Calcium and phosphorous, in the form of phosphate, are essential elements in many biological processes. Not only are they required for proper cellular function and signaling, but they also combine to make biological crystals; most notably, hydroxyapatite found in bone. Because of their essential nature, their anatomic distribution is tightly regulated with 10,000 times more calcium and phosphate circulating in the extracellular space, as opposed to the intracellular microenvironment. This ideal biological gradient provides excess calcium and phosphate for cellular function; however, calcium and phosphate are therefore at their saturation points when circulating in plasma. While this is ideal for maintaining bone integrity, calcium and phosphate can aggregate in soft tissue such as muscle, skin, and blood vessels. The fact that most soft tissues are free of calcium phosphate aggregates indicates that there are specialized biological mechanisms in place to prevent aberrant aggregation.
Pyrophosphate directly inhibits calcium and phosphate aggregation preventing in vivo mineralization (Fig. 1). Circulating levels of pyrophosphate are maintained by “pyrophosphate pumps” which in turn provide the front line defense against soft tissue calcification.1 It follows that genetic mutations to elements of the pyrophosphate pump are considered the potential underlying cause of soft tissue calcification disorders such as Pseudoxanthoma elasticum (PXE– ABCC6 mutation) and generalized arterial calcification of infancy (GACI – ENPP1 mutation).2 Although these associations have been supported, future studies designed to confirm that there are decreased levels of pyrophosphate in these patients are required. If hypo-pyrophosphatemia is identified as the underlying cause of soft tissue calcification in PXE and GACI, a logical treatment would be to replenish pyrophosphate. However, pyrophosphate is quickly metabolized during gut absorption thus necessitating an alternative route of supplementation. To address this, researchers, such as Li et al., have turned to non-hydrolyzable pyrophosphate analogs, bisphosphonates, to potentially prevent aberrant mineralization in these patients by supplementing the deficient pyrophosphate system.
Figure 1.
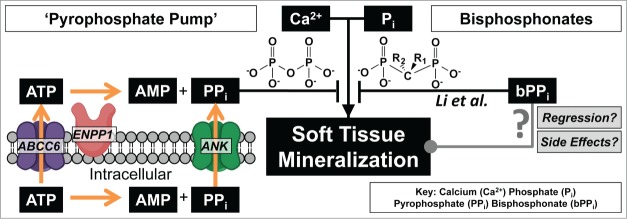
At physiologic conditions calcium and phosphate are at saturating concentrations which should cause soft tissue mineralization. One mechanism of preventing aberrant mineralization is the 'pyrophosphate pump' which provides systemic pyrophosphate that inhibits calcium phosphate aggregation. Diseases such as PXE (ABCC6 mutation) are thought to cause insufficient circulating pyrophosphate leading to soft tissue mineralization. Li. et al. demonstrate that soft tissue mineralization can be prevented by supplementing pyrophosphate with non-hydrolyzable bisphosphonates.
Bisphosphonates are a powerful family of pharmaceuticals utilized by clinicians for more than 40 y to prevent osteoporosis.3 These stable forms of pyrophosphate freely pass into cells and act as effective inhibitors of the HMG-CoA reductase pathway. Bisphosphonates have pleiotropic effects on cellular function,4 most notably attenuating osteoclast activity and reducing these cells' ability to resorb bone. However, industrial use of bisphosphonates to protect against aberrant mineralization predates their use to preserve bone by at least a century. Bisphosphonates were first used to “soften” public water supplies in the 1800s thereby preventing calcification of pipes. Additionally, the first clinical papers regarding bisphosphonates and their uses in vivo in the 1960s also focused on their ability to prevent calcium and phosphate aggregation. Despite the well-documented anti-mineralization properties of bisphosphonates, their predominate clinical application has instead been concentrated on their anti-bone resorptive properties.
Li et al. investigated the efficacy of bisphosphonate administration on mice with a deficiency of ABCC6 that are prone to developing spontaneous calcification in soft tissues.5 Specifically, the authors determined that even oral administration of bisphosphonates has the capacity to protect soft tissue from calcification in these “PXE” mice. This work represents an integral step toward the clinical application of bisphosphonates as it not only supports the concept that the soft tissue calcification caused by a loss of ABCC6 is a result of a pyrophosphate deficiency, but it also gives hope that bisphosphonates are effective means of replenishing this deficiency.
Despite these findings, the risk of bisphosphonate treatment in these patients must be carefully considered. Bisphosphonates are excellent at preventing the establishment of mineralization, but they are also known to strongly bind to pre-formed hydroxyapatite, such as bone, and persist in these areas for up to 10 y following treatment. Considering that most PXE patients already possess soft tissue calcification within their skin or retina, it is unknown what clinical effect administering bisphosphonates will have since bisphosphonates will also bind to sites of pre-existing soft tissue calcification, as they do in bone. Additionally, it is unknown what effect the bisphosphonate inhibition of the HMG-CoA reductase pathway will have on the cells of this soft tissue environment, such as dermal or retinal cells. Thus our concern for admiration of bisphosphonates is directed at both short- term and long -term side effects. Short-term side effects include: esophageal irritation, acute phase response activation, ocular inflammation, hypocalcemia, acute renal damage, and musculoskeletal pain. Long-term safety concerns also have been reported that include osteonecrosis of the jaw, and atypical femur fractures.6,7 For these reasons, further studies of the use of bisphosphonates in patients with calcification disorders, such as PXE and GACI, is warranted and necessary.
References
- 1.Dabisch-Ruthe M, et al.. J Dermatol Sci, 2014; 75:109-20; PMID:24907773; http://dx.doi.org/ 10.1016/j.jdermsci.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Uitto J, et al.. Expert Opin Orphan Drugs, 2014; 2:567-577; PMID:25383264; http://dx.doi.org/ 10.1517/21678707.2014.908702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell RG, Bone, 2011; 49:2-19; PMID:21555003; http://dx.doi.org/ 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Ohba T, et al.. Bone, 2014; 63:110-20; PMID:24636958; http://dx.doi.org/ 10.1016/j.bone.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, et al.. Cell Cycle, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pazianas M, et al.. Bone, 2011; 49:103-10; PMID:21236370; http://dx.doi.org/ 10.1016/j.bone.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Baroncelli GI, et al., Horm Res Paediatr, 2014; 82:290-302; PMID:25376487; http://dx.doi.org/ 10.1159/000365889. [DOI] [PubMed] [Google Scholar]


