Abstract
BRCA mutations are the main known hereditary factor for breast cancer. Notably, poly (ADP-ribose) polymerase 1 (PARP1) expression status plays a critical role in breast cancer progression and the clinical development of PARP1 inhibitors to treat BRCA-mutated breast cancer has advanced rapidly. However, dynamic crosstalk between BRCA1 and PARP1 remains largely unknown. Here, we showed that: (i) BRCA1 inactivation events (mutation, promoter methylation, or knockdown) were accompanied by increased PARP1 and nicotinamide adenine dinucleotide (NAD) levels, and a subsequent increase in NAD-dependent PARP1 activity in MDA-MB-231 and primary breast cancer cells; (ii) the overexpression of BRCA1 resulted in decreased PARP1 and NAD levels, and a subsequent impairment in NAD-dependent PARP1 activity in MDA-MB-231 and primary breast cancer cells; and (iii) intracellular NAD levels were largely responsible for regulating PARP1 activity in breast cancer cells, and NAD levels were positively correlated with PARP1 activity in human breast cancer specimens (R = 0.647, P < 0.001). Interestingly, the high efficiency of PARP1 triggered by BRCA1 inactivation may further inhibit BRCA1 transcription by NAD depletion. These results highlight a novel interaction between BRCA1 and PARP1, which may be beneficial for the dynamic balance between BRCA1 and PARP1-related biologic processes, especially for maintaining stable DNA repair ability. All of this may improve our understanding of the basic molecular mechanism underlying BRCA1- and PARP1-related breast cancer progression.
Keywords: BRCA1, breast cancer, DNA repair, NAD, PARP1
Abbreviations
- CtBP
C-terminal binding proteins
- DMEM
Dulbecco's Modified Eagles Medium
- ER
endoplasmic reticulum
- ETS1
protein C-ets-1
- NAD
nicotinamide adenine dinucleotide
- Nampt
nicotinamide phosphoribosyltransferase
- PARP1
poly (ADP-ribose) polymerase 1
- PCR
polymerase chain reaction
- SD
standard deviations
- shRNAs
short hairpin RNAs
- TNBC
triple-negative breast cancer
Introduction
Breast cancer is the most common malignancy and a major cause of mortality in women worldwide.1 To date, although the exact cause of breast cancer remains largely unknown, BRCA mutations are the main known hereditary factor for breast cancer.2 In 2005, 2 pivotal studies showed that BRCA-deficient cells were especially sensitive to chemical inhibitors of poly (ADP-ribose) polymerase (PARP), which plays a critical role in single-stranded DNA break repair, presumably due to the lack of homologous recombination-dependent DNA repair.3,4 These findings have raised significant concerns about PARP and PARP inhibitors in BRCA-related cancer. BRCA1 is a tumor suppressor gene that is involved in multiple cellular processes.5 Recent research has confirmed that BRCA1 is an important transcriptional regulator, and BRCA1 depletion results in changes to approximately 7% of the mRNAs expressed in cancer cells.5 Our recent study also indicated that there are wide ranges of DNA damage repair, transcriptional regulation, epigenetic patterns, and metabolic differences between cases of BRCA1 dysfunction and the basal phenotype.6-12 Notably, despite ongoing trials of PARP inhibitors in the treatment of BRCA1-related breast cancer. However, crosstalk between BRCA1 and PARP1 remains largely unknown. In addition, emerging evidence has suggested a functional link between BRCA1 and PARP1 status: (i) PARP1 expression is significantly associated with BRCA1 phenotype in basal-like and triple-negative breast cancer (TNBC);13 (ii) PARP1 knockdown inhibits BRCA1-deficient cells proliferation by induction of apoptosis;14 and (iii) PARP1 is a nicotinamide adenine dinucleotide (NAD)-dependent nuclear enzyme that generates poly(ADP-ribose) polymer from NAD.15 Also, NAD is a target of BRCA1, as confirmed by our recent data.7 For this reason, the present study was undertaken to investigate the crosstalk between BRCA1 and PARP1 status, and to provide novel insights into the regulatory mechanism of BRCA1 and PARP1-related breast cancer progression.
Results
BRCA1 may be involved in NAD synthesis, PARP1 expression and activity induction
Intracellular NAD levels were dramatically increased in BRCA1-mutated breast cancer compared with adjacent normal tissue (Fig. 1A). However, NADH levels were not affected by BRCA1 patterns (Fig. 1B). Therefore, the elevated NAD/NADH ratio was mainly dependent on the increased NAD levels in BRCA1-mutated breast cancer tissue (Fig. 1C). Although intracellular PARP1 levels (Figs. 1D and E) and activity (Fig. 1F) were increased in non-BRCA1-mutated breast cancer compared with adjacent normal tissue, BRCA1-mutated breast cancer showed dramatically increased intracellular PARP1 levels (Figs. 1D and E) and activity (Fig. 1F) compared with the 3 other groups. Therefore, these results suggest that BRCA1 inactivation may be involved in the induction of NAD synthesis, PARP1 expression, and elevated PARP1 activity.
Figure 1.

Intracellular NAD levels, PARP1 levels and activity in non-mutated and BRCA1-mutated breast cancer. (A—C) NAD and NADH levels, and the NAD/NADH ratio were measured in 41 pairs of non-mutated and BRCA1-mutated breast cancer and their adjacent normal tissue. (D—F) PARP1 protein and mRNA levels, and PARP1 activity were measured in 41 pairs of non-mutated and BRCA1-mutated breast cancer samples and the adjacent normal tissue. Bar graphs show mean ± SD.
BRCA1 inactivity mediated by promoter hypermethylation is accompanied by increased NAD synthesis, PARP1 levels and activity
In mammals, promoter methylation is an epigenetic modification that is involved in regulating gene expression.9,11,12 Consistent with this idea, we showed that breast cancer tissue with a hypermethylated BRCA1 promoter (Figs. 2A and B) displayed reduced expression of BRCA1 mRNA (Fig. 2C) compared with the adjacent normal tissue. Based on these considerations, the low levels of BRCA1 mediated by promoter hypermethylation were an appropriate model for investigating the physiological relationship between BRCA1 status, NAD synthesis, PARP1 expression and activity. Notably, there was a marked increase in NAD levels (Fig. 2D) accompanied by enhanced PARP1 activity (Fig. 2F), and a significant increase in PARP1 levels (Fig. 2E), along with a hypermethylated promoter-mediated BRCA1 deficiency. In addition, we analyzed the correlation between BRCA1 levels or NAD levels, and PARP1 levels or PARP1 activity in 53 breast cancer samples (Figs. 2G–J). It is interesting to note that a significant positive correlation was only observed between NAD levels and PARP1 activity (R = 0.647, P < 0.001; Fig. 2J). These results further indicate that BRCA1 may be responsible for the regulation of PARP1 expression, and NAD-dependent PARP1 activity.
Figure 2.
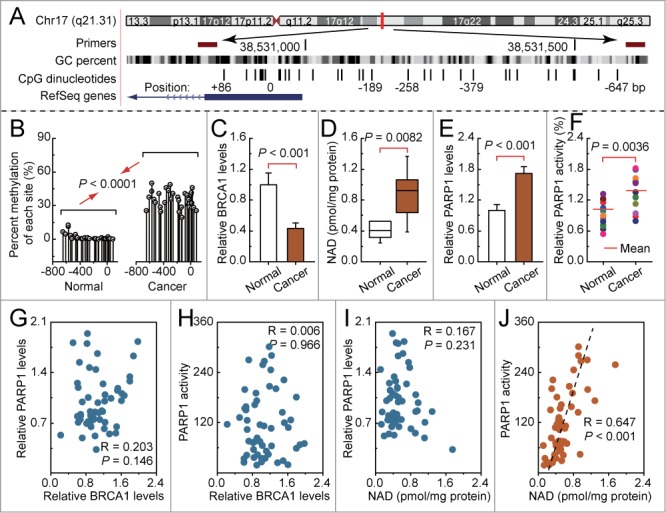
Intracellular NAD levels, PARP1 levels and activity in breast cancer with hypermethylated promoter-mediated BRCA1 inactivation. (A) the location of CpG sites in the core promoter region of BRCA1. Genomic coordinates are shown, along with the primer-amplified fragments, GC percentage, location of individual CpG dinucleotides (dashes), and the BRCA1 RefSeq gene (exon 1 is shown as a blue box and intron 1 is shown as an arrowed line). The arrow indicates the direction of transcription. (B) Summary of the methylation patterns of the BRCA1 promoter; the y-axis shows the mean methylation sites. (C—F) BRCA1 levels, NAD levels, PARP1 levels and activity were measured in a breast cancer sample with a hypermethylated BRCA1 promoter, compared with adjacent normal tissue (unmethylated BRCA1 promoter). Bar graphs show mean ± SD (Each group, n = 15). (G and H) Correlation between the BRCA1 levels, and PARP1 levels or activity in breast cancer tissues, respectively (Each group, n = 53). (I and J) Correlation between the NAD levels, and PARP1 levels or activity in breast cancer tissues, respectively (Each group, n = 53).
BRCA1 can regulate PARP1 levels and NAD-dependent PARP1 activity in breast cancer cells
To confirm the role of BRCA1 in the regulation of PARP1 expression and activity, the effects of knockdown or overexpression of BRCA1 were evaluated in estrogen receptor-negative and -positive human breast cancer cell lines (MDA-MB-231 and MCF-7), and primary breast cancer cells with identified BRCA1 mutations or no BRCA1 mutations. The results indicated that (i) there were no significant changes in NAD levels after the knockdown or overexpression of BRCA1 in MCF-7 cells; (ii) overexpression of BRCA1 could effectively reduce the NAD levels in MDA-MB-231 and primary non-mutated and BRCA1-mutated breast cancer cells; and (iii) knockdown of BRCA1 was an effective way to induce an increase in NAD levels in MDA-MB-231 and primary non-mutated breast cancer cells, but NAD levels were not sensitive to the BRCA1 knockdown in primary BRCA1-mutated breast cancer cells (Fig. 3A). Interestingly, the changes in intracellular NAD levels were consistent with the tendency of PARP1 activity (Fig. 3C), rather than the intracellular PARP1 levels (Fig. 3B). In addition, knockdown or overexpression of BRCA1 was shown to be an effective way to induce or inhibit PARP1 expression, respectively (Fig. 3B), but PARP1 levels were not sensitive to BRCA1 knockdown in primary BRCA1-mutated breast cancer cells.
Figure 3.

Effects of BRCA1 on intracellular NAD levels, PARP1 levels and activity. (A—C) NAD levels, PARP1 levels and activity after knockdown or overexpression of BRCA1 in MDA-MB-231 and MCF-7 cells (repeated 12 times), and primary non-mutated and BRCA1-mutated breast cancer cells (n = 12). Bar graphs show mean ± SD. Sh, shRNAs; Op, overexpression. *P < 0.05 vs. control.
Intracellular NAD can induce PARP1 activity, rather than PARP1 expression in breast cancer cells
To further confirm the role of intracellular NAD levels in the regulation of PARP1 expression and activity, the effects of incubation with different concentrations of NAD and knockdown or overexpression of Nampt were evaluated in MDA-MB-231, and primary breast cancer cells with identified BRCA1 mutations or no BRCA1 mutations, due to its sensitivity to changes in NAD levels mediated by BRCA1 (Fig. 3A). PARP1 activity was increased (Fig. 4B), along with increased levels of intracellular NAD (Fig. 4A). However, intracellular PARP1 levels were not sensitive to incubation with extracellular NAD in breast cancer cells (Fig. 4C). Nampt is a rate-limiting enzyme in the regeneration of NAD in mammals. The results indicated that knockdown or overexpression of Nampt could effectively reduce or increase NAD levels in breast cancer cells (Fig. 4D). Also, reduced or increased NAD levels mediated by Nampt could effectively inhibit or induce PARP1 activity (Fig. 4E), rather than influencing the PARP1 levels in breast cancer cells (Fig. 4F). These results suggested that BRCA1-mediated NAD synthesis may be largely responsible for regulating PARP1 activity.
Figure 4.

Effects of intracellular NAD on PARP1 expression and activity. (A—C) NAD levels, PARP1 levels and activity after incubation with different concentrations of NAD in MDA-MB-231 (repeated 12 times), and primary non-mutated and BRCA1-mutated breast cancer cells (n = 12). 1–5: incubation with 0, 1, 10, 100, or 1000 μM NAD. Bar graphs show mean ± SD. *P < 0.05 vs. control. (D—F) NAD levels, PARP1 levels and activity after knockdown or overexpression of Nampt in MDA-MB-231 (repeated 12 times), and primary non-mutated and BRCA1-mutated breast cancer cells (n = 12). Bar graphs show mean ± SD. Sh, shRNAs; Op, overexpression. *P < 0.05 vs. control.
Intracellular NAD can feedback activate BRCA1 expression in breast cancer cells
As already known, PARP1 overactivation results in NAD depletion. Our previous study suggested that NAD is a positive regulator for BRCA1 transcription in ovarian cancer cells. Therefore, it can be speculated that PARP1 overactivation-mediated NAD consumption may inhibit BRCA1 function. To confirm the role of PARP1-related NAD levels in the regulation of BRCA1 levels, the effects of knockdown or overexpression of PARP1 and incubation with different concentrations of NAD were evaluated in MDA-MB-231 and MCF-7 cells, and primary breast cancer cells with identified BRCA1 mutations or no BRCA1 mutations. Notably, we observed that BRCA1 expression was upregulated, along with increased levels of intracellular NAD (Fig. 5A), in MDA-MB-231 and in primary breast cancer cells with identified BRCA1 mutations or no BRCA1 mutations. In addition, increased or reduced NAD levels (Fig. 5B) mediated by the knockdown or overexpression of PARP1 could effectively induce or inhibit BRCA1 expression in MDA-MB-231, as well as in primary breast cancer cells with identified BRCA1 mutations or no BRCA1 mutations (Fig. 5C). Notably, the estrogen receptor-positive breast cancer cells MCF-7 were was an exception, as BRCA1 transcription can not be induced by increased intracellular NAD levels (Figs. 5B and C).
Figure 5.

Effects of PARP1-mediated intracellular NAD consumption on BRCA1 levels. (A) BRCA1 levels after incubation with different concentrations of NAD in MDA-MB-231 and MCF-7 cells (repeated 12 times), and primary non-mutated and BRCA1-mutated breast cancer cells (n = 12). 1–5: incubation with 0, 1, 10, 100, or 1000 μM NAD. Bar graphs show mean ± SD. *P < 0.05 vs. control. (B and C) NAD and BRCA1 levels after knockdown or overexpression of PARP1 in MDA-MB-231 and MCF-7 cells (repeated 12 times), and primary non-mutated and BRCA1-mutated breast cancer cells (n = 12). Bar graphs show mean ± SD. Sh, shRNAs; Op, overexpression. *P < 0.05 vs. control.
Discussion
In this study, we report for the first time that BRCA1 inactivity was a positive regulator for PARP1 levels and NAD-dependent PARP1 activity, and the high efficiency of PARP1-mediated NAD consumption can feedback inhibit BRCA1 transcription, and prevent the increased NAD synthesis triggered by BRCA1 inactivation (Fig. 6). Interestingly, the work presented here provides direct evidence that BRCA1, as a switch for PARP1, may have significant physiological roles in the maintenance of PARP1-related biological processes. However, NAD levels and PARP1 expression and activity were not affected by BRCA1 knockdown in primary BRCA1-mutated breast cancer cells. One can speculate that a lack of function of BRCA1 knockdown-mediated NAD or PARP1 regulation, which may be due to BRCA1 itself, has no function along with BRCA1-mutation. In addition, crosstalk among BRCA1, NAD and PARP1 was not sensitive in the estrogen receptor-positive breast cancer cell line MCF-7. It seems that the estrogen receptor may be involved in this regulatory pathway. Similarly, (i) PARP1 expression in breast cancers was associated with estrogen receptor status;16 (ii) PARP inhibitors were particularly effective in the treatment of TNBC;16 and (iii) TNBC patients have defective DNA repair similar to that seen in BRCA1-associated tumors.17
Figure 6.
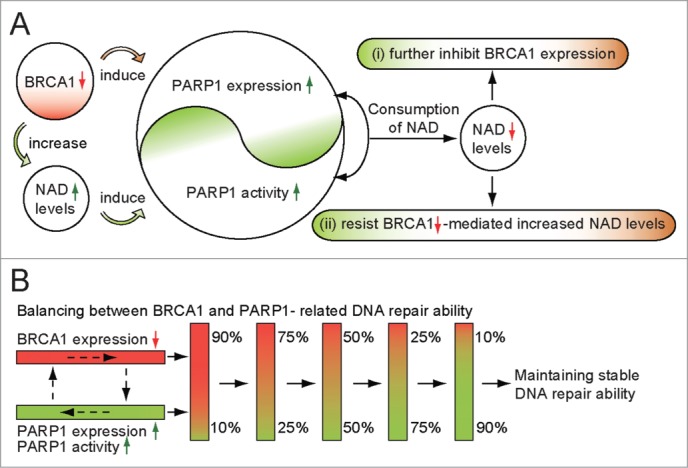
Proposed model of crosstalk between NAD-dependent BRCA1 and PARP1 in breast cancer. (A) BRCA1 inactivation events (mutation, promoter methylation, or other pathways) will induce PARP1 expression and NAD-dependent PARP1 activity. The high efficiency of PARP1-related NAD consumption may further inhibit BRCA1 expression, and prevent BRCA1 inactivation-mediated increases in NAD levels. (B) balancing mechanism between BRCA1 and PARP1, a proposed model to maintain stable DNA repair ability.
Notably, these observations further correlate the physiological properties of BRCA1 with PARP1-related metabolism. For example, (i) BRCA1-mutated ovarian cancer tissues showed the hypomethylated PARP1 promoter region, especially around the protein C-ets-1 (ETS1) motif, the mechanism involves the hypomethylated ETS1-mediated increase of histone modification H3K9ac and transcription factor ETS1 enrichment synergistically activate PARP1 expression;9 (ii) BRCA1 inactivation is not only able to increase reactive oxygen species generation, but further activates protective autophagy.18,19 PARP1 operates as an autophagy suppressor after oxidative stress.20 In this regard, the activation of PARP1 by BRCA1 inhibition may decrease the excess autophagy induced by oxidative stress; (iii) endoplasmic reticulum (ER) stress signaling plays an important role in the induction of BRCA1 expression.21 Mounting evidence has indicated that distinct NAD pools,22 NAD-dependent PARP123 and SIRT1 function24 all have an important role in the ER; and (iv) it is well known that aberrant proliferation is critical for cancer progression. A substantial body of evidence indicates that loss of function of the tumor suppressor gene BRCA1 plays an important role in promoting cancer cell proliferation and survival.25,26 Consistent with this, BRCA1 is a metabolic switch for NAD,7 and NAD-dependent signaling pathways are widely involved in cell proliferation and metabolism;27 and recent studies have suggested a possible role for PARP1-dependent Snail in regulating cell proliferation.28 Therefore, effects of BRCA1 on cell proliferation, which may involve in PARP1 due to BRCA1 as a metabolic switch for NAD and PARP1.
However, to date, it is not fully understood how the direct or indirect interaction among BRCA1, NAD and PARP1 at the molecular level. But some insight was gained by a previous study that (i) transcriptional regulatory activity of C-terminal binding proteins (CtBP) is modulated by the NAD/NADH ratio, increased intracellular NAD/NADH ratio induced loss of CtBP from the BRCA1 promoter, leading to elevated BRCA1 levels;29,30 (ii) BRCA1 has been shown to serve as a positive regulator of NAD-dependent histone deacetylase SIRT1 by binding to the promoter region,31,32 decreased levels of SIRT1 triggered by BRCA1 inactivation, may reduce the intracellular NAD consumption, which plays a partial role in the elevated NAD levels; and (iii) the results together with our previous finding7 provided the novel pathway of BRCA1-NAD-PARP1 activity and PARP1-NAD-BRCA1 expression. The emerging picture arising from these studies indicates an important functional link between BRCA1 and PARP1. The crosstalk may be beneficial for the dynamic balance between BRCA1-related biological processes, such as homologous recombination repair, transcriptional regulation, ubiquitination, apoptosis, cell cycle checkpoints, and PARP1-related single-strand break repair, chromatin remodelling, telomere maintenance, replicative stress, and cell cycle control.
These results highlight a novel interaction between BRCA1 and PARP1. All of this may improve our understanding of the basic molecular mechanism underlying BRCA1- and PARP1-related breast cancer progression.
Materials and Methods
Ethical statements
Investigation has been conducted in accordance with the ethical standards and according to the Helsinki Declaration of 1975.
Patients and tissue collection
This study was approved by the Institutional Review Board at China Medical University. Invasive ductal carcinomas patients were enrolled between 2007 and 2009, and all patients gave informed consent. Fresh breast cancer and adjacent normal breast tissues were obtained at the time of primary surgery before any chemotherapy or radiotherapy (41 pairs of BRCA1-mutated or not, 15 pairs with hypermethylated BRCA1 promoter or not). Hematoxylin and eosin staining of the samples for histopathological diagnosis and grading were performed by 3 staff pathologists using the Nottingham Combined Histologic Grade. The tumor stages were classified according to the National Comprehensive Cancer Network guidelines. Their characteristics and BRCA1-mutated information are given in Supplementary Table 1.
Cell culture and lentiviral infection
Detailed isolation and cultivation protocols were established as previously described.11,12 Briefly, tissues were washed, minced, and digested in 0.1% collagenase type III (Sigma) overnight at 37°C. The suspension was filtered through a 100-μm nylon mesh to remove the remaining clumps. Following gentle centrifugation at 100 g for 5 min, the epithelial and stromal fractions were cultured in Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum (Invitrogen) for 24 h to promote cell attachment. Breast epithelial cells were maintained in CnT-27 mammary epithelium medium (CELLnTEC), and used in all experiments were passage 2 to passage 5. Human breast cancer cell lines MDA-MB231 and MCF-7 were maintained in DMEM with 10% fetal bovine serum (Invitrogen). Lentiviral vectors expressing short hairpin RNAs (shRNAs) against BRCA1 (NM_007299) were obtained from GeneChem Co., Ltd, and synthesized sequences as shown in Supplementary Table 2. The non-silencing shRNA sequence was used as a negative control, and synthesized sequences as shown in Supplementary Table 2. The shRNAs lentiviral particles of nicotinamide phosphoribosyltransferase (Nampt) (sc-45843-V) and PARP1 (sc-29437-V) were purchased from Santa Cruz Biotechnology. For overexpression of BRCA1, Nampt, or PARP1, the open reading frame of BRCA1 (NM_007299), Nampt (NM_005746), or PARP1 (NM_001618) were respectively cloned into the lentiviral vector GV287 (Ubi-MCS-3FLAG-SV40-EGFP; GeneChem Co., Ltd), and these experiment were performed by GeneChem Co., Ltd. Transfections were performed using polybrene and enhanced infection solution (GeneChem Co., Ltd) according to the manufacturer's recommended protocol. The efficiency of BRCA1, Nampt and PARP1 knockdown and overexpression was previously described.7
Real-time quantitative PCR
Total RNA was extracted using Trizol reagents (Invitrogen) according to the manufacturer's protocol. DNA contamination was removed by adding DNase I (Invitrogen) according to the manufacturer's protocol. Total RNA was then reverse-transcribed from 2 μg of RNA using the PrimeScript RT Master Mix kit (TaKaRa) and amplified by SYBR Premix Ex TaqTM II (TaKaRa) in a Roche LightCycler 2.0 instrument (Roche Diagnostics). The specific primer sequences for BRCA1, PARP1 and Nampt were shown in Supplementary Table 2. GAPDH mRNA was amplified as an internal control for normalization of each sample. All samples were analyzed in triplicate using the 2−ΔΔCT method.
Western blotting for PARP1
Briefly, 30 μg protein was separated by 8% SDS polyacrylamide gels, and transferred to polyvinyl difluoride membranes (Millipore Corp.). The membranes were blocked in TBS containing 0.1% Tween-20 and 5% non-fat dry milk for 60 min at room temperature, and incubated with antibody to PARP1 (sc-8007) (1:300; Santa Cruz Biotechnology) overnight at 4°C. Then, the membranes were washed by PBS-Tween followed by 1 h incubation at room temperature with horseradish peroxidase-conjugated secondary antibody (1:5000; Santa Cruz Biotechnology) and detected using the enhanced chemiluminescence (Amersham Life Science).
Bisulfite sequencing for BRCA1 promoter
All the tissues were used for bisulfite sequencing from the non-BRCA1-mutated cases. Genomic DNA extracted from breast cancer and normal breast tissue with a TIANamp Genomic DNA kit (Tiangen Biotech) was subjected to bisulfite conversion using the EZ DNA Methylation-Direct kit (Zymo Research) following the manufacturer's instructions; the conversion efficiency was estimated to be at least 99.6%. It was then amplified by nested PCR. After gel purification, cloning and transformation into E. coli Competent Cells JM109 (TaKaRa), 10 positive clones of each sample were sequenced to ascertain the methylation patterns of each CpG locus. The primers were used for BRCA1 gene promoter (Accession number: NG_005905) were shown in Supplementary Table 2. The conditions were as follows: 95°C for 2 min, 40 cycles of 30s at 95°C, 30s at 56°C and 45s at 72°C, then 72°C for 7 min.
NAD incubation
MDA-MB-231, MCF-7 and primary breast cancer cells were incubated with 0, 1, 10, 100 ορ 100 μM NAΔ (Sigma) for 3 h at 37°C.
NAD levels assay
For NAD assay, 20 mg of freezed breast tissue, or 20 μl packed cultured cells were homogenized in 400 μl BioVsion NAD/NADH Extraction Buffer (BioVsion). The homogenate was ultrafiltered using BioVsion 10-kD cut-off filters (14000 g, 30 min, 4°C). Assays were performed using the NAD/NADH Quantification Kits according to the manufacturer's instructions (BioVsion).
PARP1 activity assay
PARP1 activity was evaluated by using a commercially available HT colorimetric PARP/apoptosis assay kit (Trevigen), according to the manufacturer's protocols.
Statistical analysis
Regression analysis was used to examine the possible relationship between BRCA1 levels or NAD levels, and PARP1 levels or PARP1 activity in breast cancer samples. The data are presented as means ± SD. Statistical differences in the data were evaluated by Student's t-test or one-way ANOVA as appropriate, and were considered significant at P < 0.05.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Natural Science Foundation of China (nos. 81402130, 31171259 and 31271364) and Doctoral Start-up Foundation of Liaoning Province (no. 20141045).
References
- 1. DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin 2014; 64:52-62; PMID:24114568; http://dx.doi.org/10.3322/caac.21203 [DOI] [PubMed] [Google Scholar]
- 2. Pruthi S, Gostout BS, Lindor NM. Identification and management of women Wwth BRCA mutations or hereditary predisposition for breast and ovarian cancer. Mayo Clin Proc 2010; 85:1111-20; PMID:21123638; http://dx.doi.org/ 10.4065/mcp.2010.0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005; 434:913-7; PMID:15829966; http://dx.doi.org/ 10.1038/nature03443 [DOI] [PubMed] [Google Scholar]
- 4. Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005; 434:917-21; PMID:15829967; http://dx.doi.org/ 10.1038/nature03445 [DOI] [PubMed] [Google Scholar]
- 5. Dacheux E, Vincent A, Nazaret N, Combet C, Wierinckx A, Mazoyer S, Diaz JJ, Lachuer J, Venezia ND. BRCA1-dependent translational regulation in breast cancer cells. PLoS One 2013; 8:e67313; PMID: 23805307; http://dx.doi.org/ 10.1371/journal.pone.0067313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bi FF, Li D, Yang Q. Promoter hypomethylation, especially around the E26 transformation-specific motif, and increased expression of poly (ADP-ribose) polymerase 1 in BRCA-mutated serous ovarian cancer. BMC Cancer 2013; 13:90; PMID:23442605; http://dx.doi.org/ 10.1186/1471-2407-13-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li D, Chen NN, Cao JM, Sun WP, Zhou YM, Li CY, Wang XX. BRCA1 as a nicotinamide adenine dinucleotide (NAD)-dependent metabolic switch in ovarian cancer. Cell Cycle 2014; 16:2564-2571; http://dx.doi.org/10.4161/15384101.2015.942208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fang YY, Li D, Cao C, Li CY, Li TT. Glucocorticoid receptor repression mediated by BRCA1 inactivation in ovarian cancer. BMC Cancer 2014; 14:188; PMID:24629067; http://dx.doi.org/ 10.1186/1471-2407-14-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li D, Bi FF, Cao JM, Cao C, Li CY, Liu B, Yang Q. Poly (ADP-ribose) polymerase 1 transcriptional regulation: a novel crosstalk between histone modification H3K9ac and ETS1 motif hypomethylation in BRCA1-mutated ovarian cancer. Oncotarget 2014; 5:291-7; PMID:24448423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li D, Bi FF, Cao JM, Cao C, Li CY, Yang Q. Effect of BRCA1 on epidermal growth factor receptor in ovarian cancer. J Exp Clin Cancer Res 2013; 32:102; PMID:24321281; http://dx.doi.org/ 10.1186/1756-9966-32-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li D, Bi FF, Cao JM, Cao C, Liu B, Yang Q. Regulation of DNA methyltransferase 1 transcription in BRCA1-mutated breast cancer: a novel crosstalk between E2F1 motif hypermethylation and loss of histone H3 lysine 9 acetylation. Mol Cancer 2014; 13:26; PMID:24502362; http://dx.doi.org/ 10.1186/1476-4598-13-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li D, Bi FF, Chen NN, Cao JM, Sun WP, Zhou YM, Cao C, Li CY, Yang Q. Epigenetic repression of phosphatidylethanolamine, N-methyltransferase (PEMT) in BRCA1-mutated breast cancer. Oncotarget 2014; 5:1315-25; PMID:2467547621409392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Domagala P, Huzarski T, Lubinski J, Gugala K, Domagala W. PARP-1 expression in breast cancer including BRCA1-associated, triple negative and basal-like tumors: possible implications for PARP-1 inhibitor therapy. Breast Cancer Res Treat 2011; 127:861-9; PMID:21409392; http://dx.doi.org/ 10.1007/s10549-011-1441-2 [DOI] [PubMed] [Google Scholar]
- 14. Goldberg MS, Xing D, Ren Y, Orsulic S, Bhatia SN, Sharp PA. Nanoparticle-mediated delivery of siRNA targeting Parp1 extends survival of mice bearing tumors derived from Brca1-deficient ovarian cancer cells. Proc Natl Acad Sci U S A 2011; 108:745-50; PMID: 21187397; http://dx.doi.org/ 10.1073/pnas.1016538108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piao L, Kang D, Suzuki T, Masuda A, Dohmae N, Nakamura Y, Hamamoto R. The histone methyltransferase SMYD2 methylates PARP1 and promotes poly(ADP-ribosyl)ation activity in cancer cells. Neoplasia 2014; 16:257-64; PMID:24726141; http://dx.doi.org/ 10.1016/j.neo.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ossovskaya V, Koo IC, Kaldjian EP, Alvares C, Sherman BM. Upregulation of poly (ADP-ribose) polymerase-1 (PARP1) in triple-negative breast cancer and other primary human tumor types. Genes Cancer 2010; 1:812-21; PMID:21779467; http://dx.doi.org/ 10.1177/1947601910383418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez AA, Makris A, Wu MF, Rimawi M, Froehlich A, Dave B, Hilsenbeck SG, Chamness GC, Lewis MT, Dobrolecki LE, et al. DNA repair signature is associated with anthracycline response in triple negative breast cancer patients. Breast Cancer Res Treat 2010; 123:189-96; PMID:20582464; http://dx.doi.org/ 10.1007/s10549-010-0983-zZ [DOI] [PubMed] [Google Scholar]
- 18. Martinez-Outschoorn UE, Balliet RM, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Hereditary ovarian cancer and two-compartment tumor metabolism: epithelial loss of BRCA1 induces hydrogen peroxide production, driving oxidative stress and NFkappaB activation in the tumor stroma. Cell Cycle 2012; 11:4152-66; PMID:23047606; http://dx.doi.org/ 10.4161/cc.22226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang MK, Kwong A, Tam KF, Cheung AN, Ngan HY, Xia W, Wong AS. BRCA1 deficiency induces protective autophagy to mitigate stress and provides a mechanism for BRCA1 haploinsufficiency in tumorigenesis. Cancer Lett 2014; 346:139-47; PMID:24378767; http://dx.doi.org/ 10.1016/j.canlet.2013.12.026 [DOI] [PubMed] [Google Scholar]
- 20. Wyrsch P, Blenn C, Bader J, Althaus FR. Cell death and autophagy under oxidative stress: roles of poly(ADP-Ribose) polymerases and Ca(2+). Mol Cell Biol 2012; 32:3541-53; PMID:22751932; http://dx.doi.org/ 10.1128/MCB.00437-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fan S, Meng Q, Auborn K, Carter T, Rosen EM. BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br J Cancer 2006; 94:407-26; PMID:16434996; http://dx.doi.org/ 10.1038/sj.bjc.6602935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Girolamo M, Fabrizio G, Scarpa ES, Di Paola S. NAD(+)-dependent enzymes at the endoplasmic reticulum. Curr Top Med Chem 2013; 13:3001-10; PMID:24171768 [DOI] [PubMed] [Google Scholar]
- 23. Yang JH, Choe ES. Repeated cocaine administration increases cleaved poly(ADP-ribose) polymerase-1 expression in the rat dorsal striatum. Neurosci Lett 2010; 471:58-61; PMID:20079403; http://dx.doi.org/ 10.1016/j.neulet.2010.01.011 [DOI] [PubMed] [Google Scholar]
- 24. Suzuki M, Bartlett JD. Sirtuin1 and autophagy protect cells from fluoride-induced cell stress. Biochim Biophys Acta 2014; 1842:245-55; PMID:24296261; http://dx.doi.org/ 10.1016/j.bbadis.2013.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burga LN, Tung NM, Troyan SL, Bostina M, Konstantinopoulos PA, Fountzilas H, Spentzos D, Miron A, Yassin YA, Lee BT, et al. Altered proliferation and differentiation properties of primary mammary epithelial cells from BRCA1 mutation carriers. Cancer Res 2009; 69:1273-8; PMID:19190334; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Promkan M, Liu G, Patmasiriwat P, Chakrabarty S. BRCA1 modulates malignant cell behavior, the expression of survivin and chemosensitivity in human breast cancer cells. Int J Cancer 2009; 125:2820-8; PMID:19551867; http://dx.doi.org/ 10.1002/ijc.24684 [DOI] [PubMed] [Google Scholar]
- 27. Chiarugi A, Dolle C, Felici R, Ziegler M. The NAD metabolome-a key determinant of cancer cell biology. Nat Rev Cancer 2012; 12:741-52; PMID:23018234; http://dx.doi.org/ 10.1038/nrc3340 [DOI] [PubMed] [Google Scholar]
- 28. Lin Y, Zhou BP. Doxorubicin enhances SnailLSD1-mediated PTEN suppression in a PARP1-dependent manner. Cell Cycle 2014; 13:1708-16; PMID: 24675890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deng Y, Liu J, Han G, Lu SL, Wang SY, Malkoski S, Tan AC, Deng C, Wang XJ, Zhang Q. Redox-dependent Brca1 transcriptional regulation by an NADH-sensor CtBP1. Oncogene 2010; 29:6603-8; PMID: 20818429; http://dx.doi.org/ 10.1038/onc.2010.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Di LJ, Fernandez AG, De Siervi A, Longo DL, Gardner K. Transcriptional regulation of BRCA1 expression by a metabolic switch. Nat Struct Mol Biol 2010; 17:1406-13; PMID:21102443; http://dx.doi.org/ 10.1038/nsmb.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hiraike H, Wada-Hiraike O, Nakagawa S, Koyama S, Miyamoto Y, Sone K, Tanikawa M, Tsuruga T, Nagasaka K, Matsumoto Y, et al. Identification of DBC1 as a transcriptional repressor for BRCA1. Br J Cancer 2010; 102:1061-7; PMID:20160719; http://dx.doi.org/ 10.1038/sj.bjc.6605577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanikawa M, Wada-Hiraike O, Nakagawa S, Shirane A, Hiraike H, Koyama S, Miyamoto Y, Sone K, Tsuruga T, Nagasaka K, et al. Multifunctional transcription factor TFII-I is an activator of BRCA1 function. Br J Cancer 2011; 104:1349-55; PMID:21407215; http://dx.doi.org/ 10.1038/bjc.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


