Abstract
As our society ages, neurodegenerative disorders like Parkinson`s disease (PD) are increasing in pandemic proportions. While mechanistic understanding of PD is advancing, a treatment with well tolerable drugs is still elusive. Here, we show that administration of the naturally occurring polyamine spermidine, which declines continuously during aging in various species, alleviates a series of PD-related degenerative processes in the fruit fly Drosophila melanogaster and the nematode Caenorhabditis elegans, two established model systems for PD pathology. In the fruit fly, simple feeding with spermidine inhibited loss of climbing activity and early organismal death upon heterologous expression of human α-synuclein, which is thought to be the principal toxic trigger of PD. In this line, administration of spermidine rescued α-synuclein-induced loss of dopaminergic neurons, a hallmark of PD, in nematodes. Alleviation of PD-related neurodegeneration by spermidine was accompanied by induction of autophagy, suggesting that this cytoprotective process may be responsible for the beneficial effects of spermidine administration.
Keywords: autophagy, aging, α-synuclein, dopaminergic neuron loss, motor dysfunction, neurodegeneration, Parkinson's disease, Spermidine
Introduction
Exploration of the molecular basis underlying the pathology of age-related neurodegenerative diseases has revealed an intricate interplay between several cellular processes that differentially contribute to continual neuronal demise, including mitochondrial (dys)function and an impairment of autophagy.1-4 Neurons are mainly quiescent cells surviving for decades; therefore, they might be particularly vulnerable to the slow but progressive accumulation of organellar and molecular damage, a characteristic feature of aging, as well as to defects in continuous organelle and protein turnover, which is governed by the ubiquitin proteasome system (UPS) and macroautophagy (henceforth called autophagy). A pathological hallmark of various chronic neurodegenerative disorders including Amyotrophic lateral sclerosis, Alzheimer's (AD), Huntington's (HD), and Parkinson's (PD) disease is the accumulation of large, intracellular inclusions composed of misfolded (and often ubiquitinated) proteins in disease-specific regions of the brain.5,6 The important involvement of the UPS in the degradation of these protein aggregates is for instance highlighted by the fact that the occurrence of an elongated and stable ubiquitin protein variant termed UBB+ 1 inhibits the UPS and is tightly connected to AD and HD-associated pathologies.7-10 The UBB+ 1 protein is generated by molecular misreading of the ubiquitin B mRNA11 and decreases the proteasomal activity (at least in part) via inhibition of specific deubiquitinating enzymes.12 Besides the UPS, autophagy as the other main system of the protein quality control has emerged as a decisive factor in the pathology of numerous age-associated neurodegenerative diseases, including AD, HD and PD.13-20
Spermidine is a naturally occurring polyamine that counteracts age-associated cell death (at least in part) via an activation of the autophagic machinery. It belongs to an ubiquitous family of organic polycations, the polyamines, which exert diverse roles in cell proliferation, differentiation, survival and death.21 An age-dependent decrease of polyamine levels has been described in many organisms and tissues,22 including the brain of Drosophila, rodents and humans.23-25 External administration of spermidine prolonged the lifespan of yeast, flies, worms and human PBMCs (peripheral blood mononuclear cells) via induction of autophagy.26 Interestingly, dietary spermidine supplementation as well as genetically enforced biosynthesis of spermidine protected against cognitive aging in Drosophila, preventing age-induced memory loss through activation of the autophagic machinery.23 Spermidine (as well as other activators of autophagy) was able to reduce neurodegeneration in a mouse model for TDP-43- pathology.27 Besides this cytoprotective activity related to aging, spermidine conferred a higher resistance toward environmental stress, leading to increased heat tolerance in yeast, protection against oxidative stress in yeast and flies, and reduction of age-related oxidative damage in mice.26 Drosophila subjected to oxidative stress exhibited improved motor function and survival upon supplementation of food with spermidine.28 Notably, this beneficial effect was autophagy-dependent upon challenge with the superoxide generator paraquat but not upon hydrogen peroxide treatment.28 Thus, depending on the specific scenario, spermidine-mediated cytoprotection seems to engage alternative mechanisms other than autophagy.
In this study, we show that spermidine is able to alleviate PD-associated cellular demise using heterologous expression of human α-synuclein (αSyn) in the fruit fly Drosophila melanogaster and the nematode Caenorhabditis elegans, two established model systems for PD pathology.29-32 The protein αSyn is thought to play a pivotal role in the degenerative processes during PD, as it (i) represents the main protein component of the intracellular protein aggregates called Lewy bodies, a pathological hallmark of PD, and (ii) point mutations in as well as duplication/ triplication of the gene locus coding for αSyn are linked to familial PD.33 Here, we demonstrate that supplementation of food with spermidine protects against αSyn-facilitated motor dysfunction and organismal death in flies and reduces dopaminergic neuron loss in nematodes while causing an activation of autophagy.
Results
Spermidine treatment is able to extend the life- and healthspan of various model organisms. We therefore hypothesized that its age-protective function might as well be effective during typical age-related neurodegenerative disorders. By using flies expressing human αSyn under the control of the UAS-GAL4 system,34 an established model for PD-associated neurotoxicity, we evaluated the effect of spermidine supplementation on organismal survival. The sole expression of αSyn per se only caused a very modest decrease in viability during regular aging (data not shown). This result prompted us to combine this genetic trigger of PD (αSyn) with the administration of manganese, which represents a known environmental risk factor for PD.35 Employing this experimental setup,31,32 pan-neuronal elav-GAL4-driven expression of αSyn killed the flies within 4–6 days (Fig. 1A). Simultaneous supplementation of food with spermidine could largely protect from αSyn-induced organismal death, extending both the mean and the maximum lifespan (Fig. 1A and B). In fact, while the mean lifespan in flies expressing αSyn decreased by 20% upon manganese treatment, spermidine supplementation led to an almost complete restoration of mean lifespan (Fig. 1B). Of note, we could not detect any effect of spermidine on the expression levels of αSyn after 24 h of manganese treatment (Fig. 1C ).
Figure 1.
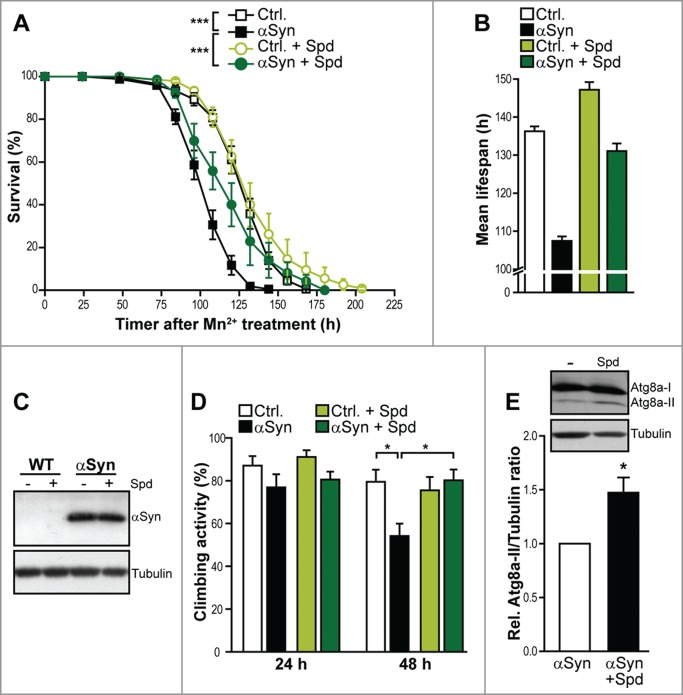
Spermidine prevents ɑSyn-induced motor dysfunction and death of D. melanogaster. (A) Survival of male flies expressing human α-synuclein (αSyn) driven by x chromosome-linked elav-GAL4 and corresponding isogenic w1118 wild type flies (Ctrl.) upon supplementation of food (10% sucrose) with 20 mM Mn2+. Survival has been determined at indicated time points. Data represent means ± s.e.m., n = 5–6 with 30–40 flies per experiment. ***P < 0.001. (B) Mean lifespan calculated using Kaplan Meier estimate of the flies described in (A) upon supplementation of food with 20 mM Mn2+. (C) Immunoblot analysis of brain lysates of flies expressing human α-synuclein (αSyn) driven by x chromosome-linked elav-GAL4 and corresponding isogenic wild type flies (Ctrl.) upon supplementation of food (10% sucrose) with 20 mM Mn2+ and 5 mM spermidine as indicated for 24 h. Blots have been probed with antibodies directed against human αSyn or tubulin as loading control and respective secondary antibodies. (D) Climbing activity of male flies described in (A) after 24 h and 48 h of Mn2+treatment. Data represent means ± s.e.m. For each genotype and condition, 150–180 flies were tested (n = 6 with 25–30 flies per experiment). *P < 0.05. (E) Immunoblotting to analyze Atg8a levels in brain lysates of flies expressing human αSyn upon supplementation of food with 20 mM Mn2+ for 72 h. Food has been supplemented with or without 5 mM spermidine. A representative blot is shown. Atg8a-II signals have been quantified densitometrically and normalized to α-tubulin levels. Data represent means ± s.e.m., n = 4, *P < 0.05.
In addition, we monitored the effect of spermidine supplementation on motor dysfunction, a typical pathological feature of PD. The expression of αSyn caused a significant defect in motor function, which was already detectable after 48 h of manganese treatment as indicated by a significant reduction in climbing ability (Fig. 1D). Again, spermidine supplementation was able to completely inhibit this pathological consequence of αSyn expression, suggesting that spermidine not only protects against organismal death induced by neurotoxicity but also prevents typical symptoms prior to final demise (Fig. 1D).
As mentioned above, accumulating data point to an involvement of autophagy in neuronal demise during PD in general and the toxic consequences of αSyn in particular. In addition, spermidine-mediated lifespan prolongation in several model organisms has been shown to largely depend on an intact autophagic machinery. Thus, we tested whether the neuroprotective function of spermidine involves an activation of autophagy, as well. To this end, we analyzed the protein levels of Atg8a (autophagy-related gene 8a) in brain lysates of flies expressing αSyn. Atg8a is a Drosophila member of the Atg8/LC3 protein family, which is cleaved and lipidated during early autophagosome formation. The level of Atg8a-II, which represents the lipidated, autophagosome-associated form of this protein, significantly increased upon spermidine administration (Fig. 1E), indicating an enhanced autophagosome formation. Thus, the neuroprotective effect of spermidine might well be exerted via induction of autophagy in the fly brain.
We next tested the ability of spermidine to protect against αSyn–associated neurodegeneration in a C. elegans model of PD.36 For that purpose, we analyzed nematodes expressing human αSyn under the control of a dopamine-specific neuronal promoter (Pdat-1) for survival of the anterior CEP (cephalic) dopaminergic neurons, which were visualized via co-expression of GFP driven by the dat-1 gene promoter. Consistent with previous findings,31,32,36,37 the expression of αSyn caused severe dopaminergic neuron loss in 7-day-old adult worms compared to same-staged animals expressing GFP alone (Fig. 2A), leading to less than 10% of worms with healthy, wild-type like CEPs (Fig. 2B). Supplementation of food with 5 mM spermidine significantly decreased this αSyn-induced neuronal degeneration (Fig. 2A and B).
Figure 2.
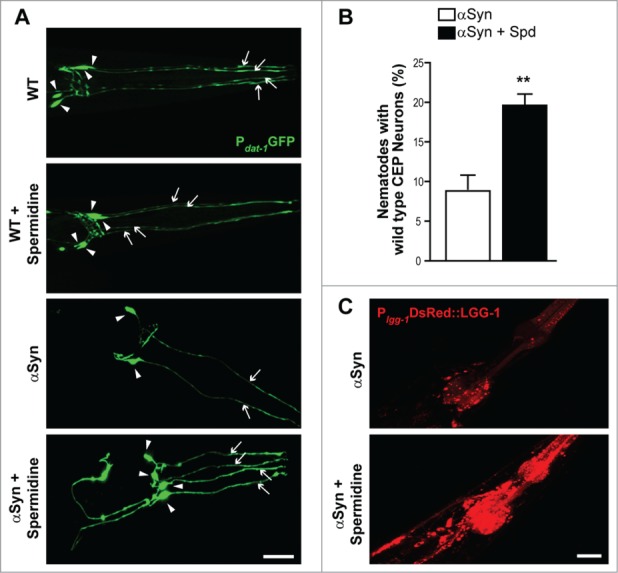
Spermidine reduces ɑSyn neurotoxicity in C. elegans and induces autophagy (A and B) Survival of anterior CEP (cephalic) dopaminergic neurons in wild type (WT) nematodes expressing GFP under the control of a dopaminergic neuron specific promoter (Pdat-1GFP) and nematodes expressing Pdat-1GFP and Pdat-1ɑSyn. Food was supplied with or without 5 mM spermidine. Representative confocal images of the head region (A) are shown, with arrowheads indicating neuronal cell bodies and arrows indicating intact neuronal processes. Scale bar represents 20 μm. In (B), the percentage of worms preserving all 4 CEPs at day 7 of adulthood was quantified with 30–40 animals per condition in each of 4 independent experiments. Data represent mean ± s.e.m., **P < 0.01, Student's t test. (C) Confocal images of nematodes (baIn11[pdat-1αSyn, pdat-1GFP]; N2Ex[plgg-1DsRED::LGG-1]) expressing ɑSyn in dopaminergic neurons as well as the autophagosomal marker LGG-1 fused to DsRED driven by the endogenous lgg-1 promoter following supplementation of food with 5 mM spermidine compared to age-matched untreated animals.
Finally, we aimed at corroborating a possible decisive role of autophagy in spermidine's cytoprotective action upon αSyn expression, as suggested by our results obtained with Drosophila. To this end, we crossed αSyn-expressing nematodes with those carrying extrachromosomal arrays of full length LGG-1 fused to DsRED driven by the endogenous lgg-1 promoter (plgg-1 DsRED::LGG-1).38 The gene lgg-1 encodes a ubiquitin-like protein belonging to the Atg8/LC3 protein family, and the respective DsRED::LGG-1 translational fusion thus allows to monitor autophagosome formation via fluorescence microscopy. We observed that spermidine treatment drastically enhanced DsRed::LGG-1 punctae frequency and intensity compared to the basal levels displayed in untreated animals (Fig. 2C) demonstrating that the ability of spermidine to mitigate αSyn toxicity in C. elegans is accompanied by the induction of autophagy.
Discussion
Deregulation of autophagy has emerged as culprit in diverse neurodegenerative processes. An accumulation of autophagosomes has been reported in post-mortem brain tissue from AD, PD, and HD patients as well as in diverse cell culture, fly and mouse models of these diseases.4,39 This phenotype most probably results from lysosomal depletion and defective lysosomal clearance.2,40 Several genetic factors implicated in the pathology of PD, including αSyn, the leucine-rich repeat kinase LRRK2, the ubiquitin ligase parkin or the PTEN-induced putative kinase PINK1, have been shown to differentially influence autophagic processes. Vice versa, modulation of autophagy alters the cellular consequences of these factors’ toxic gain-of-function or loss-of-function, though exact mechanisms remain yet to be elucidated.4,13,14,41
In mouse models for AD and PD, overexpression of the pro-autophagic regulator Beclin-1 (Atg6) ameliorated signs of neurodegeneration,42,43 and mice conditionally lacking neuronal Atg5 or Atg7 displayed severe behavior and motor deficits, abundant cellular protein inclusions, and neurodegeneration in several brain regions.44,45 These results affirm the importance of basal autophagy to maintain neuronal homeostasis. In the same lines, the pharmacological induction of autophagy is thought to represent a potential strategy to ameliorate neurodegenerative demise. Treatment with rapamycin, for example, which induces autophagy via inactivation of mTOR, has been demonstrated to be neuroprotective in several cell culture and animal models of PD, HD and AD.1,46,47 Nevertheless, though autophagy has been demonstrated to be a pro-survival process in most studies, and genetic or chemical induction of autophagy generally provided neuroprotection, excessive autophagy has also been suggested to contribute to non-apoptotic neuronal cell death.4,40 For instance, neurodegeneration after brain injury could be prevented by conditional Atg7 deficiency in mice,48 and Beclin-1 silencing or chemical inhibition of autophagy protected from cell death in cell culture models of AD.49 Similarly, pharmacological induction of autophagy via rapamycin turned out to be neurotoxic in alternative scenarios than those where protection was observed.50,51 These data indicate that a tightly controlled balance of autophagic processes is mandatory for neuronal survival.
The herein presented data show that spermidine-mediated neuroprotection in both D. melanogaster and C. elegans models for αSyn-toxicity is accompanied by an induction of autophagy. Even though the causal involvement of autophagic processes in this protection remains to be elucidated, these results are in line with studies reporting an impairment of autophagy upon high gene doses of αSyn52-54 and enhanced neuroprotection upon induction of autophagy by pharmacological and genetic means such as treatment with resveratrol, trehalose, metformin, or rapamycin or overexpression of Beclin-1 or TFEB, the major transcriptional regulator of the autophagic pathway.43, 55-58 Altogether these studies place the fine-tuning of autophagy regulation rather than autophagy itself, which as a degradative process is not intrinsically protective, at the core of neuronal viability during PD. Our results describe a scenario relevant at the organismal level that may help unveil active regulatory elements involved in protective neuroautophagy upon αSyn-toxicity.
Materials and Methods
Fly stocks and manganese resistance
All experiments were performed in isogenized w1118 background and flies were kept on standard cornmeal-molasses medium at 25°C with a 12/12 hours light/dark cycle. The line UAS-α-synuclein (#8146) was obtained from the Bloomington Stock Center (Indiana University, USA). A chromosome x-linked elav-GAL4 line was used to drive expression of αSyn in a temporally and spatially controlled way in all pan-neuronal cells. To determine survival and climbing activity upon challenge with manganese, 1- to 3-day-old male F1 flies (separated under mild CO2 anesthesia) were kept in small vials with fresh food for 24 h at 25°C to allow regeneration and transferred to 29°C for additional 24 h for enhancement of expression. Subsequently, flies were transferred into fresh vials with filter papers soaked with solution containing 10% sucrose and 20 mM MnCl2 (pH 6.0) as previously described.31,32 For spermidine treatment, 5 mM spermidine (Sigma-Aldrich S2626) was added to the sucrose/manganese solution and the pH was adjusted to pH 6.0. Throughout the experiments, flies were kept at 25°C, filters were kept wet at all times and dead flies were counted at indicated time points. For each genotype and condition, 5–6 cohorts of flies with 30–40 flies per cohort were tested and results were analyzed using 2-way ANOVA followed by Bonferroni post-hoc test.
Locomotor activity
Locomotion ability of manganese-stressed flies was performed as described previously.28 Briefly, flies were transferred to an empty vial and were allowed to adapt to the dark for half an hour. Under red light conditions the flies were gently tapped to the bottom of the vial and the number of animals that could climb a defined height (7 cm) was recorded after 15 seconds. The same flies were tested after 24 h and 48 h of manganese treatment and additional supplementation with or without 5 mM spermidine. For each condition, 150–180 flies were tested and results were analyzed using one-way ANOVA followed by Bonferroni post-hoc test.
Immunoblotting
For protein extraction 20–30 fly heads were homogenized in 2% SDS and 1% Triton X-100 final concentration. Laemmli buffer was added and head extracts were incubated at 95°C for 5 minutes. After centrifugation at 13.400 rpm for 2 minutes to pellet residual fly head debris, an equivalent of 2 fly heads for each condition was loaded onto 12% acrylamide gels for protein separation using standard SDS-PAGE followed by immunoblotting. Blots were probed with the following antibodies: rabbit anti-α-Synuclein (Sigma; 1:1000), mouse anti-α-Tubulin (Sigma; 1:5000), goat anti-Rabbit IgG horseradish peroxidase conjugated (Dianova; 1:5000), goat anti-Mouse IgG horseradish-conjugated (Dianova; 1:5000). Rabbit anti-Atg8a (1:500) was kindly gifted from Gabor Juhasz, Eotvos Lorand University, Budapest, Hungary.59 Immunoblots were densitometrically quantified, and Atg8a-II signal was normalized to respective α-Tubulin signal to measure any change in Atg8a-II signal.
C. elegans strains, genetics and neurodegeneration analysis
We followed standard procedures for C. elegans strain maintenance and genetic crosses.60 Nematode rearing temperature was kept at 20°C. The following strains were used in this study: BZ555: egIs1[pdat-1GFP], UA44: baIn11[pdat-1 αSyn, pdat-1GFP], N2Ex[plgg-1DsRED::LGG-1]; baIn11[pdat-1αSyn, pdat-1GFP]. The BZ555 and UA44 strains were generously provided by Guy Caldwell (Department of Biological Sciences, University of Alabama). Seven-day old adult animals were used for the analysis of αSyn-induced neurodegeneration. The 4 CEP (cephalic) dopaminergic neurons in the head of the worm were scored as described previously.37 Thirty to forty worms were analyzed per condition and experiment and the average of at least 4 independent experiments was reported. Statistical analysis was performed using the GraphPad Prism software package (GraphPad Software Inc.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Austrian Science Fund FWF (grant V235-B09 to SB, and grants SFB-LIPOTOX F3007, F3012, P23490-B12, P24381-B20, W1226-B18, and DK-MCD to FM), the European Research Council (ERC, to NT), the Deutsche Forschungsgemeinschaft (Exc257: FOR1363 and SI 8494-1 to SJS) as well as the Freie Universität Berlin (Focus Area DynAge to SJS). TE is recipient of an APART fellowship of the Austrian Academy of Sciences at the Institute of Molecular Biosciences, University of Graz. In addition, this work was supported by grants to GK from the Ligue Nationale contre le Cancer (Equipes labellisée), Agence Nationale pour la Recherche (ANR), the Longevity Research Chair of the AXA Foundation, Association pour la Recherche sur le Cancer, European Commission (ArtForce), European Research Council (Advanced Investigator Award), Fondation pour la Recherche Médicale, Institut National du Cancer, Cancéropôle Ile-de-France, Fondation Bettencourt-Schueller, the LabEx Onco-Immunology, and the Paris Alliance of Cancer Research Institutes.
References
- 1. Bové J, Martínez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci 2011; 12:437-52; http://dx.doi.org/ 10.1038/nrn3068 [DOI] [PubMed] [Google Scholar]
- 2. García-Arencibia M, Hochfeld WE, Toh PPC, Rubinsztein DC. Autophagy, a guardian against neurodegeneration. Semin Cell Dev Biol 2010; 21:691-8; http://dx.doi.org/ 10.1016/j.semcdb.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006; 443:787-95; PMID:17051205; http://dx.doi.org/ 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- 4. Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci 2010; 13:805-11; PMID:20581817; http://dx.doi.org/ 10.1038/nn.2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jansen AHP, Reits EAJ, Hol EM. The ubiquitin proteasome system in glia and its role in neurodegenerative diseases. Front Mol Neurosci 2014; 7:73; PMID:25152710; http://dx.doi.org/ 10.3389/fnmol.2014.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dantuma NP, Bott LC. The ubiquitin-proteasome system in neurodegenerative diseases: precipitating factor, yet part of the solution. Front Mol Neurosci 2014; 7:70; PMID:25132814; http://dx.doi.org/ 10.3389/fnmol.2014.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Leeuwen FW, de Kleijn DP, van den Hurk HH, Neubauer A, Sonnemans MA, Sluijs JA, Köycü S, Ramdjielal RD, Salehi A, Martens GJ, et al. Frameshift mutants of beta amyloid precursor protein and ubiquitin-B in Alzheimer's and Down patients. Science 1998; 279:242-7; PMID:9422699; http://dx.doi.org/ 10.1126/science.279.5348.242 [DOI] [PubMed] [Google Scholar]
- 8. De Pril R, Fischer DF, Maat-Schieman MLC, Hobo B, de Vos RAI, Brunt ER, Hol EM, Roos RAC, van Leeuwen FW. Accumulation of aberrant ubiquitin induces aggregate formation and cell death in polyglutamine diseases. Hum Mol Genet 2004; 13:1803-13; PMID:15198995; http://dx.doi.org/ 10.1093/hmg/ddh188 [DOI] [PubMed] [Google Scholar]
- 9. De Pril R, Hobo B, van Tijn P, Roos RAC, van Leeuwen FW, Fischer DF. Modest proteasomal inhibition by aberrant ubiquitin exacerbates aggregate formation in a Huntington disease mouse model. Mol Cell Neurosci 2010; 43:281-6; PMID:20005957; http://dx.doi.org/ 10.1016/j.mcn.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 10. De Vrij FM, Sluijs JA, Gregori L, Fischer DF, Hermens WT, Goldgaber D, Verhaagen J, Van Leeuwen FW, Hol EM. Mutant ubiquitin expressed in Alzheimer's disease causes neuronal death. FASEB J Off Publ Fed Am Soc Exp Biol 2001; 15:2680-8. [DOI] [PubMed] [Google Scholar]
- 11. Dennissen FJA, Kholod N, Steinbusch HWM, Van Leeuwen FW. Misframed proteins and neurodegeneration: a novel view on Alzheimer's and Parkinson's diseases. Neurodegener Dis 2010; 7:76-9; PMID:20173331; http://dx.doi.org/ 10.1159/000285510 [DOI] [PubMed] [Google Scholar]
- 12. Krutauz D, Reis N, Nakasone MA, Siman P, Zhang D, Kirkpatrick DS, Gygi SP, Brik A, Fushman D, Glickman MH. Extended ubiquitin species are protein-based DUB inhibitors. Nat Chem Biol 2014; 10:664-70; PMID:24997605; http://dx.doi.org/ 10.1038/nchembio.1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chu CT. Diversity in the regulation of autophagy and mitophagy: lessons from Parkinson's disease. Park Dis 2011; 2011:789431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lachenmayer ML, Yue Z. Genetic animal models for evaluating the role of autophagy in etiopathogenesis of Parkinson disease. Autophagy 2012; 8:1837-8; PMID:22931754; http://dx.doi.org/ 10.4161/auto.21859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plotegher N, Civiero L. Neuronal Autophagy, α-Synuclein Clearance, and LRRK2 Regulation: A Lost Equilibrium in Parkinsonian Brain. J Neurosci 2012; 32:14851-3; PMID:23100407; http://dx.doi.org/ 10.1523/JNEUROSCI.3588-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viscomi MT, D’Amelio M. The “Janus-faced role” of autophagy in neuronal sickness: focus on neurodegeneration. Mol Neurobiol 2012; 46:513-21; PMID:22773113; http://dx.doi.org/ 10.1007/s12035-012-8296-3 [DOI] [PubMed] [Google Scholar]
- 17. Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med 2013; 19:983-97; PMID:23921753; http://dx.doi.org/ 10.1038/nm.3232 [DOI] [PubMed] [Google Scholar]
- 18. Nixon RA, Yang D-S. Autophagy failure in Alzheimer's disease-locating the primary defect. Neurobiol Dis 2011; 43:38-45; PMID:21296668; http://dx.doi.org/ 10.1016/j.nbd.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol 2012; 8:108-17; http://dx.doi.org/ 10.1038/nrneurol.2011.200 [DOI] [PubMed] [Google Scholar]
- 20. Renna M, Jimenez-Sanchez M, Sarkar S, Rubinsztein DC. Chemical Inducers of Autophagy That Enhance the Clearance of Mutant Proteins in Neurodegenerative Diseases. J Biol Chem 2010; 285:11061-7; PMID:20147746; http://dx.doi.org/ 10.1074/jbc.R109.072181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging 2011; 3:716-32; PMID:21869457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishimura K, Shiina R, Kashiwagi K, Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem (Tokyo) 2006; 139:81-90; http://dx.doi.org/ 10.1093/jb/mvj003 [DOI] [PubMed] [Google Scholar]
- 23. Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, Kramer JM, Liu KSY, Schroeder S, Stunnenberg HG, et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci 2013; 16:1453-60; PMID:23995066; http://dx.doi.org/ 10.1038/nn.3512 [DOI] [PubMed] [Google Scholar]
- 24. Liu P, Gupta N, Jing Y, Zhang H. Age-related changes in polyamines in memory-associated brain structures in rats. Neuroscience 2008; 155:789-96; PMID:18621105; http://dx.doi.org/ 10.1016/j.neuroscience.2008.06.033 [DOI] [PubMed] [Google Scholar]
- 25. Vivó M, de Vera N, Cortés R, Mengod G, Camón L, Martínez E. Polyamines in the basal ganglia of human brain. Influence of aging and degenerative movement disorders. Neurosci Lett 2001; 304:107-11; http://dx.doi.org/ 10.1016/S0304-3940(01)01776-1 [DOI] [PubMed] [Google Scholar]
- 26. Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 2009; 11:1305-14; PMID:19801973; http://dx.doi.org/ 10.1038/ncb1975 [DOI] [PubMed] [Google Scholar]
- 27. Wang I-F, Guo B-S, Liu Y-C, Wu C-C, Yang C-H, Tsai K-J, Shen C-KJ. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc Natl Acad Sci U S A 2012; 109:15024-9; PMID:22932872; http://dx.doi.org/ 10.1073/pnas.1206362109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Minois N, Carmona-Gutierrez D, Bauer MA, Rockenfeller P, Eisenberg T, Brandhorst S, Sigrist SJ, Kroemer G, Madeo F. Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis 2012; 3:e401; PMID:23059820; http://dx.doi.org/ 10.1038/cddis.2012.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature 2000; 404:394-8; PMID:10746727; http://dx.doi.org/ 10.1038/35006074 [DOI] [PubMed] [Google Scholar]
- 30. Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 2006; 313:324-8; PMID:16794039; http://dx.doi.org/ 10.1126/science.1129462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Büttner S, Faes L, Reichelt WN, Broeskamp F, Habernig L, Benke S, Kourtis N, Ruli D, Carmona-Gutierrez D, Eisenberg T, et al. The Ca(2+)/Mn(2+) ion-pump PMR1 links elevation of cytosolic Ca(2+) levels to α-synuclein toxicity in Parkinson's disease models. Cell Death Differ 2013. Mar;20(3):465-77; PMID:23154387; http://dx.doi.org/ 10.1038/cdd.2012.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Büttner S, Habernig L, Broeskamp F, Ruli D, Vögtle FN, Vlachos M, Macchi F, Küttner V, Carmona-Gutierrez D, Eisenberg T, et al. Endonuclease G mediates α-synuclein cytotoxicity during Parkinson's disease. EMBO J 2013; 32:3041-54; PMID:24129513; http://dx.doi.org/ 10.1038/emboj.2013.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C. Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat 2010; 31:763-80; PMID:20506312; http://dx.doi.org/ 10.1002/humu.21277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Dev Camb Engl 1993; 118:401-15 [DOI] [PubMed] [Google Scholar]
- 35. Powers KM, Smith-Weller T, Franklin GM, Longstreth WT. Jr, Swanson PD, Checkoway H. Parkinson's disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology 2003; 60:1761-6; PMID:12796527; http://dx.doi.org/ 10.1212/01.WNL.0000068021.13945.7F [DOI] [PubMed] [Google Scholar]
- 36. Cao S, Gelwix CC, Caldwell KA, Caldwell GA. Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. J Neurosci Off J Soc Neurosci 2005; 25:3801-12; http://dx.doi.org/ 10.1523/JNEUROSCI.5157-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qiao L, Hamamichi S, Caldwell KA, Caldwell GA, Yacoubian TA, Wilson S, Xie ZL, Speake LD, Parks R, Crabtree D, et al. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. MolBrain 2008; 1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Samara C, Syntichaki P, Tavernarakis N. Autophagy is required for necrotic cell death in Caenorhabditis elegans. Cell Death Differ 2008; 15:105-12; PMID:17901876; http://dx.doi.org/ 10.1038/sj.cdd.4402231 [DOI] [PubMed] [Google Scholar]
- 39. Levine B, Kroemer G. Autophagy in the Pathogenesis of Disease. Cell 2008; 132:27-42; PMID:18191218; http://dx.doi.org/ 10.1016/j.cell.2007.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jaeger PA, Wyss-Coray T. All-you-can-eat: autophagy in neurodegeneration and neuroprotection. Mol Neurodegener 2009; 4:16; PMID:19348680; http://dx.doi.org/ 10.1186/1750-1326-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pan P-Y, Yue Z. Genetic causes of Parkinson's disease and their links to autophagy regulation. Parkinsonism Relat Disord 2014; 20 Suppl 1:S154-7; PMID:24262170; http://dx.doi.org/ 10.1016/S1353-8020(13)70037-3 [DOI] [PubMed] [Google Scholar]
- 42. Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J Clin Invest 2008 Jun;118(6):2190-9; PMID: 18497889; http://dx.doi.org/ 10.1172/JCI33585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 Gene Transfer Activates Autophagy and Ameliorates the Neurodegenerative Pathology in α-Synuclein Models of Parkinson's and Lewy Body Diseases. J Neurosci 2009; 29:13578-88; PMID:19864570; http://dx.doi.org/ 10.1523/JNEUROSCI.4390-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006; 441:885-9; PMID:16625204; http://dx.doi.org/ 10.1038/nature04724 [DOI] [PubMed] [Google Scholar]
- 45. Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006; 441:880-4; PMID:16625205; http://dx.doi.org/ 10.1038/nature04723 [DOI] [PubMed] [Google Scholar]
- 46. Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O’Kane CJ, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet 2006; 15:433-42; PMID:16368705; http://dx.doi.org/ 10.1093/hmg/ddi458 [DOI] [PubMed] [Google Scholar]
- 47. Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 2004; 36:585-95; PMID:15146184; http://dx.doi.org/ 10.1038/ng1362 [DOI] [PubMed] [Google Scholar]
- 48. Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, et al. Inhibition of Autophagy Prevents Hippocampal Pyramidal Neuron Death after Hypoxic-Ischemic Injury. Am J Pathol 2008; 172:454-69; PMID:18187572; http://dx.doi.org/ 10.2353/ajpath.2008.070876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang H, Ma J, Tan Y, Wang Z, Sheng C, Chen S, Ding J. Amyloid-β1-42 Induces Reactive Oxygen Species-Mediated Autophagic Cell Death in U87 and SH-SY5Y Cells. J Alzheimers Dis 2010; 21:597-610; PMID:20571221 [DOI] [PubMed] [Google Scholar]
- 50. Chu CT, Zhu J, Dagda RK. Beclin 1-Independent Pathway of Damage-Induced Mitophagy and Autophagic Stress: Implications for Neurodegeneration and Cell Death. Autophagy 2007; 3:663-6; PMID:17622797; http://dx.doi.org/ 10.4161/auto.4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Plowey ED, Cherra SJ, Liu Y-J, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem 2008; 105:1048-56; PMID:18182054; http://dx.doi.org/ 10.1111/j.1471-4159.2008.05217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Song J-X, Lu J-H, Liu L-F, Chen L-L, Durairajan SSK, Yue Z, Zhang H-Q, Li M. HMGB1 is involved in autophagy inhibition caused by SNCA/α-synuclein overexpression: A process modulated by the natural autophagy inducer corynoxine B. Autophagy 2014; 10:144-54; PMID:24178442; http://dx.doi.org/ 10.4161/auto.26751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Winslow AR, Chen C-W, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, et al. α-Synuclein impairs macroautophagy: implications for Parkinson's disease. J Cell Biol 2010; 190:1023-37; PMID:20855506; http://dx.doi.org/ 10.1083/jcb.201003122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L. Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PloS One 2009; 4:e5515; PMID:19436756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Björklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci U S A 2013; 110:E1817-26; PMID:23610405; http://dx.doi.org/ 10.1073/pnas.1305623110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pérez-Revuelta BI, Hettich MM, Ciociaro A, Rotermund C, Kahle PJ, Krauss S, Di Monte DA. Metformin lowers Ser-129 phosphorylated α-synuclein levels via mTOR-dependent protein phosphatase 2A activation. Cell Death Dis 2014; 5:e1209; http://dx.doi.org/ 10.1038/cddis.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein. J Biol Chem 2007; 282:5641-52; PMID:17182613; http://dx.doi.org/ 10.1074/jbc.M609532200 [DOI] [PubMed] [Google Scholar]
- 58. Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals 2011; 19:163-74; PMID:21778691; http://dx.doi.org/ 10.1159/000328516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nagy P, Varga A, Pircs K, Hegedus K, Juhasz G. Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in Drosophila melanogaster. PLoS Genet 2013;9(8):e1003664; PMID: 23950728; http://dx.doi.org/4366476 10.1371/journal.pgen.1003664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brenner S. The genetics of Caenorhabditis elegans. Genetics 1974; 77:71-94; PMID:4366476 [DOI] [PMC free article] [PubMed] [Google Scholar]


