Abstract
Most natural history models for type 1 diabetes (T1D) propose that overt hyperglycemia results after a progressive loss of insulin-secreting β-cell mass and/or function. To experimentally address this concept, we prospectively determined morning blood glucose measurements every other day in multiple cohorts (total n = 660) of female NOD/ShiLtJ mice starting at 8 weeks of age until diabetes onset or 26 weeks of age. Consistent with this notion, a majority of mice that developed diabetes (354 of 489 [72%]) displayed a progressive increase in blood glucose with transient excursions >200 mg/dL, followed by acute and persistent hyperglycemia at diabetes onset. However, 135 of the 489 (28%) diabetic animals demonstrated normal glucose values followed by acute (i.e., sudden) hyperglycemia. Interestingly, diabetes onset occurred earlier in mice with acute versus progressive disease onset (15.37 ± 0.3207 vs. 17.44 ± 0.2073 weeks of age, P < 0.0001). Moreover, the pattern of onset (i.e., progressive vs. acute) dramatically influenced the ability to achieve reversal of T1D by immunotherapeutic intervention, with increased effectiveness observed in situations of a progressive deterioration in euglycemia. These studies highlight a novel natural history aspect in this animal model, one that may provide important guidance for the selection of subjects participating in human trials seeking disease reversal.
Introduction
Type 1 diabetes (T1D) is a disorder resulting from an autoimmune destruction of the insulin-producing pancreatic β-cells (β-cells) (1). For decades, a predominant concept has existed suggesting that this β-cell loss in pre-T1D occurs in a linear fashion in genetically susceptible individuals after initiation of the destructive process by a still-undefined environmental insult(s) (2,3). The symptomatic onset of disease is thought to occur once a critical mass of β-cells is lost (4). That said, models contrary to this thought of linear loss have been put forward, reflecting views that β-cell loss might occur either after an acute (i.e., catastrophic) destruction event or after a period of stepwise (i.e., sporadic) loss akin to what might be considered a relapsing-remitting disease (2,5,6). Defining which of these models is reflective of reality has been difficult to achieve due to a number of limitations, including our inability to image human pancreas, ethical concerns over pancreatic biopsy, and a dependence on indirect measures of β-cell mass/function (i.e., glucose tolerance testing) (7,8).
As but one means to overcome such limitations, investigators have turned to studies of the NOD mouse model of the disease. Such efforts have been rewarding both in terms of defining immunologic and metabolic components of disease pathogenesis as well as in testing of therapeutic interventions (9,10). As evidence of the benefit for such efforts, many agents tested in this strain have effectively been translated to clinical investigations with human T1D patients (11–14). However, as of the time of this writing, no universally accepted means exists to prevent and/or reverse the disease in humans. Many reasons for this lack of therapeutic efficacy have been put forward, including the notion that a better description of disease heterogeneity is required, as reflected by the aggressiveness of β-cell autoimmunity or the degree of β-cell loss (15,16). Indeed, such information may be critical to achieving eventual therapeutic efficacy.
Previous studies may not have, in robust fashion, examined the precise metabolic parameters and pathways prior to disease onset in the NOD model. Cross-sectional studies have demonstrated impaired glucose metabolism as a marker of disease progression in prediabetic NOD females at different ages (17,18). However, these represent single time point analyses rather than longitudinal characterization of individual mice over time to identify the pattern from euglycemia to hyperglycemia in this strain. Furthermore, studies utilizing such information as a means to “predict” responses to therapeutic intervention are lacking. For these reasons, in this study, we sought to determine whether distinct metabolic signatures exist in prediabetic NOD mice and whether such information is informative to attempts seeking disease reversal.
Research Design and Methods
Mice
Female NOD/ShiLtJ (NOD) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) during the time period of 2009–2014. All mice used were maintained in a specific pathogen-free research animal facility at the University of Florida. Mice were allowed free access to food and acidified drinking water. All procedures were approved by the University of Florida’s Institutional Animal Use and Care Committee and were in compliance with the laws of the U.S.
Blood Glucose Measures Prior to Onset
Nonfasting morning blood glucose concentrations were measured by tail vein prick using a OneTouch Ultra 2 blood glucose meter (LifeScan Inc.) every other day starting at 8 weeks of age. Any reading of blood glucose >250 mg/dL was followed by a test 24 h later. Diabetes was diagnosed by two successive readings of blood glucose >250 mg/dL (19). The blood glucose levels are reported starting at 24 days prior to diagnosis, a time period considered practical for evaluation based on the starting age of study (i.e., 8 weeks) relative to the presumed time period of when the earliest cases of diabetes would occur.
Disease Reversal Studies
Diabetic mice were randomized into treatment arms in the new-onset or established treatment groups as previously described (20,21). Upon diabetes diagnosis, all mice were implanted with a subcutaneous insulin pellet (LinBit; LinShin Canada Inc., Scarborough, Ontario, Canada). Mice in the new-onset group began therapy on the day of confirmed diagnosis (new-onset day 0). Therapy groups consisted of insulin pellet as well as antithymocyte globulin (ATG) plus granulocyte colony-stimulating factor (G-CSF) therapy. Murine ATG (Genzyme, Cambridge, MA) was administered intraperitoneally at 250 µg/dose on days 0 and 3. Human G-CSF (Neulasta; Amgen, Thousand Oaks, CA) at 120 µg/dose was administered intraperitoneally on days 0 and 15. Blood glucose values were monitored after the initiation of treatment for 120 days or until animals experienced overt failure (two consecutive blood glucose values >400 mg/dL). Disease reversal was defined as animals maintaining blood glucose <250 mg/dL to day 120 post–diabetes onset.
Statistical Analysis
Unless stated otherwise, data are shown as mean ± SE. Significance (P < 0.05) was determined by one-way ANOVA with Bonferroni multiple comparison test, Student t test for two-group comparisons, linear regression analysis, or χ2 test for categorical data (GraphPad Prism 5.02, San Diego, CA).
Results
Frequency and Peak Age of T1D Onset in NOD Mice
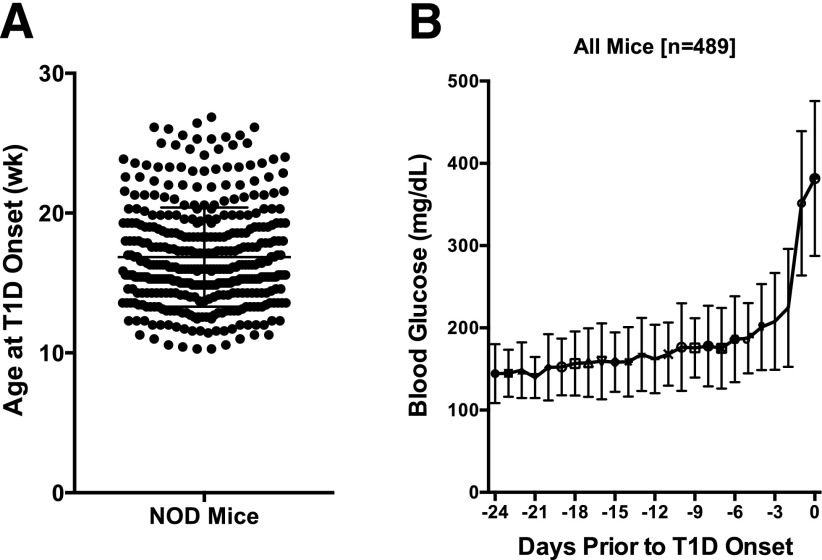
Previous studies in our animal facility using NOD/ShiLtJ female mice have historically demonstrated a T1D incidence approximating 80% by 26 weeks of age, with an incidence of 60% by 16 weeks of age (22). For this study, we intensively and prospectively monitored morning, nonfasted blood glucose values in a large cohort (n = 660) of NOD/ShiLtJ mice from 8 to 26 weeks, including a previously reported small cohort (n = 68) of animals (23). The earliest time of disease onset was 10 weeks of age, with a peak onset time of 16 weeks (Fig. 1A). By 26 weeks (end of study), the incidence in these studies was 74% (489 of 660).
Figure 1.
In NOD/ShiLtJ females, diabetes is preceded by a gradual increase in glucose levels. A: The age at T1D onset varied considerably, occurring as early as 10 and as late as 26 weeks (wk) of age (the end of the study). B: Morning glucose levels in NOD/ShiLtJ females (n = 489) were recorded every other day starting at 8 weeks of age. Results are synchronized for diabetes onset (set to day 0) defined as two consecutive glucose readings >250 mg/dL. Data are presented as mean ± SD. The data here include only the 489 mice that developed diabetes.
As a Collective, NOD Mice Show a Progressive Rise in Glycemic Values Weeks Prior to Disease Onset
In order to define the time at which blood glucose values rise in the natural history of prediabetes, we performed an analysis where all blood glucose values were plotted backward in time from the day of disease onset (Fig. 1B). From 24 days to ∼1 week prior to disease onset, morning blood glucose values were, on average, <175 mg/dL (23,24). However, within 1 week of disease onset, as a collective, blood glucose values rose rapidly, with the most rapid rise within 72 h of an overt diagnosis of T1D (Fig. 1B).
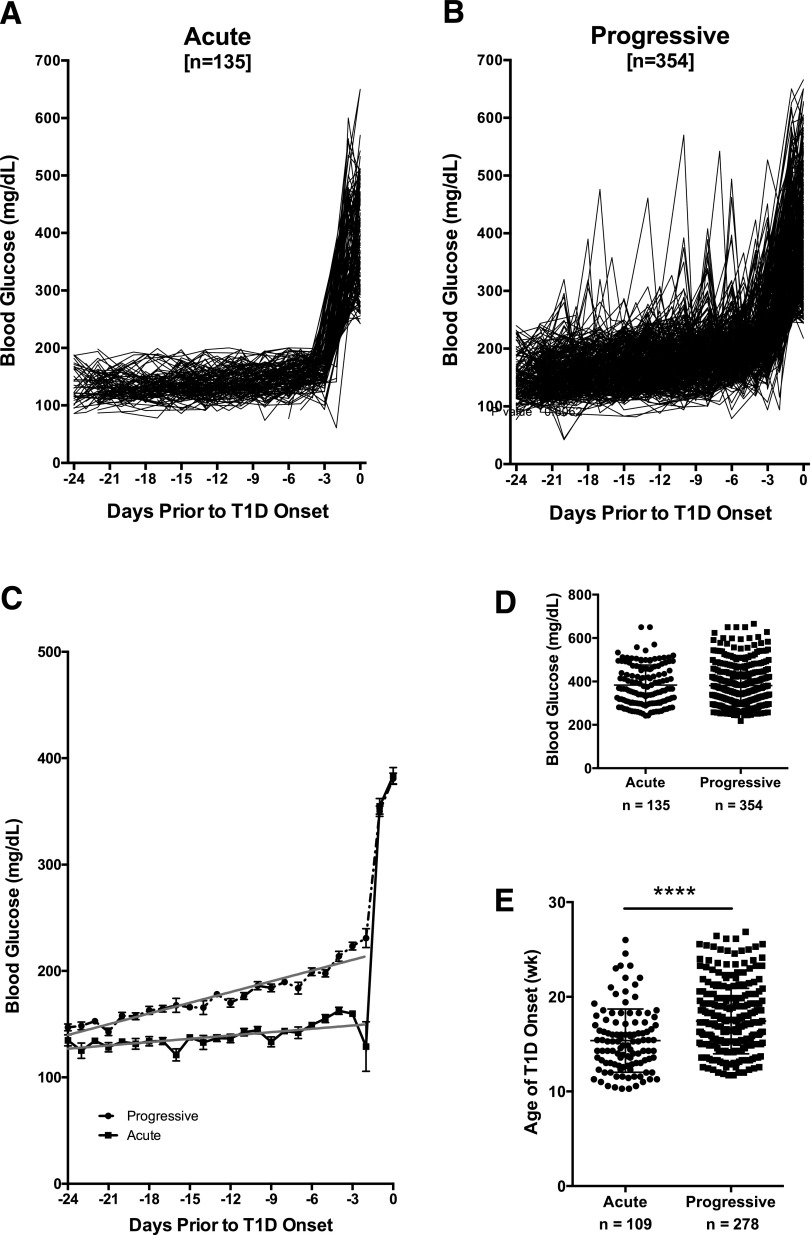
Based on Glycemic Values Prior to T1D Onset, NOD Mice Can Be Categorized as Experiencing Progressive Versus Acute Onset
When animals were evaluated collectively, NOD mice appeared to experience considerable variability in blood glucose values prior to T1D onset as noted by wide standard deviation at each time point (Fig. 1B). We separated animals into two groups based on blood glucose fluctuations in the 24 days prior to onset, characterizing those that maintained a consistent blood glucose level prior to acute onset of hyperglycemia versus those that exhibited variable blood glucose and a progressive onset of hyperglycemia. Indeed, over one-quarter (135 of 489 [28%]) of the mice experienced steady euglycemia (blood glucose <200 mg/dL) followed by sudden T1D onset (acute) (Fig. 2A), but in the remaining mice (354 of 489 [72%]), onset was preceded by at least one blood glucose excursion ≥200 mg/dL (progressive) (Fig. 2B). Linear regression analysis of blood glucose values over time prior to diagnosis (days −24 to −2) demonstrated significant differences between the two groups (slope = 3.360 progressive vs. 1.039 acute, P < 0.0001) (Fig. 2C) despite similar blood glucose levels on the day of T1D onset (Fig. 2D), suggesting two potential modalities of disease progression in NOD mice. Animals that experienced acute T1D onset were significantly younger at diagnosis compared with those with progressive disease (15.37 ± 0.3207 vs. 17.44 ± 0.2073 weeks of age, P < 0.0001) (Fig. 2E).
Figure 2.
In NOD/ShiLtJ females, diabetes onset can be categorized as acute or progressive. A: Diabetic NOD/ShiLtJ females experienced acute (n = 135) (A) or progressive (n = 354) (B) T1D onset according to their morning blood glucose levels in the 24 days prior to disease onset (set to day 0). Acute onset was defined as blood glucose <200 mg/dL prior to onset, whereas progressive was defined as at least one blood glucose reading ≥200 mg/dL prior to onset. C: Linear regression analysis of blood glucose values from day −24 to −2 prior to T1D onset (set to day 0) demonstrates significantly different disease development in progressive (dashed line) vs. acute (solid line) onset groups (P < 0.0001). D: Blood glucose levels did not differ between acute and progressive NOD mice on the day of T1D diagnosis (P = 0.966, Student t test). E: Animals belonging to the acute-onset group were significantly younger at the time of disease onset compared with mice with progressive T1D onset (****P < 0.0001, Student t test). Data are presented as mean ± SEM. wk, week.
Mode of T1D Onset Is a Significant Factor in Efficacy of Therapies Seeking T1D Reversal
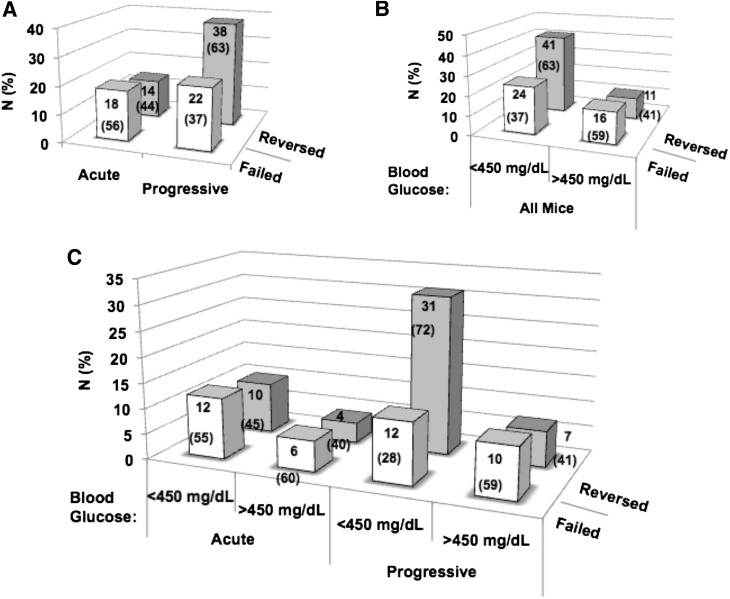
A subgroup (n = 92) of mice participating in this analysis were subjected, by experimental design, to studies monitoring the effectiveness of a previously described combination therapy aimed at reversing hyperglycemia (20,21). For this effort, after a diagnosis of diabetes, all mice were provided a slow-release insulin pellet and treated with a low dose (250 μg) of murine ATG plus G-CSF, as previously described (20). In similar form to the notions described above, 65.2% (n = 60) of mice exhibited progressive glycemic loss, with the remaining 32 animals (34.8%) demonstrating the heretofore-described acute onset of diabetes (Fig. 3).
Figure 3.
Acute vs. progressive diabetes onset in NOD mice affects ability to reverse disease with ATG + G-CSF combination therapy. Ability to reverse T1D with ATG + G-CSF combination therapy trended greater for animals with progressive T1D onset (P = 0.0815, χ2 test) (A) and mild hyperglycemia (blood glucose <450 mg/dL) (P = 0.0653, χ2 test) (B). C: Ability to reverse T1D with ATG + G-CSF combination therapy was significantly greater for animals with both progressive onset and diagnostic blood glucose <450 mg/dL at T1D onset (P < 0.05, χ2 test). Data are presented as absolute number (percentage) of animals within each category (acute vs. progressive and/or blood glucose status) that reversed (gray bars) or failed (white bars) in response to combination immune therapy.
Overall, some 56.5% (n = 52) of all treated animals demonstrated long-term diabetes reversal, whereas 43.5% (n = 40) were nonresponders to therapeutic intervention. Interestingly, the rate of disease remission trended higher in those with progressive loss of glycemia (63% [n = 38]) versus those with acute-onset disease (44% [n = 14]) (Fig. 3A).
We then examined the influence of the mode of disease onset on the therapeutic efficacy based on the degree of dysglycemia at disease intervention. As noted, NOD mice often escalated to markedly elevated blood glucose levels within hours to days of initial hyperglycemia. As a result, animals were stratified to those with blood glucose values <450 mg/dL or >450 mg/dL at therapeutic intervention based on our previous study (20). As expected and consistent with previous reports by ourselves and others (20,25), the efficacy for disease reversal was highest in those with lower starting blood glucose values, although the difference was not statistically significant (Fig. 3B). It was, however, noteworthy to observe that the mode of disease onset was a contributing factor, especially when considered as a function of the degree of dysglycemia, since reversal rate (72%, n = 31) was significantly higher for animals with both progressive T1D onset and diagnostic blood glucose levels <450 mg/dL (Fig. 3C). Here again, a combination of progressive onset and lower levels of dysglycemia was associated with the highest level of efficacy in terms of disease reversal.
Discussion
We previously reported that, like humans, NOD mice exhibit a progressive, although not necessarily linear or constant, decline in first-phase insulin response to glucose (23). It is generally accepted that the severity of glycemic dysregulation and age at onset, as factors reflecting disease aggression, are likely to influence clinical outcome with immune therapy (12,14–16). However, specific evidence in support of this notion has been lacking in murine correlates. Previous characterization of the natural history of T1D in the NOD mouse has primarily involved cross-sectional analyses of β-cell function and mass (17,18), but these studies cannot account for NOD strain heterogeneity of disease progression without an identifiable biomarker. Here for the first time, we present a longitudinal evaluation of glycemia in NOD mice demonstrating two patterns for disease development during the prediabetic period, progressive versus acute, and the effect of this designation on ability to restore normoglycemia with an immune-modulatory combination therapy, ATG + G-CSF.
Acute T1D onset occurred more frequently in young animals, whereas older mice were more likely to experience at least one hyperglycemic excursion prior to diagnosis. This reflects the clinical observation that aggressive disease (sudden symptomatic onset and more extensive β-cell ablation at diagnosis) occurs most often in young patients, whereas humans who are diagnosed as adults more often experience gradual impairment of glycemic regulation (26,27), similar to progressive onset observed here in NOD mice. Acute and progressive T1D onset may represent two potentially different pathways to disease even in genetically identical animals (28). Future studies, including those examining whether differences in pancreatic pathology (e.g., immunophenotype and β-cell mass) and glucose tolerance associate with a mode of disease onset, will be helpful to address this question. Of particular interest would be longitudinal studies to validate as well as identify the mechanisms underlying the metabolic differences using noninvasive pancreatic imaging, frequent glucose tolerance testing, or testing glycemia under fasting conditions to quantify β-cell mass and function prior to disease onset in acute versus progressive animals; however, it is important to consider the possibility that frequent fasting conditions or high volume blood collections may influence the rate and mode of diabetes development.
We believe the findings presented herein may have important clinical implications in determining likely candidates for successful intervention among distinct populations of subjects with T1D. Characterization of the underlying pathology (i.e., immune mediated, metabolic [β-cell dysfunction], and/or anatomical [β-cell mass]) differentiating these two groups is needed to direct mechanism-based intervention. We hypothesize that the reversal of acute T1D onset may be more likely to require more aggressive therapy with additional combinatorial agents, perhaps targeting β-cell function or replacement, in order to achieve therapeutic efficacy. Moving forward, the designation as acute versus progressive onset might be taken into consideration in preclinical trial design, interpretation of results, and perhaps even clinical translation.
Article Information
Acknowledgments. The authors thank Michele Youd and Scott Eisenbeis (Genzyme) for their provision of murine ATG.
Funding. These studies were supported, in part, by National Institutes of Health DK074656 (to C.E.M.) and AI42288 (to C.E.M. and M.A.A.) and were also supported by the American Diabetes Association (to C.E.M.) and JDRF (to C.E.M. and M.A.A.).
Duality of Interest. M.A.A. has intellectual property regarding the use of ATG-based therapies for T1D. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.E.M. conceived of the study, researched the data, and wrote and edited the manuscript. S.X. and A.L. researched the data. A.P. contributed to discussion, researched the data, and wrote the manuscript. Y.L.L. researched the data and reviewed and edited the manuscript. X.L., C.W., M.J.H., and D.S. contributed to discussion and reviewed and edited the manuscript. M.A.A. conceived of the study and wrote, reviewed, and edited the manuscript. M.A.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srikanta S, Ganda OP, Gleason RE, Jackson RA, Soeldner JS, Eisenbarth GS. Pre-type I diabetes. Linear loss of beta cell response to intravenous glucose. Diabetes 1984;33:717–720 [DOI] [PubMed] [Google Scholar]
- 3.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med 1986;314:1360–1368 [DOI] [PubMed] [Google Scholar]
- 4.Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab 2008;10(Suppl. 4):23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimada A, Charlton B, Taylor-Edwards C, Fathman CG. Beta-cell destruction may be a late consequence of the autoimmune process in nonobese diabetic mice. Diabetes 1996;45:1063–1067 [DOI] [PubMed] [Google Scholar]
- 6.von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol 2007;7:988–994 [DOI] [PubMed] [Google Scholar]
- 7.Akirav E, Kushner JA, Herold KC. Beta-cell mass and type 1 diabetes: going, going, gone? Diabetes 2008;57:2883–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkinson MA. Pancreatic biopsies in type 1 diabetes: revisiting the myth of Pandora’s box. Diabetologia 2014;57:656–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoda LK, Young DL, Ramanujan S, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity 2005;23:115–126 [DOI] [PubMed] [Google Scholar]
- 10.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010;464:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005;54:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 13.Ludvigsson J, Faresjö M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 14.Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009;301:1573–1579 [DOI] [PubMed] [Google Scholar]
- 15.Arif S, Leete P, Nguyen V, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes 2014;63:3835–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet 2014;383:1084–1094 [DOI] [PubMed] [Google Scholar]
- 17.Kano Y, Kanatsuna T, Nakamura N, et al. Defect of the first-phase insulin secretion to glucose stimulation in the perfused pancreas of the nonobese diabetic (NOD) mouse. Diabetes 1986;35:486–490 [DOI] [PubMed] [Google Scholar]
- 18.Reddy S, Liu W, Thompson JM, Bibby NJ, Elliott RB. First phase insulin release in the non-obese diabetic mouse: correlation with insulitis, beta cell number and autoantibodies. Diabetes Res Clin Pract 1992;17:17–25 [DOI] [PubMed] [Google Scholar]
- 19.Atkinson MA. Evaluating preclinical efficacy. Sci Transl Med 2011;3:96cm22. [DOI] [PubMed] [Google Scholar]
- 20.Parker MJ, Xue S, Alexander JJ, et al. Immune depletion with cellular mobilization imparts immunoregulation and reverses autoimmune diabetes in nonobese diabetic mice. Diabetes 2009;58:2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue S, Posgai A, Wasserfall C, et al. Combination therapy reverses hyperglycemia in NOD mice with established type 1 diabetes. Diabetes. 16 July 2015 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 22.Simon G, Parker M, Ramiya V, et al. Murine antithymocyte globulin therapy alters disease progression in NOD mice by a time-dependent induction of immunoregulation. Diabetes 2008;57:405–414 [DOI] [PubMed] [Google Scholar]
- 23.Ize-Ludlow D, Lightfoot YL, Parker M, et al. Progressive erosion of β-cell function precedes the onset of hyperglycemia in the NOD mouse model of type 1 diabetes. Diabetes 2011;60:2086–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leiter E. The NOD mouse: A model for insulin-dependent diabetes mellitus In Current Protocols in Immunology. Hoboken, NJ, John Wiley & Sons, Inc., 1997, p. 15.19.11–15.19.23 [DOI] [PubMed] [Google Scholar]
- 25.Sherry NA, Chen W, Kushner JA, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 2007;148:5136–5144 [DOI] [PubMed] [Google Scholar]
- 26.Karjalainen J, Salmela P, Ilonen J, Surcel HM, Knip M. A comparison of childhood and adult type I diabetes mellitus. N Engl J Med 1989;320:881–886 [DOI] [PubMed] [Google Scholar]
- 27.Caillat-Zucman S, Garchon HJ, Timsit J, et al. Age-dependent HLA genetic heterogeneity of type 1 insulin-dependent diabetes mellitus. J Clin Invest 1992;90:2242–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simecek P, Churchill GA, Yang H, et al. Genetic analysis of substrain divergence in non-obese diabetic (NOD) mice. G3 (Bethesda) 2015;5:771–775 [DOI] [PMC free article] [PubMed] [Google Scholar]