of T1D onset was set to day 0 (C–G) or day −14 (H–K) for the new-onset and established (delay) treatment groups, respectively, so that curves reflect blood glucose values after the initiation of treatment. L: Insulitis scoring of remitted mice showed that despite euglycemia at 120 days, the numbers of remaining islets with no to minimal inflammation (scores 0–2) were significantly reduced in all treatment groups compared with age-matched control nondiabetic NOD mice (P < 0.0001).
In new-onset mice, histological scoring of remitted animals showed fewer islets with insulitis with DPP-4i+PPI therapy and fewer islets with less than 50% insulitis (Fig. 1L); however, remitted mice with ATG+G-CSF and AGDP therapy had similar scoring. In mice with established diabetes, insulitis was similar among all three therapies, with ATG-G-CSF therapy showing a minor decrease in islets with insulitis compared with the other therapies.
AGDP Therapy Results in Disease Remission Despite Insignificant Increases in β-Cell Mass in a Cross-Sectional Study of Mechanism
Similar to the reversal studies described above, female NOD mice received a slow-release subcutaneous insulin pellet and were randomized into treatment groups at diabetes onset. However, end points were day 30 or treatment failure. In new-onset mice, AGDP and ATG+G-CSF groups trended higher than controls in circulating C-peptide levels, number of insulin+ islets in the pancreas, and pancreatic fractional insulin area, but the difference was not significant (P = 0.1115, P = 0.7776, and P = 0.7237, respectively) (Fig. 2A–C). In mice with established disease, one outlier from the DDP-4i+PPI group demonstrated an elevated fractional insulin area, but overall, the number of insulin+ islets and the fractional insulin area did not differ among the treatment groups (Fig. 2B and C). We were unable to evaluate the effect of therapy on Ki67+ β-cells as a measure of β-cell replication because many animals had no detectable insulin+ cells, rendering the number too low for statistical analysis.
Figure 2.
AGDP combination therapy and pancreatic insulin content. In a cross-sectional study, NOD mice with new-onset and established disease were implanted with a subcutaneous insulin pellet and treated with ATG+G-CSF, DPP-4i+PPI, or AGDP or not treated (control), and tissues were harvested 30 days after the initiation of treatment. A: Serum C-peptide (n = 6–7 per group), as measured via ELISA, trended higher in new-onset animals that received ATG+G-CSF and AGDP therapy compared with insulin-only controls (P = 0.1115, Kruskal-Wallis test). Estab., established. Pancreata (n = 4 per group) were stained for insulin via IHC and analyzed for the number of insulin-positive (B) islets and fractional insulin area (C). The difference between treatment groups was not significant, but there was a trend toward increased insulin-positive islets and insulin fractional area in new-onset mice treated with ATG+G-CSF and AGDP therapy (P = 0.7776 and P = 0.7237, respectively; one-way ANOVA). Pancreata (n = 2–3 per treatment group and age-matched nondiabetic NOD controls) were processed for total protein via acid-ethanol extraction and analyzed for total proinsulin (D), C-peptide (E), and insulin (F) via ELISA. Compared with insulin-treated animals, there was a trend toward increased proinsulin in new-onset mice treated with ATG+G-CSF and AGDP therapies, but all treatment groups demonstrated significantly reduced proinsuiln, C-peptide, and insulin compared with nondiabetic controls. Data are presented as mean ± SEM. ****P < 0.0001, all (one-way ANOVA).
Pancreata from two to three mice per group and two nondiabetic control NOD mice were analyzed for total proinsulin, C-peptide, and insulin content by ELISA. Results were normalized against total protein extracted from the pancreata. As expected, pancreata from nondiabetic NOD mice contained significantly higher levels of proinsulin, C-peptide, and insulin. Remarkably, despite disease reversal, AGDP therapy did not rescue pancreatic proinsulin, C-peptide, or insulin content to levels comparable with nondiabetic animals. In new-onset mice, treatment with ATG+G-CSF and AGDP was associated with an insignificant increase in total pancreatic proinsulin, C-peptide, and insulin (P < 0.0001; Fig. 2C–E). These data suggest that only a fraction of the β-cell mass, undetectable by current methods, must be preserved for T1D reversal and insulin independence.
Initial Blood Glucose Remission Rate Bias Is Ameliorated in AGDP Therapy
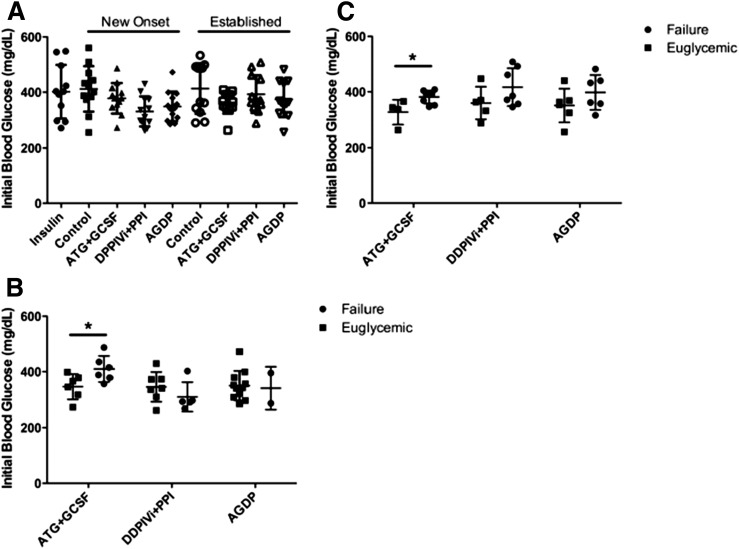
Previous NOD reversal studies have shown a correlation between higher initial blood glucose levels and failure rate in some therapies (3), including ATG alone (5). Accordingly, we determined if a similar correlation exists under these therapy conditions. Initial blood glucose levels were similar in mice randomized to all treatment groups (P = not significant [NS]) (Fig. 3A), implying no bias occurred during the randomization to treatment. However, the failure rate in mice treated with ATG+G-CSF correlated with higher initial blood glucose levels in the new-onset group and in the established group (P < 0.05) (Fig. 3B and C). This was not surprising given that suboptimal ATG dosing was used. The initial blood glucose bias in DPP-4i+PPI–treated mice was not significant (P = NS), whereas the addition of these two drugs in AGDP therapy ameliorated the initial blood glucose bias present with ATG+G-CSF therapy. Therefore, AGDP therapy was more effective than ATG+G-CSF therapy in mice with elevated initial blood glucose, whereas the influence of DPP-4i+PPI in this study was inconclusive.
Figure 3.
AGDP combination therapy and initial blood glucose. A: Initial blood glucose values were similar between mice in all therapy groups (n = 11–13 per group). P = NS, one-way ANOVA. In new-onset mice (B) and established T1D mice (C), ATG+G-CSF therapy was less effective at reversing diabetes in those with higher initial blood glucose. Data are presented as scatter with mean ± SD. *P < 0.05 (Student t test).
AGDP Therapy Induces T-Cell and NK-Cell Immunomodulation
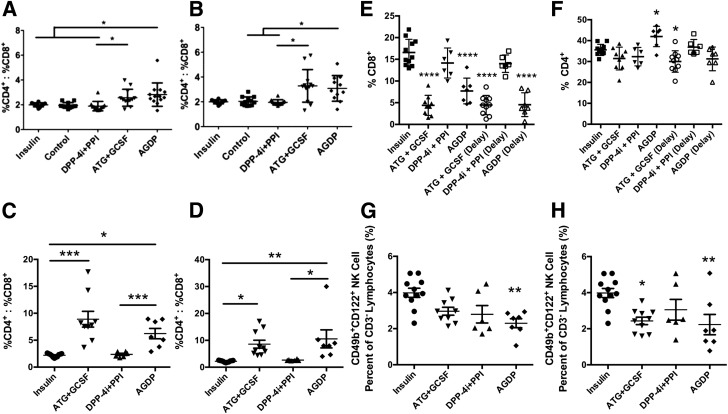
We next determined the effects of therapy on T-cell subsets. To determine the ratio of CD4+ to CD8+ T cells, splenocytes were isolated and analyzed by flow cytometry at the study end point (i.e., at reversal failure or 120 days). In mice with new-onset and established disease, treatment with AGDP significantly increased the CD4+-to-CD8+ T-cell ratio (P < 0.05, Fig. 4A and B). Treatment with ATG+G-CSF trended higher in new-onset and established disease but did not reach statistical significance, whereas DPP-4i+PPI did not have an effect on this phenotype (P = NS). These effects were even more robust in cross-sectional analysis. AGDP and ATG+G-CSF therapy significantly increased the splenocyte CD4+-to-CD8+ T-cell ratio in the new-onset and established-disease groups compared with controls, and AGDP significantly increased the CD4+-to-CD8+ T-cell ratio compared with those treated with DPP-4i+PPI (P < 0.001 and P = 0.0016, respectively) (Fig. 4C and D). These effects were largely due to CD8+ T-cell depletion, which was evident in all ATG+G-CSF– and AGDP-treated animals with new-onset and established disease (Fig. 4E). Meanwhile, splenic CD4+ T-cell frequencies were only modestly modulated in response to therapy—increased in new-onset mice with AGDP treatment, decreased in ATG+G-CSF–treated animals with established disease, and unchanged in all other groups, relative to control (Fig. 4F). Furthermore, mechanistic studies revealed that NK-cell frequency in the spleen was significantly reduced in new-onset mice treated with AGDP and in established-disease mice treated with AGDP or ATG+G-CSF (P = 0.0015 and P = 0.005, respectively) (Fig. 4G and H). Interestingly, mice that received DPP-4i+PPI treatment also trended toward reduced NK-cell frequencies in new-onset and established-disease groups, but these differences were not statistically significant (Fig. 4E and F).
Figure 4.
Combination therapy induces T-cell and NK-cell immunomodulation. T-cell subsets were analyzed by flow cytometry on treated mice (n = 11–13 per treatment group) or unmanipulated nondiabetic NOD mice (n = 9) at the study end point (therapy failure or 120 days). In new-onset (A) and established (B) T1D mice, AGDP therapy increased the CD4+-to-CD8+ T-cell ratio compared with control mice and mice treated with DPP-4i+PPI therapy, whereas ATG+G-CSF therapy had an increased ratio compared with DPP-4i+PPI therapy. These data were validated in a cross-sectional study of NOD mice with new-onset (C) and established (D) disease, where tissues were harvested 30 days after the initiation of treatment (n = 6–11 per group). ATG+G-CSF and AGDP therapies increased the CD4+-to-CD8+ T-cell ratio compared with control mice and mice treated with DPP-4i+PPI therapy (P < 0.0001 and P = 0.0016, respectively; one-way ANOVA). E: Splenocyte CD8+ T-cell frequency within the lymphocyte population was significantly reduced by ATG+G-CSF and AGDP therapy in mice with new-onset as well as established T1D, relative to insulin pellet controls (P < 0.0001, one-way ANOVA). F: Compared with control mice, splenic CD4+ T-cell frequency was increased in new-onset AGDP-treated animals but decreased with ATG+G-CSF treatment in mice with established disease (P < 0.05). In NOD mice with new-onset T1D (G), AGDP therapy decreased the splenic NK-cell frequency compared with insulin-treated control mice (P = 0.0015; one-way ANOVA), whereas in NOD mice with established disease (H), ATG+G-CSF and AGDP therapies decreased the NK-cell frequency (P = 0.005). Data are presented as scatter with mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
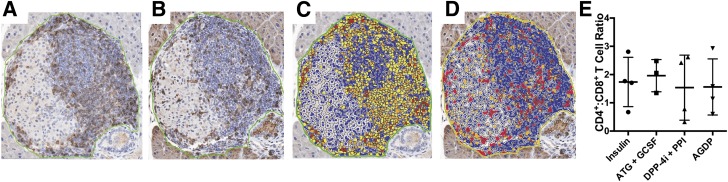
Pancreata harvested for cross-sectional studies were stained by IHC for CD4 and CD8, and digitally scanned images were manually annotated to exclude exocrine tissues from analysis. Within the pancreatic islet area, the CD4+-to-CD8+ T-cell ratios did not differ across treatment groups (Fig. 5). Moreover, absolute numbers of CD4+ and CD8+ T cells within the insulitic lesion were not significantly different (data not shown). However, these data do not consider potential modulation of T-cell phenotype or function within the pancreas or draining lymph node, and with the small sample size (n = 3–4 per group), possible immunomodulatory effects of therapy within the target organ cannot be excluded, providing an important topic of investigation for future studies.
Figure 5.
At day 30 after initiation of treatment, pancreata were fixed and embedded in paraffin. Serial sections were stained via IHC for CD4 (A) and CD8 (B). The scanned images were annotated for quantification of the percentage of cells staining positive for CD4 (C) and CD8 (D) within the islet area using CytoNuclear IHC Quantification software. (E) The CD4+-to-CD8+ T-cell ratio within the pancreatic islets was not significantly different across treatment groups.
To analyze CD4+ and CD8+ naïve, memory, and Tregs, remitted mice were compared with therapy failures and with age-matched nondiabetic female NOD mice, which were used to control for natural shifts in cell populations with age. With new-onset animals, remitted mice from all three therapy groups had an elevated percentage of splenic CD4+CD25+FoxP3+ Tregs compared with nondiabetic control mice (P < 0.05) (Fig. 6A). Within the ATG+G-CSF and DPP-4i+PPI groups, remitted mice showed significantly higher levels of Tregs than failures (P < 0.05); remitted mice in the AGDP group appear to have higher Tregs than the one failed mouse. However, the low number prohibits statistical analysis. Similarly, with established diabetes, remitted mice in all three therapy groups had elevated Tregs compared with failures (P < 0.05, Fig. 6B). Remitted mice in the DPP-4i+PPI and AGDP groups also had elevated Tregs compared with nondiabetic controls.
Figure 6.
Combination therapy induces T-cell immunoregulation. In new-onset (A) and established (B) T1D mice, remitted mice receiving AGDP, ATG+G-CSF, and DPP-4i+PPI therapies had elevated Tregs compared with failures. B: With the exception of ATG+G-CSF therapy in established disease, remitted mice from all three therapies had elevated Tregs compared with control nondiabetic mice. In new-onset (C) and established (D) T1D mice, remitted AGDP animals exhibited increased CD8+ Tregs at 120 days compared with nondiabetic controls (C) or failed AGDP (D) mice, respectively. *P < 0.05 (one-way ANOVA).
CD8+CD25+FoxP3+ Tregs were elevated in remitted mice in the new-onset AGDP therapy group versus the nondiabetic controls. In this same group, the DPP4i+PPI successfully treated mice had higher CD8+CD25+FoxP3+ Tregs than the failed mice (P < 0.05, Fig. 6C). In the established-disease group, only the successfully treated AGDP animals had elevated CD8+CD25+FoxP3+ relative to the failed animals (P < 0.05, Fig. 6D). Collectively, we interpret this as evidence of immunoregulation by any of these combinations as measured by CD4 or CD8 Tregs and that the successful treatment yielded higher immunoregulatory cell frequency over those that failed. In established mice, this trended lower, implying that endocrine or metabolic issues may underlie failure as opposed to failed immunoregulation. Additional studies will be needed to further investigate these mechanisms. DPP4i+PPI therapy also revealed a potential mechanistic basis in both CD4 and CD8 Treg modulation, perhaps adding further evidence of immunomodulatory effects beyond those favoring incretin hormone and β-cell survival mechanisms.
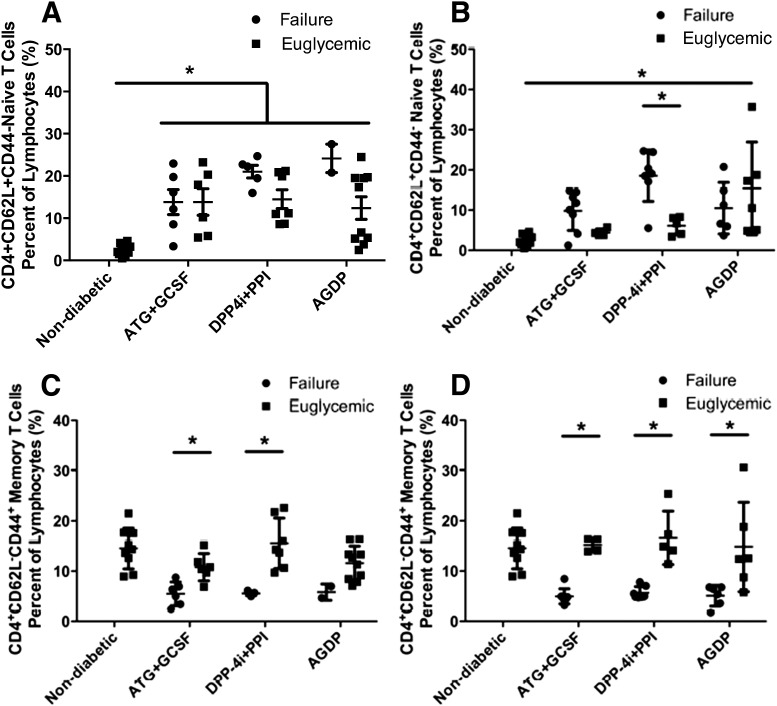
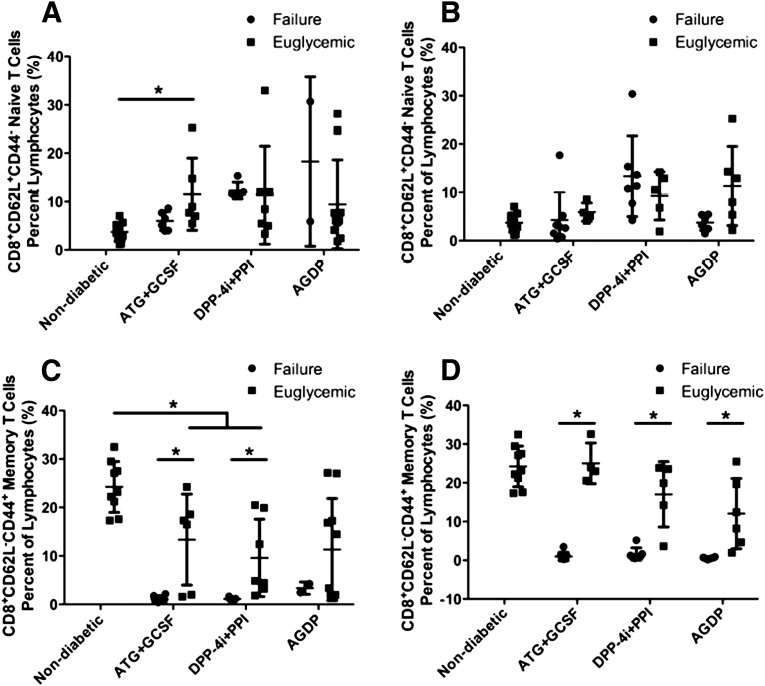
We next determined CD4+ naïve (CD4+CD62L+CD44−) and memory (CD4+CD62L−CD44+) T-cell populations in the therapy groups. In all new-onset treatment groups and in AGDP therapy in the established-disease treatment group, treated mice had a higher percentage of naïve CD4 T cells than age-matched nondiabetic controls. The only difference in failure versus success was in the DPP4i+PPI group, wherein failed mice were left with more naïve CD4 T cells (P < 0.05, Fig. 7A and B). Memory CD4 T cells, however, did associate with successfully treated mice tending to have more of these cells than failed animals. This held true for all groups, in both new-onset and established disease, except for the new-onset AGDP group, where the small sample size in the failed group did not allow for statistical significance to be achieved (Fig. 7C and D). No significance was observed for naïve CD8 T-cell frequency in any group with respect to successful reversal versus failure. There was an increase in naïve CD8+ T cells in the successfully treated new-onset ATG+G-CSF therapy relative to nondiabetic controls (Fig. 8A and B). In remitted mice from the new-onset treatment group, but not the established treatment group, ATG+G-CSF and DPP-4i+PPI therapies had a lower percentage of memory CD8+ T cells than nondiabetic age-matched controls but higher memory CD8 T-cell frequency associated with success compared with failed animals in the new-onset and established-disease groups, except again for the new-onset AGDP group, which had too few failures to evaluate statistically (P < 0.05) (Fig. 8C and D).
Figure 7.
Naïve and memory CD4+ T-cell subsets modulation by combination therapy. T-cell subsets were analyzed by flow cytometry in treated mice (n = 11–13 per treatment group) or unmanipulated nondiabetic NOD mice (n = 9) at the study end point. A: At 120 days, mice in all new-onset therapy groups, regardless of ability to reverse, had higher CD4+ naïve cell frequency than controls. B: In mice with established disease, only remitted AGDP animals corresponded to higher naïve T cells compared with nondiabetic controls, whereas naïve T-cell levels were higher in failed vs. reversed animals treated with DPP-4i+PPI. CD4+ memory T cells were not different between control and treated animals with new-onset (C) or established (D) T1D. However, successfully treated mice did show differences, as indicated, vs. mice that failed to remit. Data are presented as scatter with mean ± SD. Remitted vs. control mice analyzed by one-way ANOVA; remitted mice vs. failures by Student t test. *P < 0.05.
Figure 8.
Memory and naïve CD8+ T-cell analysis of treatment groups. Naïve CD8+ T cells were elevated at 120 days with ATG+GSCF therapy in new-onset (A) but not in established (B) T1D mice vs. nondiabetic mice (one-way ANOVA). C: Mice successfully treated with ATG+G-CSF or DPP-4i+PPI had lower levels of memory CD8+ cells than nondiabetic NOD mice at 120 days but higher levels of memory CD8 T cells than failed animals, as indicated. *P < 0.05 (one-way ANOVA). D: Memory CD8+ cells were elevated in remitted mice compared with failures for all treatment conditions in the group with established disease. Data are presented as mean ± SD. Differences between remitted and failed mice within treatment groups were analyzed by Student t test. *P < 0.05.
Discussion
Despite instances of success in preventing and even reversing hyperglycemia in the NOD mouse model of T1D, efforts at clinical translation have, for the most part, been disappointing in meeting their desired end points (2–4,15). At the same time, this statement is not intended to convey that promising therapeutic efforts, either preclinical or clinical (e.g., alefacept, imatinib), do not exist (reviewed in [16]) because efforts are, thankfully, moving forward.
In the studies presented here, we tested combinations of four drugs that have in past settings demonstrated potential utility in T1D or in other relevant autoimmune diseases with the notion that ATG+G-CSF treatment would promote immunoregulation, whereas DPP-4i+PPI therapy might preserve or even increase β-cell mass and function (5,6,8–13). We therefore hypothesized that AGDP therapy may result in synergy for the reversal of T1D. Indeed, our previous studies showing the efficacy of ATG+G-CSF therapy required higher doses of ATG to obtain similar reversal rates in new-onset mice (3,5). In this regard, AGDP therapy may be more readily translatable to human clinical trials because lower doses of ATG would potentially be safer and cause fewer adverse effects. The ability of AGDP therapy to induce disease remission in mice with established diabetes is striking, considering the paucity of β-cells remaining after such an extended period of autoimmune destruction (17). Taken together, AGDP with a lower dose of ATG is superior to ATG+G-CSF or DPP-4i+PPI. In established disease, however, a very similar profile was observed with all therapeutic combinations, alluding to potential additional β-cell loss being responsible as opposed to failure to immunomodulate.
In fact, similar immunomodulatory effects (increased Treg and memory T-cell frequencies) were observed across cured animals from ATG+G-CSF–, DPP-4i+PPI–, and AGDP-treated groups when treatment was initiated at onset or in mice with established disease. Although the benefits of increased Tregs in situations of autoimmunity are well recognized (18–20), a potential role for memory T cells is of particular interest. We would hypothesize that these observed memory T cells may possess a regulatory phenotype given previous reports of tolerance-inducing memory T cells preventing hyperglycemia in NOD mice (21–23); however, further ex vivo evaluation of the function of these cells would be needed to definitively establish their role in controlling disease.
It is curious that despite long-term remission from hyperglycemia, only modest increases in serum C-peptide and β-cell mass are evident in AGDP-treated animals versus controls. Furthermore, pancreatic insulin, proinsulin, and C-peptide content were dramatically lower in animals that received any of the tested combinations compared with nondiabetic NOD controls. Thus, it is possible that surprisingly small amounts of insulin were sufficient for blood glucose control; however, glucose tolerance in the mice was not assessed and should be a subject of further investigation. Also, we did not investigate for possible effects of AGDP treatment on insulin sensitivity and glucose counter-regulatory hormones that might have contributed to glucose control. Future efforts should also monitor for pancreatic alterations as a function of region (head, body, tail) because it may correlate with observations of heterogeneous β-cell loss in humans (24,25).
To date, one of the most promising forms of therapy to show long-term efficacy in humans (including subjects discontinuing insulin therapy) with T1D is one in which ATG, G-CSF, cyclophosphamide, and autologous stem cell therapy were combined (26,27). However, this particular therapy remains controversial, particularly its safety. Restoration of glycemic control in patients with T1D will likely require a combination therapy that confers immunoregulation as well as β-cell benefits yet is safe for use in humans (28). With this in mind, our use of low-dose ATG (as a milder form of immunosuppression/immune modulation) with the standard dose of G-CSF was taken alongside drugs that are approved by the U.S. Food and Drug Administration. AGDP treatment was well tolerated in NOD mice, with no noted adverse effects. However, there is always a potential for unexpected off-target effects with combination therapy, and future translational efforts in humans should take this into account.
We (A.R.) also recently reported our first experience in using a DPP-4i alongside a PPI, specifically, sitagliptin and lansoprazole, in humans with T1D (29). This double-blind, placebo-controlled, phase 2 trial (i.e., REPAIR-T1D) involved participants (11–36 years old) within 6 months of their T1D diagnosis. Although this study failed to meet its primary end point (i.e., C-peptide response to a mixed-meal challenge at 12 months measured as 2-h area under the curve in treated subjects vs. control subjects), no adverse or serious adverse events were related to the drug combination. Interestingly, although the primary end point was not achieved, not all participants had increases in the GLP-1 and gastrin concentrations expected for this treatment. Hence, further studies (with greater statistical power) monitored for GLP-1 and gastrin concentrations, along with the combination proposed here (AGDP), likely form an attractive future therapeutic possibility.
In combining the four drugs for this preclinical study, we have created a regimen that minimizes immunosuppression yet remains effective at reversing established T1D in NOD mice with new-onset as well as established disease. These data demonstrate the efficacy of AGDP to reverse murine T1D and the potential of this therapy in a translational context. Indeed, we believe future efforts exploring this combination in humans with T1D would appear warranted.
Article Information
Acknowledgments. The authors thank John Williams and Scott Eisenbeiss, from Genzyme, for their provision of murine ATG.
Funding. These studies were funded through support from Sanford Health, JDRF, National Institutes of Health (grant AI-42288), and the Keene Family Professorship.
Duality of Interest. M.A.A. has filed an invention disclosure related to the use of ATG in T1D. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.X. and A.P. conceived the study, researched data, and wrote the manuscript. C.W., C.E.M., and M.H. contributed to discussion and reviewed and edited the manuscript. C.M. researched data. M.C.-T. contributed to the research and to discussion. A.R., A.S., T.B., M.B., and D.S. contributed to the study design, data interpretation, and discussion, and reviewed and edited the manuscript. M.A.A. conceived the study, evaluated the data, contributed to discussion, and reviewed and edited the manuscript. M.A.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001;358:221–229 [DOI] [PubMed] [Google Scholar]
- 2.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 2005;23:447–485 [DOI] [PubMed] [Google Scholar]
- 3.Atkinson MA. Evaluating preclinical efficacy. Sci Transl Med 2011;3:96cm22. [DOI] [PubMed] [Google Scholar]
- 4.Herold KC, Bluestone JA. Type 1 diabetes immunotherapy: is the glass half empty or half full? Sci Transl Med 2011;3:95fs1. [DOI] [PubMed] [Google Scholar]
- 5.Parker MJ, Xue S, Alexander JJ, et al. Immune depletion with cellular mobilization imparts immunoregulation and reverses autoimmune diabetes in nonobese diabetic mice. Diabetes 2009;58:2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Parker M, Xia C, et al. Rabbit polyclonal mouse antithymocyte globulin administration alters dendritic cell profile and function in NOD mice to suppress diabetogenic responses. J Immunol 2009;182:4608–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haller MJ, Gitelman SE, Gottlieb PA, et al. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest 2015;125:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suarez-Pinzon WL, Cembrowski GS, Rabinovitch A. Combination therapy with a dipeptidyl peptidase-4 inhibitor and a proton pump inhibitor restores normoglycaemia in non-obese diabetic mice. Diabetologia 2009;52:1680–1682 [DOI] [PubMed] [Google Scholar]
- 9.Mu J, Woods J, Zhou YP, et al. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 2006;55:1695–1704 [DOI] [PubMed] [Google Scholar]
- 10.Pospisilik JA, Martin J, Doty T, et al. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 2003;52:741–750 [DOI] [PubMed] [Google Scholar]
- 11.Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes 2008;57:3281–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TC, Bonner-Weir S, Oates PS, et al. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J Clin Invest 1993;92:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rooman I, Lardon J, Bouwens L. Gastrin stimulates beta-cell neogenesis and increases islet mass from transdifferentiated but not from normal exocrine pancreas tissue. Diabetes 2002;51:686–690 [DOI] [PubMed] [Google Scholar]
- 14.Jang JY, Kim SW, Han JK, et al. Randomized prospective trial of the effect of induced hypergastrinemia on the prevention of pancreatic atrophy after pancreatoduodenectomy in humans. Ann Surg 2003;237:522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staeva TP, Chatenoud L, Insel R, Atkinson MA. Recent lessons learned from prevention and recent-onset type 1 diabetes immunotherapy trials. Diabetes 2013;62:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenbaum CJ, Schatz DA, Haller MJ, Sanda S. Through the fog: recent clinical trials to preserve β-cell function in type 1 diabetes. Diabetes 2012;61:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chmelova H, Cohrs CM, Chouinard JA, et al. Distinct roles of β-cell mass and function during type 1 diabetes onset and remission. Diabetes 2015;64:2148–2160 [DOI] [PubMed] [Google Scholar]
- 18.Tonkin DR, He J, Barbour G, Haskins K. Regulatory T cells prevent transfer of type 1 diabetes in NOD mice only when their antigen is present in vivo. J Immunol 2008;181:4516–4522 [DOI] [PubMed] [Google Scholar]
- 19.Tonkin DR, Haskins K. Regulatory T cells enter the pancreas during suppression of type 1 diabetes and inhibit effector T cells and macrophages in a TGF-beta-dependent manner. Eur J Immunol 2009;39:1313–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richer MJ, Lavallée DJ, Shanina I, Horwitz MS. Immunomodulation of antigen presenting cells promotes natural regulatory T cells that prevent autoimmune diabetes in NOD mice. PLoS One 2012;7:e31153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godebu E, Summers-Torres D, Lin MM, Baaten BJ, Bradley LM. Polyclonal adaptive regulatory CD4 cells that can reverse type I diabetes become oligoclonal long-term protective memory cells. J Immunol 2008;181:1798–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber SE, Harbertson J, Godebu E, et al. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J Immunol 2006;176:4730–4739 [DOI] [PubMed] [Google Scholar]
- 23.Li CR, Baaten BJ, Bradley LM. Harnessing memory adaptive regulatory T cells to control autoimmunity in type 1 diabetes. J Mol Cell Biol 2012;4:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arif S, Leete P, Nguyen V, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes 2014;63:3835–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pugliese A, Yang M, Kusmarteva I, et al. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes 2014;15:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2007;297:1568–1576 [DOI] [PubMed] [Google Scholar]
- 27.Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009;301:1573–1579 [DOI] [PubMed] [Google Scholar]
- 28.Schatz D, Gale EA, Atkinson MA. Why can’t we prevent type 1 diabetes?: maybe it’s time to try a different combination. Diabetes Care 2003;26:3326–3328 [DOI] [PubMed] [Google Scholar]
- 29.Griffin KJ, Thompson PA, Gottschalk M, Kyllo JH, Rabinovitch A. Combination therapy with sitagliptin and lansoprazole in patients with recent-onset type 1 diabetes (REPAIR-T1D): 12-month results of a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol 2014;2:710–718 [DOI] [PMC free article] [PubMed] [Google Scholar]