Abstract
Noncoding RNA and especially microRNAs (miRs) have emerged as important regulators of key processes in cell biology, including development, differentiation, and survival. Currently, over 2,500 mature miRs have been reported in humans, and considering that each miR has multiple targets, the number of genes and pathways potentially affected is huge. Not surprisingly, many miRs have also been implicated in diabetes, and more recently, some have been discovered to play important roles in the pancreatic islet, including β-cell function, proliferation, and survival. The goal of this Perspective is to offer an overview of this rapidly evolving field and the miRs involved, reveal novel networks of β-cell miR signaling, and provide an outlook of the opportunities and challenges ahead.
Introduction
The perceived role of noncoding RNA has greatly changed over the past two decades. What was once thought to be largely “junk” is now understood to play an important regulatory role within the cell. Among the types of noncoding RNAs, microRNAs (miRs) have been shown to regulate key processes of cell biology, including differentiation and survival (1,2). These short nucleotide sequences (21–23 nt) are located either intergenically or within a host gene (3,4) and are transcribed under the control of RNA polymerase II (5), although transcription via RNA polymerase III has also been observed (6). Primary miR transcripts are processed into precursor miR by Drosha (7,8) and into mature miR by Dicer (9), and the mature miR strand combines with Argonaute proteins (Ago) into a RNA-induced silencing complex (RISC) (10) that binds to target sequences generally located in the 3′ untranslated region (UTR) of mRNAs (11). This target sequence is complementary (though often imperfectly so) to the mature miR, and specificity is primarily conferred by the first seven or eight nucleotides, known as the “seed” sequence (12). Upon binding to its gene target, the miR/RISC complex causes translational repression and/or degradation of the targeted mRNA sequence, as previously reviewed in detail (13), resulting in the downregulation of the protein encoded by the gene target. There also have been a few isolated reports of noncanonical effects, including miRs interacting with the coding sequence or the 5′UTR of target genes and thereby leading to translational repression (14–16). Surprisingly, in muscle cells, miR-1 has recently been shown to enter the mitochondria and to increase rather than to repress translation of mitochondrial DNA–encoded proteins (17). This effect was still dependent on Ago2 and on specific base-pairing of the miR with the target gene mRNA. In contrast, the same miR decreased the translation of target genes in the cytoplasm (17). It was therefore hypothesized that miRs may stimulate translation if Ago2 is present but its functional partner GW182 is excluded and if the target mRNA lacks a poly-A tail and 5′ cap, all of which is the case in mitochondria. While such mitochondrial effects have yet to be shown in pancreatic β-cells, they would be expected to have a major impact, especially considering the unique susceptibility of β-cells to alteration in bioenergetics and oxidative stress (18). Interestingly, miR-184, a miR highly expressed in human β-cells, has been suggested to be involved in noncanonical pathways and has been shown to directly target Ago2 (19).
According to version 21 of the online database for miRs (www.mirbase.org), there are 2,588 mature miRs found in Homo sapiens (20). The fact that each miR has multiple targets dramatically increases the number of potential genes targeted and pathways affected. It is therefore not surprising that many miRs have also been implicated in diabetes and its complications, as previously reviewed (21,22). However, the miR field is rapidly evolving and an increasing number of miRs have recently been shown to play important roles specifically in the pancreatic islet. By combining this knowledge and putting the data into context, this Perspective helps to provide an updated overview and reveal novel miR signaling networks with the potential impact on the life and death of islet β-cells.
Identifying MiRs Expressed In Islet β-Cells
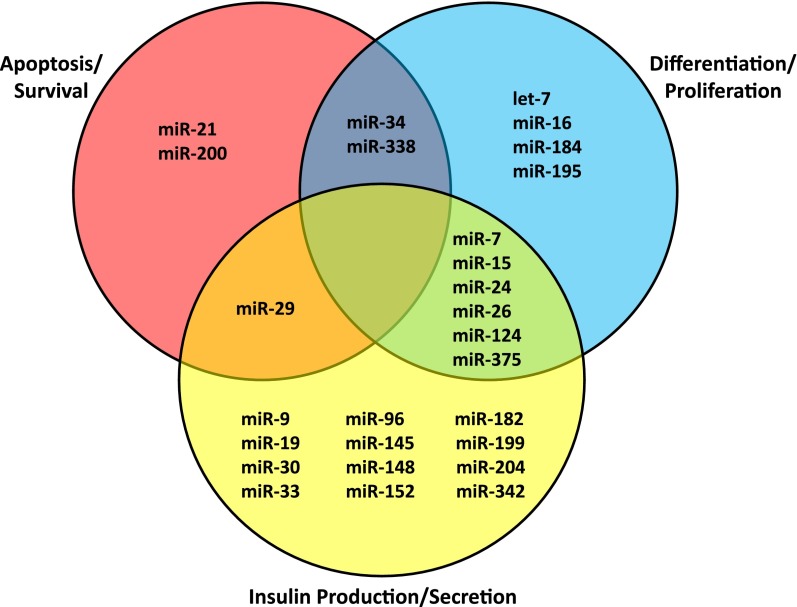
MiR profiling experiments have been performed to determine the expression of miRs in various tissues, and these studies have yielded an atlas for mammalian miR expression (23) and profiles from different species and various tissues, which are available online (smiRNAdb; http://www.mirz.unibas.ch/cloningprofiles) (24). These data have also been used to estimate relative abundance and tissue specificity of miRs in human islets and MIN6 mouse insulinoma cells (25). Furthermore, recent studies have used MIN6 cells (26), mouse islets (19), primary intact human islets (27), and human β-cells (27) sorted by flow cytometry for in-depth RNA sequencing, providing some indication of miR expression levels in pancreatic islets and β-cells. In addition, others have compared the enrichment of miR species in flow-sorted human islet α- and β-cell fractions using a quantitative PCR-based miR array platform (28). The expression levels and enrichment of the miRs reported to play a role in islet β-cell biology and discussed in this review are provided in Table 1. The most highly expressed miR was miR-375, and not surprisingly, miR-375 represents the best studied miR in β-cell biology and has been found to be involved in inhibition of insulin secretion (29) and compensatory β-cell proliferation (30). The top β-cell–enriched miR (>100-fold higher expression in β-cells as opposed to α-cells) was miR-204, and miR-204 has recently been shown to play an important role in the control of insulin production (31). These findings support the notion of expression level and/or enrichment being important; however, considering the fact that miRs are very potent regulatory molecules, it is not surprising that miRs with lower expression levels and/or no specific β-cell enrichment are capable of affecting β-cell biology (e.g., miR-34a) (32). Finally, some miRs may be expressed at very low levels or even be undetectable in human islets under normal, physiological conditions but might be upregulated under pathological circumstances, which would then affect β-cell function (e.g., miR-199a) (33). In the following paragraphs, only miRs that have been demonstrated to control key processes of islet β-cell biology, such as insulin production and secretion, proliferation or differentiation, and apoptosis or survival, are briefly discussed. Their β-cell effects are summarized in Table 2 and the overlap of their functions is shown in Fig. 1.
Table 1.
Expression and β-cell enrichment of miRs discussed
| miRs | Human β-cells | Human islets | Mouse islets | Mouse MIN6 cells | β-Cell enrichment |
|---|---|---|---|---|---|
| miR-375 | 5,804,575 | 2,046,518 | 636,788 | 369,695 | 2 |
| miR-7 | 670,521 | 259,457 | 842,839 | 423 | 3 |
| let-7a | 668,000 | 343,033 | 227,187 | 4,481 | 5 |
| miR-148a | 389,911 | 176,002 | 742,453 | 15,457 | 56 |
| miR-26a | 389,655 | 132,411 | 46,550 | 25,539 | 3 |
| miR-182 | 327,398 | 152,455 | 33,286 | 38,695 | 10 |
| miR-184 | 151,568 | 58,167 | 56,873 | 143 | |
| miR-30d | 111,933 | 149,301 | 61,834 | 12,796 | 3 |
| miR-200c | 60,837 | 72,125 | 153,868 | 1,297 | 3 |
| miR-21 | 60,031 | 284,834 | 27,077 | 1,514 | 5 |
| miR-24 | 34,032 | 69,287 | 58,673 | 390 | 6 |
| miR-204 | 32,817 | 8,346 | 890 | 11 | 108 |
| miR-200b | 22,134 | 14,969 | 27,367 | 2,236 | 2 |
| miR-30a-5p | 21,116 | 117,912 | 174,538 | 9,278 | 4 |
| miR-16 | 18,254 | 10,389 | 3,215 | 5,186 | 3 |
| miR-29a | 15,815 | 13,379 | 39,338 | 1,375 | 3 |
| miR-15a | 2,914 | 1,982 | 684 | 89 | 16 |
| miR-19b | 2,081 | 1,321 | 56 | ||
| miR-152 | 1,656 | 1,659 | 44,121 | 211 | |
| miR-29b | 1,212 | 1,500 | 1,984 | 3 | |
| miR-342 | 1,017 | 586 | 300 | 449 | |
| miR-9 | 687 | 2,028 | 275 | ||
| miR-96 | 514 | 499 | 500 | 312 | 23 |
| miR-15b | 442 | 330 | 133 | 149 | |
| miR-195 | 213 | 516 | 244 | 2 | |
| miR-34a | 190 | 503 | 62 | ||
| miR-199a-3p | 168 | 4 | |||
| miR-145 | 49 | 804 | 206 | 2 | |
| miR-124a | 41 | 120 | 17 | ||
| miR-33a | 92 | 34 | |||
| miR-338-3p | 193 | 72 |
MiRs are listed in order of their expression level in human β-cells as assessed by RNA sequencing. Numbers represent read counts in sorted human β-cells (27), intact human islets (27), C57BL/6J mouse islets (19), and MIN6 cells (26). Enrichment in human β-cells in comparison with α-cells was assessed by a quantitative PCR-based miR array; numbers represent fold enrichment (28).
Table 2.
β-Cell–relevant miRs and their genomic location and function and references
| Mature miR | Precursor miR coding regions (human) | Function in the β-cell | References |
|---|---|---|---|
| let-7 | mir-let-7a-1: chr9: 94175957-94176036 | Targets IRS2 and regulates β-cell insulin signaling. | 72 |
| mir-let-7a-2: chr11: 122146522-122146593 | |||
| mir-let-7a-3: chr22: 46112749-46112822 | |||
| miR-7 | mir-7-1: chr9: 83969748-83969857 | Inhibits insulin secretion by targeting SNARE and vesicle-related proteins. Possibly affects β-cell proliferation by targeting parts of the mTOR pathway and differentiation by targeting the transcription factor Pax6. | 40, 48, 70, 77, 78, 83–86 |
| mir-7-2: chr15: 88611825-88611934 | |||
| mir-7-3: chr19: 4770670-4770779 | |||
| miR-9 | mir-9-1: chr1: 156420341-156420429 | Inhibits insulin secretion by targeting the deacetylase Sirt1 and the transcription factor Onecut2. | 35, 60 |
| mir-9-2: chr5: 88666853-88666939 | |||
| mir-9-3: chr15: 89368017-89368106 | |||
| miR-15 | mir-15a: chr13: 50049119-50049201 | MiR-15a increases insulin biosynthesis, possibly by targeting UCP2. May also increase apoptosis by targeting Bcl-2. May alter differentiation and development by targeting Ngn3. | 64, 74, 103 |
| mir-15b: chr3: 160404588-160404685 | MiR-15b targets Ngn3, overexpression during development reduces total number of endocrine cells. | 74 | |
| miR-16 | mir-16-1: chr13: 50048973-50049061 | Targets Ngn3, overexpression reduces total number of endocrine cells. | 74 |
| mir-16-2: chr3: 160404745-160404825 | |||
| miR-19 | mir-19b-1: chr13: 91351192-91351278 | May be important in development and insulin synthesis as it targets NeuroD1 and decreases insulin mRNA. | 67 |
| mir-19b-2: chrX: 134169671-134169766 | |||
| miR-21 | mir-21: chr17: 59841266-59841337 | Sensitizes β-cells to cytokine-induced apoptosis. | 32, 101 |
| miR-24 | mir-24-1: chr9: 95086021-95086088 | Increased in db/db mice. Knockdown results in decreased insulin mRNA via de-repression of transcriptional repressors. Overexpression decreases insulin secretion and proliferation. Targets menin, Hnf1a, and NeuroD1. | 65, 66, 71 |
| mir-24-2: chr19: 13836287-13836359 | |||
| miR-26 | mir-26a-1: chr3: 37969404-37969480 | Knockdown decreases insulin mRNA via upregulation of repressors. May play a role in development by targeting TET enzymes, which are oxidizers of DNA. | 65, 73 |
| mir-26a-2: chr12: 57824609-57824692 | |||
| miR-29 | mir-29a: chr7: 130876747-130876810 | Inhibits GSIS by targeting SNARE protein syntaxin-1, Onecut2, and Mct1. Overexpression also causes β-cell apoptosis via downregulation of Mcl1. | 25, 26, 36, 57, 58 |
| mir-29b-1: chr7: 130877459-130877539 | |||
| mir-29b-2: chr1: 207802443-207802523 | |||
| miR-30 | mir-30a: chr6: 71403551-71403621 | MiR-30a-5p plays a key role in glucotoxicity and targets NeuroD1. | 38 |
| mir-30d: chr8: 134804876-134804945 | MiR-30d increases insulin expression by increasing the transcription factor MafA. Protects from TNF-α–induced toxicity by downregulating MAP4K4. | 51, 54 | |
| miR-33 | mir-33a: chr22: 41900944-41901012 | Targets ABCA1 transporter protein to control cholesterol efflux from cells and consequently decreases insulin secretion from β-cells. | 34, 63 |
| miR-34 | mir-34a: chr1: 9151668-9151777 | Expressed during differentiation of β-cells. Can cause β-cell apoptosis, possibly by targeting Bcl-2. | 32, 78, 96, 99, 101, 105 |
| miR-96 | mir-96: chr7: 129774692-129774769 | Decreases insulin secretion by increasing granuphilin and decreasing the GTPase effector Noc2. | 37 |
| miR-124 | mir-124a-1: chr8: 9903388-9903472 | Decreases insulin secretion by targeting GTPase Rab27a and indirectly affecting SNARE proteins. Highly expressed late in development. Targets FoxA2, which is involved in β-cell differentiation. | 37, 50, 51, 53 |
| mir-124a-2: chr8: 64379149-64379257 | |||
| mir-124a-3: chr20: 63178500-63178586 | |||
| miR-145 | mir-145: chr5: 149430646-149430733 | Decreases GSIS by targeting ABCA1. | 34 |
| miR-148 | mir-148a: chr7: 25949919-25949986 | Targets ABCA1. Knockdown decreases insulin mRNA via upregulation of transcriptional repressors. | 34, 65 |
| miR-152 | mir-152: chr17: 48037161-48037247 | Increased in offspring of mothers fed a low-protein diet, negatively affecting insulin secretion. | 33 |
| miR-182 | mir-182: chr7: 129770383-129770492 | Knockdown decreases insulin mRNA via upregulation of transcriptional repressors. | 65 |
| miR-184 | mir-184: chr15: 79209788-79209871 | Negatively regulates β-cell proliferation by targeting Ago2. | 19, 95, 96 |
| miR-195 | mir-195: chr17: 7017615-7017701 | Increased in regenerating mouse pancreas and decreases Ngn3. | 74, 95 |
| miR-199 | mir-199a-1: chr19: 10817426-10817496 | Mediates negative effects of low-protein maternal diet on insulin secretion by targeting mTOR signaling. | 33, 96 |
| mir-199a-2: chr1: 172144535-172144644 | |||
| miR-200 | mir-200b: chr1: 1167104-1167198 | MiR-200b increases β-cell apoptosis by targeting Zeb1. | 100 |
| mir-200c: chr12: 6963699-6963766 | MiR-200c increases β-cell apoptosis by targeting Dnajc3, Jazf1, Xiap, and Rps6kb1. | 106 | |
| miR-204 | mir-204: chr9: 70809975-70810084 | Decreases insulin transcription by targeting MafA. | 31 |
| miR-338 | mir-338: chr17: 81125883-81125949 | MiR-338-3p is reduced in states of β-cell expansion such as pregnancy and is antiproliferative and proapoptotic. | 97 |
| miR-342 | mir-342: chr14: 100109655-100109753 | Mediates negative effects of low-protein maternal diet on insulin secretion by targeting mTOR signaling. | 33 |
| miR-375 | mir-375: chr2: 219001645-219001708 | Decreases GSIS by targeting Mtpn, decreases insulin gene expression by targeting PDK1, and increases compensatory β-cell proliferation. | 26, 27, 29, 30, 38, 41, 42, 45, 46, 77, 78, 86, 95 |
MiRs with established effects on β-cell biology are listed in numerical order.
Figure 1.
Key β-cell processes and the miRs involved. MiRs are grouped based on their currently known primary function(s); those with multiple functions in the β-cell are listed in the cross sections of the Venn diagram.
MiRs and Insulin Secretion AND Production
The most prominent function of β-cells is the production and secretion of insulin, and miRs play a variety of different yet important roles in this process (Table 2).
Insulin Secretion: Preventing Excess
Several miRs (e.g., miR-7, -9, -29, -30, -33, -96, -124, -145, and -375) have been shown to target components of the secretory machinery and thereby to control insulin secretion (29,34–40). They constitute the largest group of miRs with an established role in β-cell biology (Fig. 1). Given the importance of a well-orchestrated and fine-tuned insulin response, it is not surprising that this process is controlled at an additional level by these miRs. However, it is striking that all of the miRs have an overall inhibitory effect on insulin exocytosis, suggesting that the ultimate goal is to prevent any excessive insulin secretion and any potentially life-threatening hypoglycemia.
The first miR studied functionally in pancreatic β-cells and shown to regulate insulin secretion was miR-375 (29). MiR-375 is highly and specifically expressed in human pancreatic islets (α- and β-cells) (27,41) and in MIN6 mouse insulinoma cells (29) (Table 1) and is easily detectable in INS-1 cells (42). However, it is also emerging as a miR frequently downregulated in various cancers (43). Overexpression of miR-375 decreases glucose-stimulated insulin secretion (GSIS) in MIN6 cells (29) by targeting myotrophin/V-1 (Mtpn), a protein involved in vesicular transport (44), and this results in decreased insulin exocytosis independent of intracellular calcium signaling (29). In addition, miR-375 has been linked to decreased insulin gene expression through the targeting of 3′phosphoinositide-dependent protein kinase 1 (PDK1) in INS-1E cells (45). β-Cell expression of miR-375 has been reported to be regulated by pancreatic duodenal homeobox 1 (PDX1) and neurogenic differentiation transcription factor (NeuroD1), both crucial transcription factors for β-cell development and necessary for β-cell function and insulin transcription. PDX1 and NeuroD1 were shown to bind to the miR-375 locus in NIT1 mouse insulinoma cells, as well as MIN6 cells (46). In addition, it has been demonstrated in INS-1 832/13 cells and in isolated rat islets that cyclic adenosine monophosphate (cAMP) negatively regulates miR-375 transcription by decreasing the binding of RNA polymerase II to the miR-375 promoter (42). Analysis of the miR-375 promoter further identified important cis-acting elements for the control of miR-375 expression (41,47).
MiR-7 also regulates insulin secretion via targeting of the mRNA of key vesicle fusion and SNARE proteins and proteins involved in vesicular trafficking, membrane targeting, and cytoskeletal rearrangement. β-Cell–specific deletion of miR-7a-2 in mice increases insulin secretion and significantly improves glucose tolerance (40). The precursors of miR-7 are encoded by three different genomic loci (7-1, 7-2, and 7-3 in humans and 7a-1, 7a-2, and 7b in mice) and the latter two have been shown to be induced by NeuroD1 in MIN6 cells (48). MiR-7 is also increased in mouse models of diabetes and negatively regulates GSIS (40). Consistent with the increase in mouse models of diabetes and the detrimental effects on GSIS, miR-7 was also found to be increased in cadaveric human islets of diabetic donors as compared with those of obese nondiabetic donors (40), confirming the role of this miR in human islet pathophysiology.
MiR-124a is increased in the islets of humans with type 2 diabetes (39) and targets multiple proteins related to insulin secretion by both direct and indirect regulation. Overexpression of miR-124a in MIN6 cells increases basal insulin release but causes a decrease in GSIS by directly targeting the GTPase Rab27a (37,39), which is involved in the fusion of secretory vesicles with the plasma membrane (49). Additional targets include SNARE proteins (37), Sirtuin 1 (Sirt1), NeuroD1, and the transcription factor forkhead box A2 (FoxA2) (39). It is via FoxA2 that miR-124a regulates PDX1 as well as the ATP-sensitive potassium channel subunits Kir6.2 and Sur-1 (50). Studies of the upstream regulation of miR-124a in β-cells have reported that its expression can be increased by glucose as shown by a miR microarray in MIN6 insulinoma cells (51). In contrast, incubation with high glucose was found to decrease miR-124 expression in primary rat islets (52). Consistent with this latter finding, proapoptotic and glucose-induced thioredoxin-interacting protein (TXNIP) inhibited β-cell miR-124a expression in both rat INS-1 cells and mouse islets (53).
β-Cell function has also been shown to improve in db/db mice in response to miR-30a-5p suppression, and knockdown of miR-30a-5p reversed glucotoxicity-associated β-cell dysfunction, including insulin gene expression and GSIS, in primary rat islets (38). MiR-30a-5p was further found to be upregulated in response to glucotoxicity and to directly target NeuroD1. Another family member, miR-30d, was found to be increased by high glucose in the aforementioned MIN6 miR microarray along with miR-124a (51). MiR-30d overexpression has been described to increase insulin gene expression in MIN6 cells and mouse islets, supposedly via an increase in the insulin transcription factor MafA (51,54), but the direct miR-30d target mediating this effect remains unknown. On the other hand, miR-30d has been shown to directly downregulate the tumor necrosis factor α (TNF-α)–activated protein kinase, mitogen-activated 4 protein kinase 4 (MAP4K4), and thereby protecting MIN6 β-cells from the detrimental effects of cytokine TNF-α exposure (54).
MiR-96 has been studied together with miR-124a and has been shown to inhibit insulin exocytosis and secretion in MIN6 cells by increasing granuphilin (37). Granuphilin decreases insulin exocytosis (55) by stabilizing a fusion-incompetent form of syntaxin-1a, thus increasing docking yet inhibiting fusion of insulin granules (55) and consequently decreasing secretion (37). Furthermore, miR-96 decreases the GTPase effector Noc2 (37), which also affects the β-cell capacity to respond to secretory stimuli (56).
The miR-29 family (miR-29a/b/c) has been shown by multiple reports to regulate β-cell insulin secretion and to directly target syntaxin-1, a t-SNARE protein involved in insulin exocytosis (36). MiR-29 increases in response to glucose in INS-1E β-cells and human and rat islets (57), and the corresponding decrease in syntaxin-1 has a negative effect on GSIS. Overexpression of miR-29 in MIN6 cells and in dissociated mouse islets results in a similar decrease in GSIS and occurs along with a decrease in the transcription factor Onecut2, one of the predicted targets of miR-29 that negatively regulates the expression of granuphilin (58). MiR-29a and miR-29b have also been associated with the downregulation of monocarboxylate transporter (Mct1) (25). As the ATP/ADP ratio is crucial to insulin secretion, targeting of Mct1, which allows circulating pyruvate to enter the β-cell, may play a role in the effect of miR-29 on glucose sensing and, subsequently, insulin secretion.
Like miR-29, miR-9 also decreases the expression of Onecut2, consequently increasing granuphilin and decreasing insulin exocytosis in INS-1 cells (35). In addition, miR-9 has been demonstrated to target Sirt1, an NAD-dependent deacetylase known to affect insulin secretion (59) and suspected also to be involved in the control of GSIS by miR-9 (60).
Given that they are located in introns of the sterol regulatory element–binding proteins (SREBP-1/2), miR-33a/b are typically studied in the context of insulin signaling in extrapancreatic tissues (61). However, miR-33a is also relevant to β-cell function as it has been shown to target ATP-binding cassette (ABC) transporters, such as ABCA1 (34). ABCA1 promotes the efflux of cholesterol and increased miR-33a results in β-cell cholesterol accumulation, mediating a subsequent decrease in insulin secretion in both mouse and human islets (62,63).
Another miR that decreases GSIS through ABCA1 targeting is miR-145. Investigators discovered miR-145 as a potent ABCA1 regulator by searching for miRs that may regulate ABCA1 by using a combination of eight different miR target prediction software programs (34). Inhibition of miR-145 in mouse islets resulted in an increase in ABCA1 protein levels and an improvement in GSIS. MiR-145 also regulated total islet cholesterol and was found to be decreased in response to glucose in vitro (34).
Insulin Production: Keeping It Steady
Obviously, β-cell function requires proper control not only of insulin secretion but also of insulin production, and both processes are impaired in diabetes. Recently, a few miRs have been discovered to modulate insulin production by inhibiting insulin transcription (e.g., miR-204 [31]) or enhancing insulin biosynthesis (e.g., miR-15, -24, -26, -148, and -182 [64,65]) and to mediate the epigenetic downregulation of insulin production in the context of maternal malnutrition (e.g., miR-199 and -342 [33]).
MiR-204 is the most highly enriched miR in islet β-cells (Table 1) and was first linked to diabetes based on its marked upregulation in a miR microarray study in which the prodiabetic protein TXNIP was overexpressed in INS-1 β-cells (31). Islet miR-204 expression is indeed increased in multiple diabetes models, and by directly targeting and downregulating the insulin transcription factor MafA, miR-204 causes a decrease in insulin production in INS-1 cells and human islets. MiR-204 is encoded within an intron of the transient receptor potential melastatin 3 (TRPM3) gene and therefore also shares the promoter with TRPM3. Given this information, the transcription factor mediating the TXNIP-induced increase in miR-204 was discovered. Signal transducer and activator of transcription 3 (STAT3) acts as a repressor of the TRPM3/miR-204 promoter, and TXNIP was found to decrease STAT3 phosphorylation and activity (31), making this signaling pathway one of the few with at least one known trans-acting factor capable of controlling miR expression.
In contrast, by targeting transcriptional repressors of insulin, miR-24, miR-26, miR-148, and miR-182 have been demonstrated to promote β-cell insulin content (65). After finding that a global decrease of miRs by a tamoxifen-inducible RIP-Cre Dicer knockout led to a diabetic phenotype and decreased insulin levels, the role of some miRs found to be highly expressed in β-cells was investigated further (65). Among them, knockdown of miR-24, miR-26, miR-148, and miR-182 resulted in decreased insulin mRNA levels in primary islets (65). This effect was found to be at least partially mediated by a de-repression of two transcriptional repressors of insulin, Sry-related HMG box 6 (Sox6) and basic helix-loop-helix family, member E22 (Bhlhe22). On the other hand, miR-24 also targets NeuroD1 and hepatic nuclear factor 1 homeobox A (Hnf1a), genes related to maturity-onset diabetes of the young, and inhibits insulin secretion and β-cell proliferation (66). Furthermore, miR-24 expression has been shown to be increased in db/db and high-fat diet–fed mice and in islets exposed to oxidative stress (66).
MiR-15a overexpression has also been shown to increase insulin biosynthesis in mouse islets, possibly by targeting uncoupling protein 2 (UCP2), which, in turn, alters the ATP/ADP ratio (64). Interestingly, high glucose increases miR-15a levels in the short-term, whereas exposure to high glucose downregulates miR-15a in the long-term (64), consistent with the notion of chronic glucotoxicity.
Although it had no significant effect on total insulin levels, miR-19b overexpression has been shown to inhibit insulin mRNA expression in MIN6 cells. MiR-19 also targets the 3′UTR of NeuroD1 and has been found to be increased in pancreatic progenitor cells (67).
During pregnancy, a low-protein diet for the mother can cause harmful effects to the developing fetus, including an increased susceptibility to type 2 diabetes (68). Offspring of mice fed a low-protein diet were found to have insulin secretory defects due to impaired insulin biosynthesis, reduced β-cell insulin content, decreased mammalian target of rapamycin (mTOR), and increased expression of miR-152, miR-199a-3p, miR-342, and miR-7 (33). Also, overexpression of miR-152 led to a decrease in GSIS. Furthermore, inhibition of miR-342 and miR-199a-3p restored insulin content and secretion back to normal (33). Interestingly, miR-375 expression also has been reported to be increased in offspring of low-protein diet–fed mothers, and normalization of miR-375 levels restored β-cell insulin secretion in rat models (69). Together, this suggests that multiple miRs might be affected by and involved in this epigenetic phenomenon caused by a maternal low-protein diet.
MiRs and β-Cell Proliferation and Differentiation
Proliferation and Differentiation: Maintaining the Balance
A considerable number of miRs have been associated with pancreatic β-cell development by affecting proliferation or differentiation (e.g., miR-375 [30], miR-7 [70], miR-124a [50], miR-24 [71], let-7a [72], miR-26a [73], miR-184 [19], miR-195, miR-15, and miR-16 [74]) (Table 2). Not surprisingly, many of them have also been associated with mature β-cell function (Fig. 1). On the other hand, β-cell de-differentiation has recently emerged as an important contributing factor to the loss of functional β-cell mass (75), and it is therefore conceivable that the downregulation of miRs that promote β-cell differentiation may be involved in this process. Indeed, de-differentiation of β-cell–derived cells for in vitro expansion has been found to be associated with a decrease in miR-375 expression (76). In addition, miR-7a transgenic mice have been reported to have diabetes and impaired insulin secretion and show signs of β-cell de-differentiation (40). On the other hand, an initial study to identify miRs highly expressed during development revealed miR-375 and miR-7 to be increased during differentiation of human islets (77). Investigation of miRs involved in human embryonic stem cell differentiation into insulin-producing cells further confirmed the relevance of miR-375 and miR-7 and implicated a number of others (e.g., miR-146 and -34a) in this process (78).
Interestingly, miR-375 knockout mice develop hyperglycemia despite increased β-cell insulin exocytosis (30). Although the hyperglycemia is primarily due to an increase in glucagon levels (consistent with the relative high expression level of miR-375 in α-cells), pancreatic β-cell mass was found to be severely decreased in miR-375–deficient mice. In addition, miR-375 levels were increased in islets of leptin-deficient ob/ob mice, which typically have a compensatory increase in islet mass. On the other hand, double mutant mice deficient in both leptin and miR-375 showed a more than 70% reduction in β-cell mass compared with ob/ob control mice, and further analysis determined that miR-375 targets a number of growth-inhibiting genes and thereby may enhance compensatory β-cell proliferation (30). In vitro experiments overexpressing miR-375 have also shown that miR-375 can induce mesenchymal stem cells from human placenta to become insulin-producing cells (79) and help differentiate induced pluripotent stem cells into insulin-producing islet-like cell clusters (80).
In addition to its role in insulin secretion, miR-7 has also been implicated in the control of β-cell proliferation. Interestingly, miR-7 targets five unique mRNAs involved in the signaling pathway of mTOR (70), a serine/threonine protein kinase known to be involved in β-cell proliferation (81). Inhibition of miR-7 in primary adult pancreatic islets leads to the activation of mTOR signaling and β-cell proliferation, suggesting that miR-7 acts as an inhibitor of β-cell proliferation (70). However, no alteration in β-cell mass was observed in β-cell–specific miR-7a-2 knockout mice (40). MiR-7 loss- and gain-of-function experiments in β-cells have also revealed a regulation of the transcription factor paired box 6 (Pax6). Pax6 is crucial for the development of endocrine cells and proper glucose homeostasis (82). When knocked down in cultured pancreatic rudiments of mouse embryos, an explant model of pancreatic development, the lack of miR-7 increased the number of insulin-positive cells, while the overexpression of miR-7 caused a decrease in Pax6 and insulin content (83). In contrast, whole-body knockdown of miR-7 using intrauterine morpholino injections during early embryonic development has been shown to decrease total β-cell number and insulin expression and to impair glucose tolerance postnatally (84). This suggests that the role of miR-7 expression may be dependent on the developmental stage and the cell distribution of its expression. MiR-7 has been reported to be a highly abundant miR in endocrine cells (85,86), and its expression increases throughout the progression of development and differentiation of human islets (77) as well as in the developing mouse pancreas (84). This is in alignment with its high expression level observed in sorted human β-cells (Table 1). Together, these findings implicate miR-7 as an important miR for maintaining fully differentiated β-cells, while limiting their potential for proliferation.
MiR-124a expression is upregulated later in mouse embryonic pancreatic development (e.g., embryonic day 18.5), indicating a possible role in differentiation (50). As previously mentioned, miR-124a has been demonstrated to target the transcription factor FoxA2 in MIN6 cells, which, in addition to affecting insulin secretion, is also known to play a role in differentiation via PDX1 (87).
Although miR-24 was mentioned above in regard to its role in regulating insulin content, this miR has also been demonstrated to target menin, a ubiquitously expressed nuclear protein involved in cell cycle (88) and tumor suppression (89) and the gene mutated in multiple endocrine neoplasia 1 (MEN1). Menin has also been shown to be involved in the control of pancreatic islet expansion (90) and pancreatic tumorigenesis, as demonstrated by menin knockout mice, which develop islet hyperplasia and tumors (91). In MIN6 and betalox5 cells, targeting of menin by miR-24 led to cellular proliferation and increased cell viability (71), suggesting that miR-24 may play a role in β-cell growth.
Interestingly, menin has also been shown to affect arsenite-resistant protein 2 (ARS2), which is part of the nuclear RNA cap-binding complex. This complex is important for the production of certain miRs, including the highly expressed miR let-7a. Menin promotes the processing of let-7a from primary miRs to precursor miRs (72), and let-7a targets insulin receptor substrate 2 (IRS2), which is crucial for compensatory β-cell proliferation (92). It is therefore hypothesized that the negative effect of menin on β-cell mass is mediated by an increase in let-7a and a subsequent decrease in IRS2 (72).
Pancreatic cell proliferation, endocrine differentiation, and islet cell number have been found to be significantly increased in miR-26a transgenic mice (73), and miR-26a has been shown to target ten-eleven translocation (TET) and thymine-DNA glycosylase (TDG) enzymes (73). TET enzymes are oxidizers of DNA, whereas TDG plays a role in base excision (93). Both of these miR-26a targets have been implicated in development (94).
MiR-184 targets Ago2, one of the primary components of RISC (19). Loss of miR-184 and increases in Ago2 result in increased β-cell proliferation in mice. Interestingly, miR-184 has been found in abundance in normal human islets but not in islets from glucose-intolerant donors (95), and its expression was found to be silenced in islets of humans with type 2 diabetes (96) and ob/ob mice. When miR-184 was restored in ob/ob mice, compensatory β-cell expansion was prevented (19). Together, these data from several independent studies implicate miR-184 as a negative regulator of β-cell proliferation and explain its decrease in diabetic models as an attempt at compensation in response to increased metabolic demand. They thereby support the role of this miR in the control of compensatory β-cell mass expansion.
MiR-338-3p is also thought to play a major role in the control of compensatory β-cell expansion associated with pregnancy and obesity and was found to be significantly downregulated in islets of rats at 14 days of gestation (97). Knockdown of miR-338-3p in INS-1 cells or rat islets increased β-cell proliferation. MiR-338-3p knockdown also protected against cytokine- or palmitate-induced apoptosis, whereas overexpression of miR-338-3p led to increased apoptosis in dispersed rat islets. Interestingly, the G protein–coupled receptor GPR30 activation by 17β-estradiol seemed to negatively regulate β-cell miR-338-3p expression (97), which may explain the observed in vivo downregulation of this miR during pregnancy.
During pancreas development, Neurogenin3 (Ngn3) pushes precursor cells toward an endocrine fate (98), and miR-15a/b, miR-16, and miR-195 were reported to be higher in the regenerating pancreas of partially pancreatectomized mice and to target Ngn3 (74). Inhibition of each of these miRs in regenerating mouse pancreatic tissue resulted in an increase in Ngn3, as well as in its downstream factors, Nkx2.2 and NeuroD1. Conversely, overexpression of these miRs in developing mouse pancreatic bud explants led to a reduction in the total number of endocrine cells (74), suggesting that these miRs may play an important role in the control of islet cell development and differentiation.
MiRs and β-Cell Apoptosis and Survival
Apoptosis and Survival: Walking a Fine Line
β-Cell apoptosis is a critical factor in the pathogenesis and progression of diabetes, and a number of miRs have been shown to be involved, including miR-34a (32), miR-146 (99), miR-21 (32), miR-29 (58), and, most recently, miR-200 (100) (Figs. 1 and 2 and Table 2). It is also important to note that a subset of miRs has been demonstrated to be upregulated by proinflammatory cytokines (101). Cytokines are known to damage β-cell function and contribute to β-cell apoptosis, but the exact mechanisms are still not fully understood. Interestingly, exposure of MIN6 cells or human islets to interleukin-1β (IL-1β) and TNF-α was found to cause an increase in miR-21, miR-34a, and miR-146a expression. These miRs are also increased during prediabetic insulitis in the NOD mouse model of type 1 diabetes and knockdown of miR-34a and miR-146a protected MIN6 cells from cytokine-induced cell death (101), raising the possibility that these miRs might be involved in β-cell cytokine toxicity.
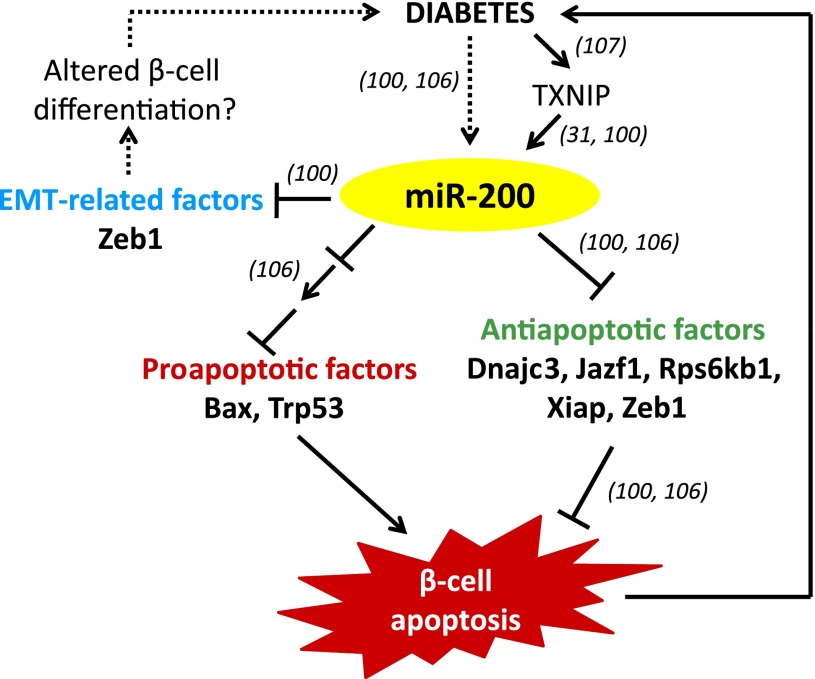
Figure 2.
MiR-200 signaling and β-cell apoptosis. MiR-200 is upregulated in diabetes and in response to glucose/diabetes-induced TXNIP. It directly targets the 3′UTR of the antiapoptotic factors Dnajc3, Jazf1, Rps6kb1, and Xiap and the antiapoptotic and EMT-related factor Zeb1. In addition, it indirectly leads to an increase in proapoptotic Bax and tumor suppressor Trp53. Together, this results in increased β-cell apoptosis and further worsening of the diabetes. By inhibiting EMT, miR-200 may also alter the state of β-cell differentiation, which, in turn, may affect diabetes development by limiting β-cell expansion. Numbers in parentheses refer to corresponding references.
Because of its regulation by the tumor suppressor p53 and its targeting of genes involved in development, differentiation, cell cycle, and apoptosis, miR-34a has been studied heavily in the context of cancer (102,103). In fact, a miR-34 mimic is currently in clinical trials for cancer (16,104). Recent reports, however, have also found a connection between this miR and β-cell apoptosis. MiR-34a was increased in islets of diabetic db/db mice, and inhibition of miR-34a partially protected palmitate-treated cells from apoptosis (99). The knockdown of miR-34a in INS-1 cells also resulted in an increase in the number of β-cells and reduced apoptosis in the absence and presence of cytokines (32). It appears that the mechanism by which miR-34a affects β-cell apoptosis is by targeting the antiapoptotic molecule B-cell lymphoma 2 (Bcl-2). Overexpression of miR-34a in MIN6 cells reduced Bcl-2 levels, whereas inhibition of miR-34a abolished the palmitate-induced decrease in Bcl-2 (105). Combined, these multiple independent β-cell studies and extensive cancer literature make a strong case for miR-34a playing a role in the regulation of β-cell death.
MiR-21 has also been positively associated with β-cell apoptosis, and overexpression resulted in decreased numbers of INS-1 β-cells in culture and an increase in their sensitivity to cytokine-induced apoptosis (32). Furthermore, miR-21 was increased alongside miR-34a and miR-146 in response to IL-1β and TNF-α in MIN6 cells and human pancreatic islets (101). Interestingly, miR-21, miR-34a, and miR-146 have also been found to be upregulated in the islets of prediabetic NOD mice (58). However, unlike miR-34a and miR-146, the knockdown of miR-21 did not protect MIN6 cells from cytokine-induced apoptosis (101).
Mentioned previously based on its ability to regulate insulin secretion, miR-29 is also increased in NOD mice with prediabetes and is upregulated in response to cytokines (58). Overexpression of miR-29 led to increased apoptosis in dispersed human islets and in MIN6 cells, and miR-29 was found to confer these effects by targeting the antiapoptotic protein and Bcl-2 family member, myeloid cell leukemia 1 (Mcl1) (58).
Most recently, two independent articles reported the involvement of miR-200 in diabetic β-cell apoptosis (100,106). A schematic summarizing the combined findings is shown in Fig. 2. Initially, members of the miR-200 family (miR-200a/b/c/141/429) were discovered to be increased in islets of diabetic ob/ob mice and in response to overexpression of the glucose-induced proapoptotic protein, TXNIP (31,100). While TXNIP has previously been shown to be highly upregulated in diabetes (107), it is not clear at this point whether these increased TXNIP levels are necessary to confer the miR-200 increase in response to diabetes. In any case, miR-200b was then shown to induce apoptosis in β-cells, as demonstrated by overexpression in INS-1 cells as well as human islets (100). MiR-200b was also found to target and decrease the expression of zinc finger E-box binding homeobox 1 (Zeb1) and knockdown of Zeb1 recapitulated the effect of miR-200b on β-cell apoptosis, indicating that targeting Zeb1 may be the mechanism conferring the apoptotic effect of miR-200b. Furthermore, the fact that Zeb1 is a crucial modulator of E-cadherin and epithelial-mesenchymal transition (EMT) (108) raised the possibility that miR-200b may also have an impact on β-cell differentiation and development (100).
Consistent with these initial findings, members of the miR-200 family that share the AAUACUG seed sequence (i.e., miR-200b, -200c, and -429) have also been demonstrated to play a crucial role in β-cell apoptosis in vivo (106). These miRs were shown to be increased in the islets of diabetic db/db mice, and overexpression of the most abundant member of the miR-200 family, miR-200c, was found to cause apoptosis in mouse islets and MIN6 cells. Importantly, the lack of miR-200 protected mice from streptozotocin-induced diabetes and from diabetes in the Akita mouse model. The study also revealed multiple miR-200c targets, the downregulation of which contributed to the β-cell apoptosis associated with the miR-200c overexpression phenotype, including DnaJ homolog, subfamily C, member 3 (Dnajc3); JAZF zinc finger 1 gene (Jazf1); ribosomal protein S6 kinase, 70kDa, polypeptide 1 gene (Rps6kb1); and X-linked inhibitor of apoptosis, E3 ubiquitin protein ligase (Xiap). In addition, Belgardt et al. (106) found that miR-200c upregulated the activity of the tumor suppressor Trp53, further promoting apoptosis. Interestingly, although the upregulation of miR-200 in diabetes and the resulting increase in β-cell apoptosis has been confirmed by both studies in various models, a number of different downstream targets and pathways have been implemented. However, this diversity is very much in line with the notions that each miR regulates multiple targets, each miR family member targets a distinct set of genes, and often a combination of different pathways is necessary to elicit the full biological effect.
Revealing a MiR-NeuroD1 Signaling Network
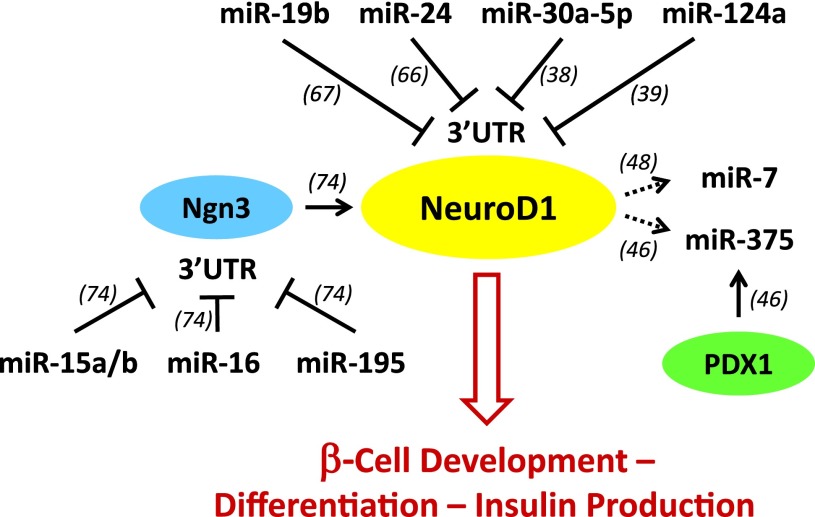
The transcription factor NeuroD1 plays a critical role in β-cell development and function (109). It was striking to recognize that a variety of different miRs regulate NeuroD1 directly or indirectly, whereas others are being regulated by NeuroD1, giving rise to an intricate signaling network (Fig. 3). MiR-19b (67), miR-24 (66), miR-30a-5p (38), and miR-124a (39) have been shown to directly target the 3′UTR of NeuroD1 and thereby downregulate its expression. In addition, NeuroD1 expression is under the control of Ngn3, and miR-15a/b, miR-16, and miR-195 have been reported to target Ngn3 and thereby indirectly regulate NeuroD1 (74). In turn, NeuroD1 has been shown to bind to the promoter region of miR-375 (46) and to activate the promoters of miR-7a-2 and miR-7b (48), raising the possibility that it might regulate the expression of these miRs. While the physiological effects of some of the miRs suggested to regulate NeuroD1 will still have to be confirmed and the effects of NeuroD1 on miR-375 and miR-7 expression remain to be determined, this emerging miR-transcription factor network represents an example of how different miRs can converge onto one important pathway to provide tight regulation and an additional level of control.
Figure 3.
MiR-NeuroD1 signaling network. MiR-19b, miR-24, miR-30a-5p, and miR-124a target the 3′UTR of NeuroD1 and thereby downregulate its expression. NeuroD1 is also under the transcriptional control of Ngn3, and miR-15a/b, miR-16, and miR-195 target the 3′UTR of Ngn3 and thereby indirectly regulate NeuroD1. In turn, NeuroD1, like PDX1, has been shown to bind to the miR-375 promoter as well as to activate the miR-7 promoter. Together, the cross talk of these miRs and transcription factors provides a multilevel control of NeuroD1 signaling, β-cell development, differentiation, and insulin production. Numbers in parentheses refer to corresponding references.
Emerging Concepts and Remaining Challenges
MiRs and Their Many Gene Targets
To date, a multitude of miRs have been linked to islet β-cell biology (Table 1 and 2). They add an additional level of control but also an enormous amount of complexity to the regulation of β-cell function, development, and survival that often still remains underappreciated. MiRs are very potent regulatory molecules, and even small changes in their expression can have major effects that are further multiplied by the fact that each miR typically has many gene targets. Consistent with this notion, several miRs were found to have multiple functions in the β-cell (Fig. 1). The number of targets from one miR locus may further increase due to slight variations in the miR sequence, resulting in multiple isoforms (known as isomiRs) (110). These variants typically involve a shift in the seed sequence of the miR due to processing or posttranscriptional editing. Moreover, a number of noncanonical pathways have recently been described for miR function, including targeting complementary sequences outside of the 3′UTR, localizing to organelles rather than the cytoplasm and leading to translational activation rather than repression of mitochondrial genes (16,17). At this point, it is not clear whether these examples are isolated cases or if they just represent the tip of the iceberg of an even more complex miR regulatory network. In any case, the potential role of such noncanonical pathways in the pancreatic β-cell still remains to be elucidated.
Among miRs and isomiRs highly expressed in β-cells, miR-29 and let-7 were identified as the top two predicted regulatory hubs in type 2 diabetes (26). This means that these miRs scored highly on a bioinformatics analysis that determined the number of conserved predicted targets for each miR among genes in the human type 2 diabetes network. These predicted targets included genes identified in type 2 diabetes genome-wide association studies, such as Camk1d, Glis3, Jazf1, and Slc16a1. Furthermore, isomiRs of miR-375 were found to be likely hubs for type 2 diabetes (26). Taking these findings into consideration, it is tempting to speculate that miRs might play a significant role in the genetics/epigenetics of diabetes, and it will be important for future studies to keep this aspect in mind. In fact, a recent study demonstrated that the expression of a cluster of miRs in an imprinted locus was downregulated in islets of organ donors with type 2 diabetes as compared with control subjects without diabetes (111). While some of these cluster miRs were predicted to target genes playing important roles in β-cell biology (e.g., IAPP), the (patho)physiological role of these miRs still remains to be proven experimentally.
MiR Expression and Upstream Regulation
Over the past 10 years since the initial discovery of miR-375 regulating β-cell insulin secretion, a lot of advances have been made in regard to miRs and their role in islet β-cell function. However, some major challenges still remain. For one, classic transient loss- or gain-of-function experiments are often not able to reveal changes in miR expression and downstream function, and more chronic alterations, such as the creation of stable cell lines and complicated knockout or transgenic mouse models, are necessary. In addition, while the tools and programs to predict putative miR targets have improved dramatically, clearly facilitating the analysis of any downstream effects, identification of the specific upstream factors regulating the expression of any given miR has remained notoriously difficult. In addition, epigenetics such as DNA methylation may play an important role in the regulation of some miRs (111), consistent with their more chronic control pattern and the often observed lack of an acute response. To date, the responsible transcription factors conferring some of the observed changes in miR expression are only known for very few β-cell miRs, e.g., miR-375 (PDX1, NeuroD1), miR-7 (NeuroD1) (46,48), and miR-204 (STAT3) (31). It obviously will be important to identify the cis- and trans-acting factors responsible for the regulation of miR expression in order to recognize novel patterns and fully characterize the physiological role of β-cell miRs.
MiR Cross Talk With Transcription Factors and Other Noncoding RNAs
Aside from the well-accepted concept of one miR targeting many different pathways, the example of the miR-NeuroD1 signaling network revealed that there might also be convergence of different miRs onto one pathway (Fig. 3). It also suggested that cross talk exists between miRs and key transcription factors. While miRs act primarily as posttranscriptional regulators of gene expression, their ability to directly target different transcription factors (e.g., NeuroD1 [38,39,66,67], MafA [31], FoxA2 [50]) also allows them to indirectly control gene transcription. On the other hand, the function of miRs has been suggested to be modulated by competitive endogenous RNAs acting as sponges. These RNAs have been reported to be circular RNAs (112), large noncoding RNAs (lncRNAs) (113), or RNAs encoding pseudogenes (114). However, more recent work has put this theory of RNAs acting as miR sponges in question by demonstrating that the abundance of miR targets does not significantly alter the miR effects on gene expression, at least in the context of normal cell biology (115).
In contrast, lncRNAs are gaining more and more recognition as a biologically important class of noncoding RNAs that is highly regulated and capable of controlling gene expression. Recent studies have demonstrated a high degree of tissue specificity for lncRNAs, revealing more than 1,100 lncRNAs specific to islet cells (116). LncRNAs are thought to be involved in β-cell differentiation, and a subset was found to be dysregulated in the islets of humans with type 2 diabetes. Some islet lncRNAs have also been mapped to genomic loci associated with type 2 diabetes. As an example of their regulatory function, knockdown of HI-LNC25 in cultured human β-cells causes a decrease in the mRNA expression of GLIS3, a gene that codes for an islet Kruppel-like zinc finger protein transcription factor and is mutated in a form of monogenic diabetes (116). On the basis of the findings in NOD islets and MIN6 cells treated with proinflammatory cytokines, lncRNAs have also recently been suggested to be differentially regulated during the development of type 1 diabetes. Moreover, their overexpression has been shown to make β-cells more susceptible to apoptosis (117). The implications of lncRNAs for islet function and development and in the context of imprinted genomic loci have recently been reviewed (118,119). However, it is obvious that this is just the beginning and a lot more work will be necessary to elucidate the role of lncRNAs in β-cell biology and the pathophysiology of diabetes.
Summary
MiRs are small but very powerful regulators of β-cell biology. Their effects are not limited to a certain pathway or any particular set of functions, and their target genes include a variety of different proteins involved in all aspects of cell biology. However, a common theme that seems to emerge is that all genes targeted play important roles in β-cell development, function, and/or survival and therefore may have been deemed “worthy” of this extra level of control. They also seem to share the distinctive feature of being part of processes that leave very little room for error and therefore require additional checks and balances. In fact, it has been suggested that miRs help canalize general genetic programs of development and thereby buffer any random deviations to ensure stable phenotypes (120). They are also thought to contribute to gene regulatory networks that promote robustness and stability of physiological processes such as glucose homeostasis (121). Thus, continuing to peel off the different layers of these regulatory networks not only will provide insight into the various roles of noncoding RNAs but also should help yield a better understanding of β-cell biology and of the molecular processes driving the development of diabetes.
Article Information
Funding. This work was supported in part by National Institutes of Health grants R01DK078752 and UC4DK104204 to A.S.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Yamakuchi M, Ferlito M, Lowenstein CJ. MiR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 2008;105:13421–13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 2010;463:621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res 2004;14:1902–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet 2011;12:846–860 [DOI] [PubMed] [Google Scholar]
- 5.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 2004;5:522–531 [DOI] [PubMed] [Google Scholar]
- 6.Faller M, Guo F. MicroRNA biogenesis: there's more than one way to skin a cat. Biochim Biophys Acta 2008;1779:663–667 [DOI] [PMC free article] [PubMed]
- 7.Tomari Y, Zamore PD. MicroRNA biogenesis: Drosha can’t cut it without a partner. Curr Biol 2005;15:R61–R64 [DOI] [PubMed] [Google Scholar]
- 8.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev 2003;17:3011–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutvágner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001;293:834–838 [DOI] [PubMed] [Google Scholar]
- 10.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 2005;123:631–640 [DOI] [PubMed] [Google Scholar]
- 11.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993;75:855–862 [DOI] [PubMed] [Google Scholar]
- 12.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005;120:15–20 [DOI] [PubMed] [Google Scholar]
- 13.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 2011;12:99–110 [DOI] [PubMed] [Google Scholar]
- 14.Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc Natl Acad Sci U S A 2007;104:9667–9672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 2008;455:1124–1128 [DOI] [PubMed] [Google Scholar]
- 16.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov 2013;12:847–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Zuo X, Yang B, et al. MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell 2014;158:607–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology 2002;143:339–342 [DOI] [PubMed] [Google Scholar]
- 19.Tattikota SG, Rathjen T, McAnulty SJ, et al. Argonaute2 mediates compensatory expansion of the pancreatic β cell. Cell Metab 2014;19:122–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 2014;42:D68–D73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Valverde SL, Taft RJ, Mattick JS. MicroRNAs in β-cell biology, insulin resistance, diabetes and its complications. Diabetes 2011;60:1825–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the microRNA perspective. Diabetes 2011;60:1832–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landgraf P, Rusu M, Sheridan R, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007;129:1401–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hausser J, Berninger P, Rodak C, Jantscher Y, Wirth S, Zavolan M. MirZ: an integrated microRNA expression atlas and target prediction resource. Nucleic Acids Res 2009;37:W266–W272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pullen TJ, da Silva Xavier G, Kelsey G, Rutter GA. MiR-29a and miR-29b contribute to pancreatic beta-cell-specific silencing of monocarboxylate transporter 1 (Mct1). Mol Cell Biol 2011;31:3182–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baran-Gale J, Fannin EE, Kurtz CL, Sethupathy P. Beta cell 5′-shifted isomiRs are candidate regulatory hubs in type 2 diabetes. PLoS One 2013;8:e73240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van de Bunt M, Gaulton KJ, Parts L, et al. The miRNA profile of human pancreatic islets and beta-cells and relationship to type 2 diabetes pathogenesis. PLoS One 2013;8:e55272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein D, Misawa R, Bravo-Egana V, et al. MicroRNA expression in alpha and beta cells of human pancreatic islets. PLoS One 2013;8:e55064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poy MN, Eliasson L, Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004;432:226–230 [DOI] [PubMed] [Google Scholar]
- 30.Poy MN, Hausser J, Trajkovski M, et al. MiR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci U S A 2009;106:5813–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu G, Chen J, Jing G, Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat Med 2013;19:1141–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Backe MB, Novotny GW, Christensen DP, Grunnet LG, Mandrup-Poulsen T. Altering β-cell number through stable alteration of miR-21 and miR-34a expression. Islets 2014;6:e27754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alejandro EU, Gregg B, Wallen T, et al. Maternal diet-induced microRNAs and mTOR underlie β cell dysfunction in offspring. J Clin Invest 2014;124:4395–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang MH, Zhang LH, Wijesekara N, et al. Regulation of ABCA1 protein expression and function in hepatic and pancreatic islet cells by miR-145. Arterioscler Thromb Vasc Biol 2013;33:2724–2732 [DOI] [PubMed] [Google Scholar]
- 35.Plaisance V, Abderrahmani A, Perret-Menoud V, Jacquemin P, Lemaigre F, Regazzi R. MicroRNA-9 controls the expression of Granuphilin/Slp4 and the secretory response of insulin-producing cells. J Biol Chem 2006;281:26932–26942 [DOI] [PubMed] [Google Scholar]
- 36.Bagge A, Dahmcke CM, Dalgaard LT. Syntaxin-1a is a direct target of miR-29a in insulin-producing β-cells. Horm Metab Res 2013;45:463–466 [DOI] [PubMed] [Google Scholar]
- 37.Lovis P, Gattesco S, Regazzi R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microRNAs. Biol Chem 2008;389:305–312 [DOI] [PubMed] [Google Scholar]
- 38.Kim JW, You YH, Jung S, et al. MiRNA-30a-5p-mediated silencing of Beta2/NeuroD expression is an important initial event of glucotoxicity-induced beta cell dysfunction in rodent models. Diabetologia 2013;56:847–855 [DOI] [PubMed] [Google Scholar]
- 39.Sebastiani G, Po A, Miele E, et al. MicroRNA-124a is hyperexpressed in type 2 diabetic human pancreatic islets and negatively regulates insulin secretion. Acta Diabetol 2015;52:523–530 [DOI] [PubMed] [Google Scholar]
- 40.Latreille M, Hausser J, Stützer I, et al. MicroRNA-7a regulates pancreatic β cell function. J Clin Invest 2014;124:2722–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avnit-Sagi T, Kantorovich L, Kredo-Russo S, Hornstein E, Walker MD. The promoter of the pri-miR-375 gene directs expression selectively to the endocrine pancreas. PLoS One 2009;4:e5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller DM, Clark EA, Goodman RH. Regulation of microRNA-375 by cAMP in pancreatic β-cells. Mol Endocrinol 2012;26:989–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan JW, Lin JS, He XX. The emerging role of miR-375 in cancer. Int J Cancer 2014;135:1011–1018 [DOI] [PubMed] [Google Scholar]
- 44.Yamakuni T, Yamamoto T, Ishida Y, et al. V-1, a catecholamine biosynthesis regulatory protein, positively controls catecholamine secretion in PC12D cells. FEBS Lett 2002;530:94–98 [DOI] [PubMed] [Google Scholar]
- 45.El Ouaamari A, Baroukh N, Martens GA, Lebrun P, Pipeleers D, van Obberghen E. MiR-375 targets 3′-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells. Diabetes 2008;57:2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keller DM, McWeeney S, Arsenlis A, et al. Characterization of pancreatic transcription factor Pdx-1 binding sites using promoter microarray and serial analysis of chromatin occupancy. J Biol Chem 2007;282:32084–32092 [DOI] [PubMed] [Google Scholar]
- 47.Avnit-Sagi T, Vana T, Walker MD. Transcriptional mechanisms controlling miR-375 gene expression in the pancreas. Exp Diabetes Res 2012;2012:891216 [DOI] [PMC free article] [PubMed]
- 48.Kredo-Russo S, Ness A, Mandelbaum AD, Walker MD, Hornstein E. Regulation of pancreatic microRNA-7 expression. Exp Diabetes Res 2012;2012:695214 [DOI] [PMC free article] [PubMed]
- 49.Aizawa T, Komatsu M. Rab27a: a new face in beta cell metabolism-secretion coupling. J Clin Invest 2005;115:227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baroukh N, Ravier MA, Loder MK, et al. MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cell lines. J Biol Chem 2007;282:19575–19588 [DOI] [PubMed] [Google Scholar]
- 51.Tang X, Muniappan L, Tang G, Ozcan S. Identification of glucose-regulated miRNAs from pancreatic beta cells reveals a role for miR-30d in insulin transcription. RNA 2009;15:287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esguerra JL, Bolmeson C, Cilio CM, Eliasson L. Differential glucose-regulation of microRNAs in pancreatic islets of non-obese type 2 diabetes model Goto-Kakizaki rat. PLoS One 2011;6:e18613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jing G, Westwell-Roper C, Chen J, Xu G, Verchere CB, Shalev A. Thioredoxin-interacting protein promotes islet amyloid polypeptide expression through miR-124a and FoxA2. J Biol Chem 2014;289:11807–11815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao X, Mohan R, Özcan S, Tang X. MicroRNA-30d induces insulin transcription factor MafA and insulin production by targeting mitogen-activated protein 4 kinase 4 (MAP4K4) in pancreatic β-cells. J Biol Chem 2012;287:31155–31164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomi H, Mizutani S, Kasai K, Itohara S, Izumi T. Granuphilin molecularly docks insulin granules to the fusion machinery. J Cell Biol 2005;171:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheviet S, Coppola T, Haynes LP, Burgoyne RD, Regazzi R. The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic beta-cell exocytosis. Mol Endocrinol 2004;18:117–126 [DOI] [PubMed] [Google Scholar]
- 57.Bagge A, Clausen TR, Larsen S, et al. MicroRNA-29a is up-regulated in beta-cells by glucose and decreases glucose-stimulated insulin secretion. Biochem Biophys Res Commun 2012;426:266–272 [DOI] [PubMed] [Google Scholar]
- 58.Roggli E, Gattesco S, Caille D, et al. Changes in microRNA expression contribute to pancreatic β-cell dysfunction in prediabetic NOD mice. Diabetes 2012;61:1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moynihan KA, Grimm AA, Plueger MM, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2005;2:105–117 [DOI] [PubMed] [Google Scholar]
- 60.Ramachandran D, Roy U, Garg S, Ghosh S, Pathak S, Kolthur-Seetharam U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic β-islets. FEBS J 2011;278:1167–1174 [DOI] [PubMed] [Google Scholar]
- 61.Ramírez CM, Goedeke L, Fernández-Hernando C. “Micromanaging” metabolic syndrome. Cell Cycle 2011;10:3249–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brunham LR, Kruit JK, Pape TD, et al. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med 2007;13:340–347 [DOI] [PubMed] [Google Scholar]
- 63.Wijesekara N, Zhang LH, Kang MH, et al. MiR-33a modulates ABCA1 expression, cholesterol accumulation, and insulin secretion in pancreatic islets. Diabetes 2012;61:653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun LL, Jiang BG, Li WT, Zou JJ, Shi YQ, Liu ZM. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pract 2011;91:94–100 [DOI] [PubMed] [Google Scholar]
- 65.Melkman-Zehavi T, Oren R, Kredo-Russo S, et al. MiRNAs control insulin content in pancreatic β-cells via downregulation of transcriptional repressors. EMBO J 2011;30:835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu Y, You W, Wang H, et al. MicroRNA-24/MODY gene regulatory pathway mediates pancreatic β-cell dysfunction. Diabetes 2013;62:3194–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang ZW, Zhang LQ, Ding L, et al. MicroRNA-19b downregulates insulin 1 through targeting transcription factor NeuroD1. FEBS Lett 2011;585:2592–2598 [DOI] [PubMed] [Google Scholar]
- 68.Sandovici I, Hammerle CM, Ozanne SE, Constância M. Developmental and environmental epigenetic programming of the endocrine pancreas: consequences for type 2 diabetes. Cell Mol Life Sci 2013;70:1575–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dumortier O, Hinault C, Gautier N, Patouraux S, Casamento V, Van Obberghen E. Maternal protein restriction leads to pancreatic failure in offspring: role of misexpressed microRNA-375. Diabetes 2014;63:3416–3427 [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Liu J, Liu C, Naji A, Stoffers DA. MicroRNA-7 regulates the mTOR pathway and proliferation in adult pancreatic β-cells. Diabetes 2013;62:887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vijayaraghavan J, Maggi EC, Crabtree JS. MiR-24 regulates menin in the endocrine pancreas. Am J Physiol Endocrinol Metab 2014;307:E84–E92 [DOI] [PubMed] [Google Scholar]
- 72.Gurung B, Muhammad AB, Hua X. Menin is required for optimal processing of the microRNA let-7a. J Biol Chem 2014;289:9902–9908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu X, Jin L, Wang X, et al. MicroRNA-26a targets ten eleven translocation enzymes and is regulated during pancreatic cell differentiation. Proc Natl Acad Sci U S A 2013;110:17892–17897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joglekar MV, Parekh VS, Mehta S, Bhonde RR, Hardikar AA. MicroRNA profiling of developing and regenerating pancreas reveal post-transcriptional regulation of neurogenin3. Dev Biol 2007;311:603–612 [DOI] [PubMed] [Google Scholar]
- 75.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012;150:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nathan G, Kredo-Russo S, Geiger T, et al. MiR-375 promotes redifferentiation of adult human β cells expanded in vitro. PLoS One 2015;10:e0122108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joglekar MV, Joglekar VM, Hardikar AA. Expression of islet-specific microRNAs during human pancreatic development. Gene Expr Patterns 2009;9:109–113 [DOI] [PubMed] [Google Scholar]
- 78.Wei R, Yang J, Liu GQ, et al. Dynamic expression of microRNAs during the differentiation of human embryonic stem cells into insulin-producing cells. Gene 2013;518:246–255 [DOI] [PubMed] [Google Scholar]
- 79.Shaer A, Azarpira N, Vahdati A, Karimi MH, Shariati M. MiR-375 induces human decidua basalis-derived stromal cells to become insulin-producing cells. Cell Mol Biol Lett 2014;19:483–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shaer A, Azarpira N, Karimi MH. Differentiation of human induced pluripotent stem cells into insulin-like cell clusters with miR-186 and miR-375 by using chemical transfection. Appl Biochem Biotechnol 2014;174:242–258 [DOI] [PubMed] [Google Scholar]
- 81.Balcazar N, Sathyamurthy A, Elghazi L, et al. mTORC1 activation regulates beta-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J Biol Chem 2009;284:7832–7842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ding J, Gao Y, Zhao J, et al. Pax6 haploinsufficiency causes abnormal metabolic homeostasis by down-regulating glucagon-like peptide 1 in mice. Endocrinology 2009;150:2136–2144 [DOI] [PubMed] [Google Scholar]
- 83.Kredo-Russo S, Mandelbaum AD, Ness A, et al. Pancreas-enriched miRNA refines endocrine cell differentiation. Development 2012;139:3021–3031 [DOI] [PubMed] [Google Scholar]
- 84.Nieto M, Hevia P, Garcia E, et al. Antisense miR-7 impairs insulin expression in developing pancreas and in cultured pancreatic buds. Cell Transplant 2012;21:1761–1774 [DOI] [PubMed] [Google Scholar]
- 85.Correa-Medina M, Bravo-Egana V, Rosero S, et al. MicroRNA miR-7 is preferentially expressed in endocrine cells of the developing and adult human pancreas. Gene Expr Patterns 2009;9:193–199 [DOI] [PubMed] [Google Scholar]
- 86.Bravo-Egana V, Rosero S, Molano RD, et al. Quantitative differential expression analysis reveals miR-7 as major islet microRNA. Biochem Biophys Res Commun 2008;366:922–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee CS, Sund NJ, Vatamaniuk MZ, Matschinsky FM, Stoffers DA, Kaestner KH. Foxa2 controls Pdx1 gene expression in pancreatic beta-cells in vivo. Diabetes 2002;51:2546–2551 [DOI] [PubMed] [Google Scholar]
- 88.Kaji H, Canaff L, Goltzman D, Hendy GN. Cell cycle regulation of menin expression. Cancer Res 1999;59:5097–5101 [PubMed] [Google Scholar]
- 89.Agarwal SK, Guru SC, Heppner C, et al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell 1999;96:143–152 [DOI] [PubMed] [Google Scholar]
- 90.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science 2007;318:806–809 [DOI] [PubMed] [Google Scholar]
- 91.Crabtree JS, Scacheri PC, Ward JM, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci U S A 2001;98:1118–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kubota N, Tobe K, Terauchi Y, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 2000;49:1880–1889 [DOI] [PubMed] [Google Scholar]
- 93.He YF, Li BZ, Li Z, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011;333:1303–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ito S, Shen L, Dai Q, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011;333:1300–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bolmeson C, Esguerra JL, Salehi A, Speidel D, Eliasson L, Cilio CM. Differences in islet-enriched miRNAs in healthy and glucose intolerant human subjects. Biochem Biophys Res Commun 2011;404:16–22 [DOI] [PubMed] [Google Scholar]
- 96.Nesca V, Guay C, Jacovetti C, et al. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia 2013;56:2203–2212 [DOI] [PubMed] [Google Scholar]
- 97.Jacovetti C, Abderrahmani A, Parnaud G, et al. MicroRNAs contribute to compensatory β cell expansion during pregnancy and obesity. J Clin Invest 2012;122:3541–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A 2000;97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lovis P, Roggli E, Laybutt DR, et al. Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes 2008;57:2728–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Filios SR, Xu G, Chen J, Hong K, Jing G, Shalev A. MicroRNA-200 is induced by thioredoxin-interacting protein and regulates Zeb1 protein signaling and beta cell apoptosis. J Biol Chem 2014;289:36275–36283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roggli E, Britan A, Gattesco S, et al. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes 2010;59:978–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen F, Hu SJ. Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. J Biochem Mol Toxicol 2012;26:79–86 [DOI] [PubMed] [Google Scholar]
- 103.Tarasov V, Jung P, Verdoodt B, et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle 2007;6:1586–1593 [DOI] [PubMed] [Google Scholar]
- 104.Bader AG. MiR-34—a microRNA replacement therapy is headed to the clinic. Front Genet 2012;3:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin X, Guan H, Huang Z, et al. Downregulation of Bcl-2 expression by miR-34a mediates palmitate-induced Min6 cells apoptosis. J Diabetes Res 2014;2014:258695 [DOI] [PMC free article] [PubMed]
- 106.Belgardt B-F, Ahmed K, Spranger M, et al. The microRNA-200 family regulates pancreatic beta cell survival in type 2 diabetes. Nat Med 2015;21:619–627 [DOI] [PubMed] [Google Scholar]
- 107.Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology 2005;146:2397–2405 [DOI] [PubMed] [Google Scholar]
- 108.Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10:593–601 [DOI] [PubMed] [Google Scholar]
- 109.Bernardo AS, Hay CW, Docherty K. Pancreatic transcription factors and their role in the birth, life and survival of the pancreatic beta cell. Mol Cell Endocrinol 2008;294:1–9 [DOI] [PubMed] [Google Scholar]
- 110.Kim YK, Heo I, Kim VN. Modifications of small RNAs and their associated proteins. Cell 2010;143:703–709 [DOI] [PubMed] [Google Scholar]
- 111.Kameswaran V, Bramswig NC, McKenna LB, et al. Epigenetic regulation of the DLK1-MEG3 microRNA cluster in human type 2 diabetic islets. Cell Metab 2014;19:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature 2013;495:384–388 [DOI] [PubMed] [Google Scholar]
- 113.Wang Y, Xu Z, Jiang J, et al. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell 2013;25:69–80 [DOI] [PubMed] [Google Scholar]
- 114.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 2010;465:1033–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Denzler R, Agarwal V, Stefano J, Bartel DP, Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol Cell 2014;54:766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morán I, Akerman I, van de Bunt M, et al. Human β cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab 2012;16:435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Motterle A, Gattesco S, Caille D, Meda P, Regazzi R. Involvement of long non-coding RNAs in beta cell failure at the onset of type 1 diabetes in NOD mice. Diabetologia 2015;58:1827–1835 [DOI] [PubMed] [Google Scholar]
- 118.Esguerra JL, Eliasson L. Functional implications of long non-coding RNAs in the pancreatic islets of Langerhans. Front Genet 2014;5:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kameswaran V, Kaestner KH. The Missing lnc(RNA) between the pancreatic β-cell and diabetes. Front Genet 2014;5:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hornstein E, Shomron N. Canalization of development by microRNAs. Nat Genet 2006;38(Suppl.):S20–S24 [DOI] [PubMed] [Google Scholar]
- 121.Herranz H, Cohen SM. MicroRNAs and gene regulatory networks: managing the impact of noise in biological systems. Genes Dev 2010;24:1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]