Inhibition of placental mechanistic target of rapamycin (mTOR) signalling, which activates NEDD4-2 (neural precursor cell expressed developmentally down-regulated protein 4-2) ubiquitin ligase leading to increased sodium-coupled neutral amino acid transporter 2 (SNAT-2) ubiquitination and removal from the syncytiotrophoblast plasma membrane may constitute a key mechanism underlying decreased placental amino acid transport in human IUGR.
Keywords: maternal–fetal exchange, mechanistic target of rapamycin (mTOR), pregnancy
Abstract
Placental amino acid transport is decreased in intrauterine growth restriction (IUGR); however, the underlying mechanisms remain largely unknown. We have shown that mechanistic target of rapamycin (mTOR) signalling regulates system A amino acid transport by modulating the ubiquitination and plasma membrane trafficking of sodium-coupled neutral amino acid transporter 2 (SNAT-2) in cultured primary human trophoblast cells. We hypothesize that IUGR is associated with (1) inhibition of placental mTORC1 and mTORC2 signalling pathways, (2) increased amino acid transporter ubiquitination in placental homogenates and (3) decreased protein expression of SNAT-2 in the syncytiotrophoblast microvillous plasma membrane (MVM). To test this hypothesis, we collected placental tissue and isolated MVM from women with pregnancies complicated by IUGR (n=25) and gestational age-matched women with appropriately grown control infants (n=19, birth weights between the twenty-fifth to seventy-fifth percentiles). The activity of mTORC1 and mTORC2 was decreased whereas the protein expression of the ubiquitin ligase NEDD4-2 (neural precursor cell expressed developmentally down-regulated protein 4-2; +72%, P<0.0001) and the ubiquitination of SNAT-2 (+180%, P<0.05) were increased in homogenates of IUGR placentas. Furthermore, IUGR was associated with decreased system A amino acid transport activity (–72%, P<0.0001) and SNAT-1 (–42%, P<0.05) and SNAT-2 (–31%, P<0.05) protein expression in MVM. In summary, these findings are consistent with the possibility that decreased placental mTOR activity causes down-regulation of placental system A activity by shifting SNAT-2 trafficking towards proteasomal degradation, thereby contributing to decreased fetal amino acid availability and restricted fetal growth in IUGR.
CLINICAL PERSPECTIVES
-
•
IUGR is a common pregnancy complication associated with neonatal morbidity and mortality and development of metabolic and cardiovascular disease later in life. The pathophysiological mechanisms leading to restricted fetal growth are poorly understood and no specific intervention is available. Placental amino acid transport is decreased in human IUGR, which is believed to contribute to decreased fetal amino acid availability and restricted fetal growth. However, the mechanisms causing decreased placental amino acid transport in IUGR are largely unknown.
-
•
We provide evidence consistent with the possibility that down-regulation of placental amino acid transport in the IUGR placenta is caused by inhibition of mTORC1 and mTORC2 signalling, which activates NEDD4-2 ubiquitin ligase leading to increased ubiquitination and decreased plasma membrane expression of the system A amino acid transporter isoform SNAT-2.
-
•
These findings will help us better understand the mechanisms underlying human IUGR and facilitate the development of specific intervention strategies.
INTRODUCTION
Intrauterine growth restriction (IUGR) is a pregnancy complication involving failure of the fetus to achieve its genetically determined growth potential. IUGR is not only associated with increased perinatal morbidity and mortality but also constitutes a risk factor for metabolic and cardiovascular disease in adult life [1,2]. The aetiology of IUGR is multi-factorial; however, the most common cause of restricted fetal growth in the industrialized world is placental dysfunction or insufficiency, which is associated with alterations in placental morphology, blood flow and nutrient and oxygen delivery [3]. The activity of several placental amino acid transporters is reduced in pregnancies complicated by IUGR. For example, the activity of system A, a Na+-dependent transporter mediating the uptake of non-essential amino acids, has been consistently shown to be lower in syncytiotrophoblast microvillous plasma membranes (MVM) isolated from IUGR placentas [4–6]. In addition, the activity of system A in MVM is related to the degree of fetal compromise in IUGR because term IUGR fetuses are less affected than preterm IUGR [6] and the most severe cases of IUGR, as defined by abnormal pulsatility index in the umbilical artery and abnormal fetal heart rate tracings, are associated with the most pronounced decreases in MVM system A activity [5]. Similarly, the activity of transporters of essential amino acids, including system β (mediating trans-port of taurine) and system L (transporting essential amino acids such as leucine), is reduced in MVM and/or syncytial basal plasma membranes of IUGR placentas [7,8]. These in vitro findings are consistent with studies using stable isotope techniques in pregnant women demonstrating that placental transfer of the essential amino acids leucine and phenylalanine is reduced in IUGR pregnancies at term [9]. However, although maternal nutrient restriction in animal models has been shown to cause down-regulation of specific amino acid transporter isoforms in the placental barrier [10], the mechanisms underlying the decreased placental amino acid transport in human IUGR and which specific transporter isoforms are affected remain unknown.
The serine/threonine kinase mechanistic target of rapamycin (mTOR) is a signalling pathway regulating cell growth and metabolism in response to nutrient availability and growth factor signalling by modulating gene expression and protein translation [11]. mTOR forms two distinct complexes, mTORC1 and mTORC2. In mTORC1, mTOR interacts with regulatory-associated protein of mTOR (raptor), whereas mTOR associates with the rapamycin-insensitive companion of mTOR (rictor) in mTORC2. The function of mTORC1 is mediated by phosphorylation of downstream targets, primarily eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) and p70 S6 kinase (S6K). Activation of mTORC2 promotes phosphorylation of protein kinase B (Akt), protein kinase C α (PKCα) and serum and glucocorticoid-regulated kinase 1 (SGK1). Placental mTORC1 signalling has been reported to be inhibited in human IUGR [12,13] and animal models of restricted fetal growth, such as maternal nutrient restriction [10]. Although some preliminary indications suggest that placental mTORC2 is inhibited in response to high altitude hypoxia [14], this placental signalling pathway has not previously been studied in pregnancies associated with placental insufficiency.
We have previously shown that both mTORC1 and mTORC2 constitute powerful positive regulators of amino acid transport in cultured primary human trophoblast cells [15,16] mediated by influencing the trafficking of specific transporter isoforms to the plasma membrane [16]. Neural precursor cell expressed developmentally down-regulated protein 4-2 (NEDD4-2) is an ubiquitin ligase that targets proteins for ubiquitination, which will lead to internalization and degradation. Hatanaka et al. [17] demonstrated that NEDD4-2 regulates system A amino acid transport activity in adipocytes by influencing the ubiquitination and therefore plasma membrane expression of the SNAT-2 (sodium-coupled neutral amino acid transporter 2) isoform. Consistent with these findings, we previously reported that mTORC1 silencing in cultured primary human trophoblast cells activates the ubiquitin ligase NEDD4-2 resulting in increased transporter ubiquitination, which removes the specific amino acid transporter isoforms SNAT-2 and LAT-1 (L-type amino acid transporter) from the plasma membrane [18].
In the present study, we tested the hypothesis that IUGR in human pregnancy is associated with (1) inhibition of placental mTORC1 and mTORC2 signalling pathways, (2) increased amino acid transporter ubiquitination in placental homogenates and (3) decreased protein expression of SNAT-2 in syncytiotrophoblast MVM.
MATERIALS AND METHODS
Study subjects and tissue collection
The present study was approved by the University of Western Ontario Health Sciences Research Ethics Board. Pregnant women attending St. Joseph's Health Care Centre, London, Ontario, Canada, were enrolled after informed consent was obtained. The recruitment procedure, including inclusion and exclusion criteria, has been described previously [19]. Briefly, women with singleton pregnancies delivered by either vaginal delivery or caesarean section were included. Pregnancies complicated by congenital abnormalities, diabetes, thyroid disorders, chronic hypertensive disorders or pre-eclampsia were exclusion criteria. The gestational age was determined from last menstrual period and corrected by ultrasound at first trimester. Control placentas were obtained from preterm deliveries (n=12, range 27–36 weeks of gestation) and from uncomplicated term pregnancies (n=7) with a birth weight between the twenty-fifth and seventy-fifth percentiles for corresponding gestational age. IUGR was defined as a birth weight less than the third percentile for gestational age. The placentas of the IUGR group were obtained from preterm (n=14, range 27.9–36.4 weeks of gestation) and term (n=11) deliveries. Placentas were obtained immediately after delivery. After removing decidua basalis and chorionic plate, 10 pieces of chorionic villus tissue were dissected from random locations representing the whole placenta and immediately snap frozen in liquid nitrogen. Prior to shipping samples to University of Colorado for analysis, samples and clinical information were de-identified.
Isolation of membrane vesicles
Syncytiotrophoblast MVM was prepared according to established protocols [20] with modifications [21]. In brief, after initial centrifugation steps to remove debris (at 4°C, in 250 mM sucrose, 10 mM Hepes–Tris and protease inhibitors, pH 7.4), MVM were isolated from placental homogenates by Mg2+ precipitation. Samples were frozen in liquid nitrogen and stored in–80°C until use. The protein concentration was determined using BCA assay and the MVM enrichment was assessed by measuring alkaline phosphatase activity [22]. MVM alkaline phosphatase activity in the control (26.1±4.8-fold, n=19) and IUGR (21.6±3.0-fold, n=25) groups was similar (P=0.407).
Western blot
Western blot analysis was performed, as previously described [10] and the sources of antibodies are provided in Supplementary Data. In brief, 7 μg (homogenates) or 3 μg (MVM) of total protein, was loaded on to a NuPAGE Novex (Invitrogen) precast 4%–12% Bis–Tris gels. Electrophoresis was performed at a constant voltage of 200 V for 40 min and proteins were transferred to nitrocellulose membranes at a constant 40 V. After transfer, membranes were blocked in 5% milk in Tris-buffered saline plus 0.1% Tween-20 for 1 h at room temperature. Membranes were incubated in primary antibodies overnight at 4°C and followed by incubation in corresponding secondary antibody for 1 h at room temperature. After washing, bands were visualized using ECL detection reagents (Pierce Biotechnology) and images obtained by G:Box Chemi system (Syngene). Target band densities were adjusted to loading by using β-actin or Ponceau Red as a control. There was no statistically significant difference in β-actin expression between the control and the IUGR groups (result not shown), validating the use of β-actin as a loading control. Blots were analysed by using ImageJ software (National Institutes of Health: imagej.nih.gov/ij/). For each target, the mean density of control sample bands was arbitrarily assigned a value of 1.0 and all individual density values were expressed relative to this mean for both groups.
Immunoprecipitation
Ubiquitin was immunoprecipitated from placental homogenates (n=5 in both IUGR and control groups with matched gestational age) using the Pierce Crosslink Immunoprecipitation Kit (Thermo Fisher Scientific).
System A activity
The system A transporter activity was determined by a modification of the protocol described by Mahendran et al. [4]. Vesicles were preloaded in incubation buffer (at 4°C, at 300 mM mannitol and 10 mM Hepes–Tris, pH 7.4) overnight. On the next day, vesicles were re-suspended in a small volume of the same buffer and then kept on ice until immediately prior to transport measurements when samples were warmed to 37°C. At time zero, 30 μl of vesicles were rapidly mixed with 37°C incubation buffer (150 mM NaCl, 10 mM Hepes–Tris, pH 7.4) containing 14C-methyl-aminoisobutyric acid (MeAIB, 150 μM) in a ratio of 1:2. After 30 s, uptake of MeAIB was terminated by adding 2 ml of ice-cold PBS and rapidly separating vesicles from the substrate medium by filtration on mixed ester filters (0.45 μm pore size, Millipore Corporation) and washed three times with 2 ml of PBS. Filters were dissolved in 2 ml of liquid scintillation fluid and counted. The uptake was expressed as pmol/(mg protein × 30 s). The Na+-dependent uptake of MeAIB, representing system A activity, was calculated by subtracting Na+-independent uptakes (using Na+ free buffers in which sodium chloride was replaced by choline chloride) from total uptakes.
Data presentation and statistics
The number of experiments (n) represents the number of different placentas studied. In the amino acid uptake experiments, each condition was studied in triplicate and data were averaged to represent a value for each placenta. Data are presented as mean ± S.E.M. or mean + S.E.M. Statistical significance of differences between control and IUGR groups was assessed using Student's t test or Mann–Whitney test (GraphPad Prism). A P-value <0.05 was considered significant.
RESULTS
Selected clinical data for the control and IUGR groups are listed in Table 1. There was no significant difference in maternal age, body mass index (BMI) or gestational age between the control and the IUGR groups. Birth weight was 28% lower (P<0.01) and placental weight was reduced by 36% (P<0.001) in the IUGR group compared with controls.
Table 1. Selected clinical data.
Data are presented as means ± S.E.M. Abbreviations: C, caesarean section; F, female; M, male; n, numbers; V, vaginal delivery. *n=10 (control) and 18 (IUGR); ‡by corresponding gestational age; †P<0.05; ∥P<0.01; § P<0.0001.
| Control (n=19) | IUGR (n=25) | |
|---|---|---|
| Maternal age (years) | 25.9±1.29 | 28.7±1.23 |
| BMI (kg/m2)* | 28.3±2.6 | 26.8±2.0 |
| Gestational age (weeks) | 33.9±0.95 | 35.7±0.61 |
| Birth weight (g) | 2493±236 | 1804±110† |
| Birth weight percentile‡ | 55.9±4.6 | 2.4±0.3§ |
| Placental weight (g) | 566±42.0 | 394±18.4║ |
| Fetal sex (M/F) | 7/12 | 8/17 |
| Mode of delivery (C/V) | 6/13 | 15/10 |
mTORC1 signalling
Placental mTORC1 activity was assessed by the phosphorylation of 4E-BP1, S6K1 and mTOR. Phosphorylation of 4E-BP1 at Thr37/46 was significantly decreased (–53%, P<0.05) in the IUGR group compared with control, whereas phosphorylation of 4E-BP1 at Thr70 was not significantly different between the two groups (Figures 1A and 1B). In contrast, total expression of 4E-BP1 (+119%, P<0.05) was increased in the IUGR group (Figures 1A and 1B). However, total and phosphorylated S6K1 and mTOR were unaffected by IUGR.
Figure 1. mTORC1 signalling.
(A) Representative Western blots for mTORC1 downstream targets: P-4E-BP1 Thr37/46, P-4E-BP1 Thr70, 4E-BP1, P-S6K1 Thr389, S6K1, P-mTOR Ser2448 and mTOR. (B) Histogram summarizing the Western blot data. *P<0.05; Mean + S.E.M.; unpaired Student's t test. Abbreviations: C, control; I, IUGR.
mTORC2 signalling
We used phosphorylation of Akt at Ser473, PKCα at Ser657 and SGK1 at Ser422 as functional read-outs for mTORC2 activity. In the IUGR group, both phosphorylation of Akt (–42%, P<0.05) and PKCα (–33%, P<0.01) were decreased as compared with control (Figures 2A and 2B). In addition, total expression of Akt was increased in the IUGR group (36%, P<0.05). No significant alteration was demonstrated in phosphorylation of SGK1 or total expression of PKCα and SGK1. The phosphorylation of Akt and PKCα was positively correlated with gestational age (r=0.44, P<0.05 and r=0.40, P<0.05, respectively) in the IUGR group (Figures 2C and 2D). In addition, phosphorylation of PKCα, but not Akt, was positively correlated with gestational age in the control group (r=0.529, P<0.05; Figure 2D).
Figure 2. mTORC2 signalling.
(A) Representative Western blots for P-Akt Ser473, Akt, P-PKCα Ser657, PKCα, P-SGK1 Ser422 and SGK1. (B) Histogram summarizing the Western blot data. *P<0.05; **P<0.01; Mean + S.E.M.; unpaired Student's t test. (C) There was a positive correlation between gestational age and placental P-Akt Ser473 in the IUGR group but not in the control group. (D) P-PKCα Ser657 was positively correlated to gestational age in both control and IUGR group. Abbreviations: C, control; I, IUGR.
Endoplasmic reticulum stress signalling and signal transducer and activator of transcription 3 signalling
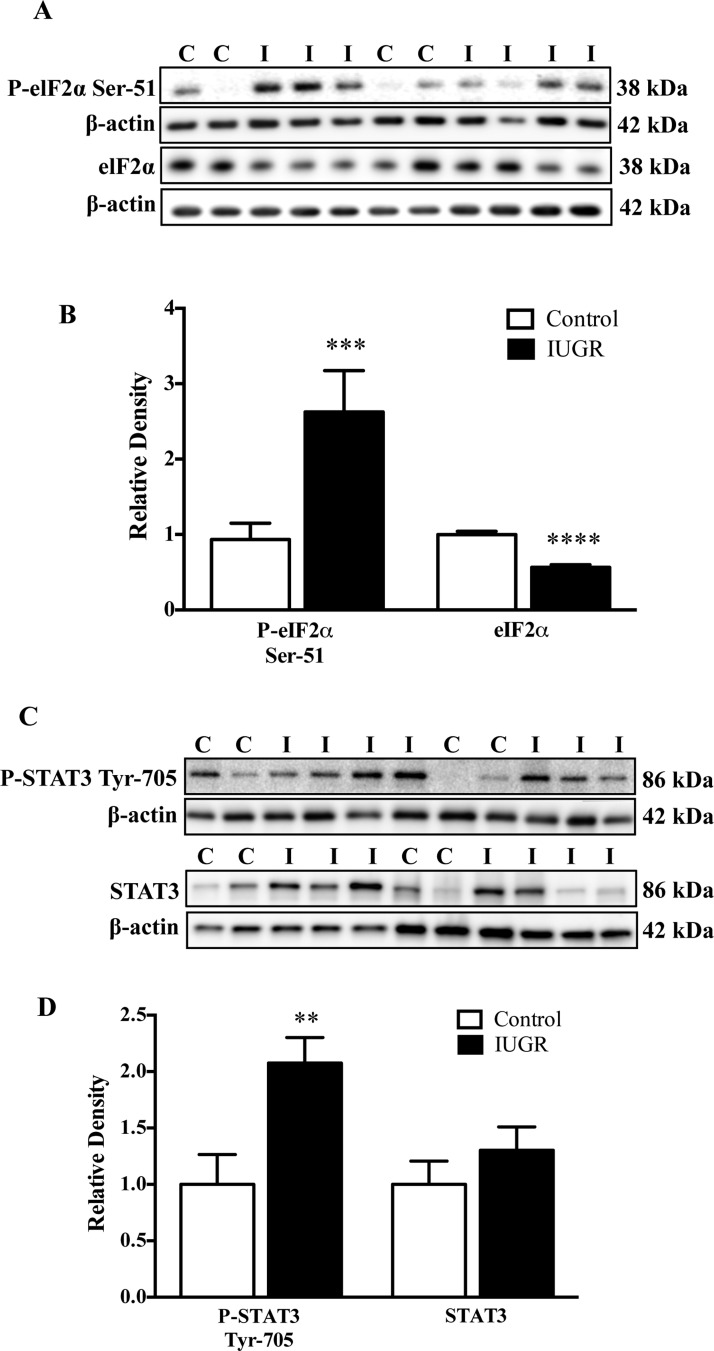
Endoplasmic reticulum (ER) stress has been implicated in IUGR pathophysiology and phosphorylation of eukaryotic initiation factor 2α (eIF2α) is associated with ER stress [13]. Phosphorylation of eIF2α at Ser51 was increased (+162%, P<0.001) whereas total eIF2α expression was decreased (–43%, P<0.0001) in IUGR placentas compared with control (Figures 3A and 3B). Signal transducer and activator of transcription 3 (STAT3) has been shown to be involved in the regulation of placental amino acid transporters [23]. Phosphorylation of STAT3 at Tyr705 was increased in IUGR placentas (+107%, P<0.01) whereas total STAT3 expression was not altered (Figures 3C and 3D).
Figure 3. ER stress signalling.
(A) Representative Western blots for P-eIF2α and eIF2α. (B) Histogram summarizing the Western blots data. (C) Representative Western blots for P-STAT3 Tyr705 and STAT. (D) Histogram summarizing the Western blots data. **P<0.01, ***P<0.001 and ****P<0.0001; mean + S.E.M.; unpaired Student's t test. Abbreviations: C, control; I, IUGR.
MVM nutrient transporter protein expression
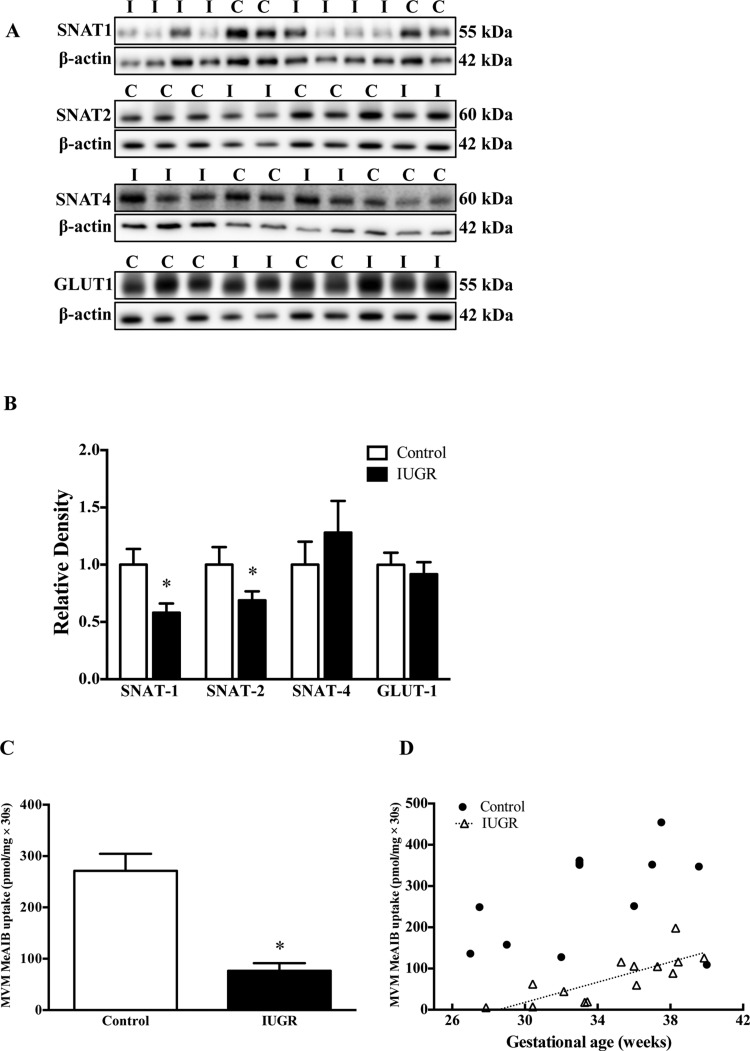
The protein expression of the system A amino acid transporter isoforms SNAT-1 (–42%, P<0.05) and SNAT-2 (–31%, P<0.05) was decreased in IUGR MVMs (Figures 4A and 4B). However, no significant change could be observed in the expression of the SNAT-4 isoform. The expression of SNAT-1 and SNAT-2 in placental homogenates was similar in IUGR and control groups (Supplementary Figure S1). Furthermore, the MVM expression of glucose transporter isoform GLUT-1 (glucose transporter 1) was not significantly altered (Figures 4A and 4B).
Figure 4. Protein expression of amino acid and glucose transporter isoforms and system A uptake in MVMs.
(A) Representative Western blots for SNAT-1, SNAT-2, SNAT-4 and GLUT-1. (B) Histogram summarizes the Western blot data. (C) MeAIB uptake into MVMs. (D) There was a significant positive correlation between gestational age and MVM System A activity in the IUGR group, but not in the control group. *P<0.05 compared with control; mean + S.E.M.; unpaired Student's t test. Abbreviations: C, control; I, IUGR.
MVM system A activity
The system A activity (76.5±14.9 pmol/mg × 30 s) in IUGR group was decreased by 72% as compared with the control group (271.1±33.3 pmol/mg × 30 s; Figure 4C, P<0.0001). In addition, the MVM system A activity was positively correlated to gestational age in the IUGR group (r=0.79, P<0.001), whereas no such relation was observed for the controls (Figure 4D).
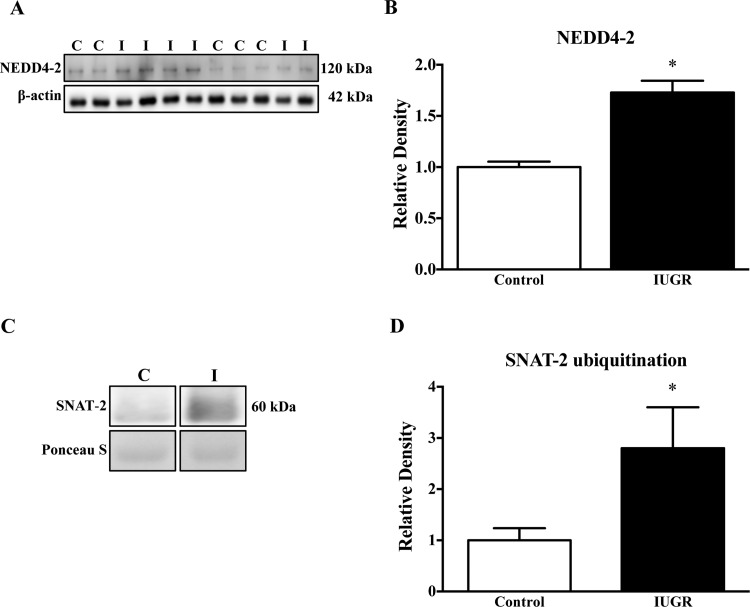
NEDD4-2 expression and ubiquitination of SNAT-2
The expression of NEDD4-2 in homogenates of IUGR placentas was significantly increased (+72%, P<0.0001) as compared with the controls (Figures 5A and 5B). To examine the ubiquitination of SNAT-2 in placental homogenates, we first performed immunoprecipitation for ubiquitin in the placental homogenates. Subsequently, the abundance of ubiquitinated SNAT-2 was assessed by immunoblotting. As shown in Figures 5(C) and 5(D), the ubiquitination of SNAT-2 in the IUGR group was markedly increased (+180%, P<0.05).
Figure 5. NEDD4-2 expression and ubiquitination of SNAT-2.
(A) Representative Western blots for NEED4-2 (B) Histogram summarizing the Western blot data. *P<0.05 compared with control; mean + S.E.M.; unpaired Student's t test. (C) Representative Western blots for SNAT-2 after immunoprecipitation for ubiquitin. (D) Histogram summarizing the Western blot data. *P<0.05 compared with control; mean + S.E.M.; non-parametric Mann–Whitney test. Abbreviations: C, control; I, IUGR.
DISCUSSION
We report for the first time a decreased protein expression of SNAT-2, an isoform of the system A amino acid transporter, in MVM isolated from human IUGR placentas, which was associated with increased SNAT-2 ubiquitination. Another novel aspect of our study is that we extend previous preliminary reports showing that mTORC2 signalling is inhibited in the IUGR placenta. These findings are consistent with the possibility that decreased placental mTOR activity causes down-regulation of placental system A activity by shifting SNAT-2 trafficking towards proteasomal degradation, thereby contributing to decreased fetal amino acid availability and restricted fetal growth in IUGR.
In the current study, we confirm previous reports of inhibition of placental mTORC1 signalling in the IUGR placenta [12,13]; however, only the 4E-BP1 branch of mTORC1 signalling was inhibited whereas the S6K signalling was unaffected. These findings are in general agreement with the demonstration that 4E-BP1 phosphorylation was decreased but total 4E-BP1 expression was markedly increased and S6K signalling was unaffected in a small number of placentas collected at high altitude [14]. 4E-BP1 prevents translation initiation by binding eukaryotic translation initiation factor 4E (eIF4E) and phosphorylation of 4E-BP1 interferes with this binding, thereby activating protein translation [24]. As a consequence, both the increased total expression of 4E-BP1 and the decreased phosphorylation of 4E-BP1 at Thr37/46 will promote binding of eIF4E resulting in inhibition of cap-dependent translation initiation in the IUGR placenta. Furthermore, we observed increased phosphorylation of eIF2α at Ser51 indicating ER-stress in the IUGR placenta, confirming a previous report [13]. Phosphorylation of eIF2α inhibits eIF2B and thus impairs general RNA translation [25]. Because previous high-resolution transcriptome-scale ribosome profiling following mTOR inhibition suggests that mTORC1 regulates mRNA translation largely mediated by the 4E-BPs [26], together with increased phosphorylation of eIF2α, we propose that one of the functional consequences of these changes is decreased protein synthesis in the IUGR placenta, which may contribute to decreased placental growth and ultimately fetal growth restriction.
mTORC2 regulates cellular metabolism through modulating Akt/PKB by phosphorylating this kinase on Ser473. In addition, phosphorylation of Thr308 by phosphoinositide-dependent kinase 1 (PDK1) [27] is required for full activation of Akt/PKB. Previously, Yung et al. [14] studied three placentas collected at 3600 m in Colorado, using placentas collected in London, England as controls. Although not statistically significant, fetal weights at high altitude tended to be lower than at sea level and high altitude hypoxia was associated with a marked inhibition of Akt phosphorylation at Ser473. In the present study, we extend these observations and show that phosphorylation of Akt (Ser473) and PKCα (Ser657) was decreased in IUGR placentas. mTORC2 has also been implicated in regulation of the cytoskeleton through PKCα [28]. Phosphorylation of SGK1, another downstream target of mTORC2, however was similar in IUGR and control groups. One possible explanation is that SGK1 is also regulated by kinases other than mTORC2, including PDK1 and phosphatidylinositide-3-kinase (PI3K) [29] and the unchanged phosphorylation of SGK1 Ser422 may be due to other kinases targeting this particular residue.
GLUT-1 expression was not altered in MVM isolated from IUGR placentas, in agreement with previous studies [6]. Several investigators have reported a decreased system A activity in MVM isolated from IUGR placentas [4–6], which we confirm in the present study. Of the three system A isoforms expressed in the human placenta, SNAT-2 is the one most often subjected to regulation. For example, MVM SNAT-2 expression and system A activity is increased in obese women giving birth to large babies [30]. In addition, interleukin (IL)-6 stimulates system A amino acid transporter activity in primary human trophoblast cells through STAT3 and increased gene expression of SNAT-2 [23] and placental SNAT-2 is down-regulated in response to maternal protein restriction in the rat [31] and following maternal calorie restriction in the baboon [10]. We have previously shown that both mTORC1 and mTORC2 regulate SNAT-2 in cultured primary human trophoblast cells by influencing the trafficking of this transporter isoform to the plasma membrane [16]. The finding that SNAT-2 protein expression was decreased in the MVM but not in total placental homogenate strongly suggests that the observed responses are caused by changes in SNAT-2 plasma membrane trafficking, implicating the inhibition of mTORC1 and mTORC2 as the mechanism underlying the decreased MVM SNAT-2 expression in IUGR.
Ubiquitination regulates degradation of cellular proteins by the ubiquitin–proteasome protein degradation pathway, controlling protein half-life and expression levels. This process involves sequential action of three distinct enzymes. NEDD4-2 is an ubiquitin ligase shown to be involved in the degradation of SNAT-2 in adipocytes [17] and we have previously reported a mechanistic link between mTOR inhibition, activation of NEDD4-2, increased SNAT-2 ubiquitination and decreased system A amino acid transport activity in cultured primary human trophoblast cells [18]. Our finding of increased SNAT-2 ubiquitination in the IUGR placenta in the present study supports the model that decreased placental mTOR activity causes down-regulation of placental system A activity by decreasing the plasma membrane abundance of SNAT-2. The exact mechanism involved remains to be established; however, the unchanged SNAT-2 protein abundance in homogenates could indicate intracellular retention of ubiquitinated protein, rather than increased protein breakdown.
Previous studies have reported that the reduction in SNAT-2 gene expression in IUGR placentas is associated with neither epigenetic modifications in SNAT-2 intron-1 nor single nt polymorphisms in that region [32]. In addition, decreased placental SNAT-2 gene expression could only be found in the most severe IUGR cases [32]. These findings suggest that the expression of SNAT-2 in IUGR placentas may not be regulated at transcriptional level and our results provide a possible mechanism for the SNAT-2 down-regulation in IUGR.
In contrast with SNAT-2, SNAT-1 appears not to be regulated by mTOR signalling [15] and the mechanisms mediating the decreased SNAT-1 protein expression in MVM isolated from IUGR placentas remain to be established. We previously reported that STAT3 is involved in the regulation of placental amino acid transporters [23] and decreased placental STAT3 phosphorylation was found in animal models with maternal protein restriction [31]. Nevertheless, we observed an increased placental STAT3 phosphorylation in IUGR and additional studies are required to elucidate the underlying mechanisms. Because STAT3 is regulated by many cytokines and hormones [33], one possibility is that increased inflammation in IUGR placentas may activate the STAT3 signalling pathway. However, our study did not address this hypothesis.
We have previously reported that, although the total expression or phosphorylation of many placental proteins is not influenced by labour, a small sub-group of proteins is [34]. Specifically, the phosphorylation of selected downstream targets of mTOR were found to be decreased in response to labour [34], raising some concerns about the different distribution of caesarean sections/vaginal deliveries between the control and the IUGR group in our study (Table 1). We therefore reassessed the key mTOR targets that were shown to have decreased phosphorylation in the IUGR group in the current study by comparing the phosphorylation of these targets between vaginal and caesarean deliveries. We found that the phosphorylation of 4E-BP1 Thr37/36, Akt Ser473 and PKCα Ser657 was not significantly different between vaginal and caesarean deliveries in the control or the IUGR group (result not shown). Importantly, the difference in phosphorylation between the control and the IUGR groups remained when vaginal deliveries and caesarean sections were analysed separately (Supplementary Figure S2), albeit this difference did not reach significance in the caesarean section group, most probably because of the relatively low number of caesarean sections in the control group. This suggests that our results are not significantly influenced by the different distribution of delivery modes between the two groups.
Fetal growth is highly dependent on amino acid availability and animal experiments have provided evidence in support of a causative link between changes in placental amino acid transport and altered fetal growth. For example, in pregnant rats subjected to protein restriction, down-regulation of placental amino acid transporter preceded the onset of IUGR [35]. We have identified one potential mechanism, involving inhibition of placental mTORC1 and mTORC2 signalling, activation of NEDD4-2 ubiquitin ligase and increased degradation of MVM SNAT-2, which contributes to decreased fetal amino acid availability and restricted fetal growth in human IUGR.
Abbreviations
- 4E-BP1
eukaryotic initiation factor 4E-binding protein 1
- Akt
protein kinase B
- BMI
body mass index
- eIF2α
eukaryotic initiation factor 2α
- eIF4E
eukaryotic translation initiation factor 4E
- ER
endoplasmic reticulum
- IUGR
intrauterine growth restriction
- LAT
L-type amino acid transporter
- MeAIB
14C-methyl-aminoisobutyric acid
- mTOR
mechanistic target of rapamycin
- MVM
microvillous plasma membrane
- NEDD4-2
neural precursor cell expressed developmentally down-regulated protein 4-2
- PDK1
phosphoinositide-dependent kinase 1
- PKCα
protein kinase C α
- S6K
p70 S6 kinase
- SGK1
serum and glucocorticoid-regulated kinase 1
- SNAT
sodium-coupled neutral amino acid transporter
- STAT3
signal transducer and activator of transcription 3
AUTHOR CONTRIBUTION
Yi-Yung Chen, Theresa Powell and Thomas Jansson were responsible for designing the experiments, interpretation of results and writing the manuscript. Majida Shehab and Madhulika Gupta acquired ethical approval, collected human placental samples and assisted in data interpretation and in writing the manuscript. Yi-Yung Chen and Fredrick Rosario conducted the experiments. All authors approved the paper for publication.
FUNDING
This work was supported by the National Institute of Health [grant number HD068370].
References
- 1.Zeitlin J., El Ayoubi M., Jarreau P.H., Draper E.S., Blondel B., Kunzel W., Cuttini M., Kaminski M., Gortner L., Van Reempts P., et al. Impact of fetal growth restriction on mortality and morbidity in a very preterm birth cohort. J. Pediatr. 2010;157:733–739. doi: 10.1016/j.jpeds.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Barker D.J., Gluckman P.D., Godfrey K.M., Harding J.E., Owens J.A., Robinson J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-A. [DOI] [PubMed] [Google Scholar]
- 3.Maulik D., Frances Evans J., Ragolia L. Fetal growth restriction: pathogenic mechanisms. Clin. Obstet. Gynecol. 2006;49:219–227. doi: 10.1097/00003081-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Mahendran D., Donnai P., Glazier J.D., D'Souza S.W., Boyd R.D., Sibley C.P. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr. Res. 1993;34:661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Glazier J.D., Cetin I., Perugino G., Ronzoni S., Grey A.M., Mahendran D., Marconi A.M., Pardi G., Sibley C.P. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr. Res. 1997;42:514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Jansson T., Ylven K., Wennergren M., Powell T.L. Glucose transport and system a activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- 7.Norberg S., Powell T.L., Jansson T. Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatr. Res. 1998;44:233–238. doi: 10.1203/00006450-199808000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Jansson T., Scholtbach V., Powell T.L. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr. Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Paolini C.L., Marconi A.M., Ronzoni S., Di Noio M., Fennessey P.V., Pardi G., Battaglia F.C. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J. Clin. Endocrinol. Metab. 2001;86:5427–5432. doi: 10.1210/jcem.86.11.8036. [DOI] [PubMed] [Google Scholar]
- 10.Kavitha J.V., Rosario F.J., Nijland M.J., McDonald T.J., Wu G., Kanai Y., Powell T.L., Nathanielsz P.W., Jansson T. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J. 2014;28:1294–1305. doi: 10.1096/fj.13-242271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Roos S., Jansson N., Palmberg I., Saljo K., Powell T.L., Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J. Physiol. 2007;582:449–459. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yung H.W., Calabrese S., Hynx D., Hemmings B.A., Cetin I., Charnock-Jones D.S., Burton G.J. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 2008;173:451–462. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yung H.W., Cox M., Tissot van Patot M., Burton G.J. Evidence of endoplasmic reticulum stress and protein synthesis inhibition in the placenta of non-native women at high altitude. FASEB J. 2012;26:1970–1981. doi: 10.1096/fj.11-190082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos S., Kanai Y., Prasad P.D., Powell T.L., Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am. J. Physiol. Cell Physiol. 2009;296:C142–C150. doi: 10.1152/ajpcell.00330.2008. [DOI] [PubMed] [Google Scholar]
- 16.Rosario F.J., Kanai Y., Powell T.L., Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J. Physiol. 2013;591:609–625. doi: 10.1113/jphysiol.2012.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatanaka T., Hatanaka Y., Setou M. Regulation of amino acid transporter ATA2 by ubiquitin ligase Nedd4-2. J. Biol. Chem. 2006;281:35922–35930. doi: 10.1074/jbc.M606577200. [DOI] [PubMed] [Google Scholar]
- 18.Rosario F., Powell T., Jansson T. Regulation of trophoblast amino acid transporter trafficking by mTOR complex 1 is mediated by the ubiquitin ligase nedd4-2. Reprod. Sci. 2012;19:122A. doi: 10.1177/1933719112436515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta M.B., Seferovic M.D., Liu S., Gratton R.J., Doherty-Kirby A., Lajoie G.A., Han V.K.M. Altered proteome profiles in maternal plasma in pregnancies with fetal growth restriction.pdf. Clin. Proteomics. 2006;2:169–184. doi: 10.1007/BF02752499. [DOI] [Google Scholar]
- 20.Illsley N.P., Wang Z.Q., Gray A., Sellers M.C., Jacobs M.M. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim. Biophys. Acta. 1990;1029:218–226. doi: 10.1016/0005-2736(90)90157-J. [DOI] [PubMed] [Google Scholar]
- 21.Johansson M., Jansson T., Powell T.L. Na(+)-K(+)-ATPase is distributed to microvillous and basal membrane of the syncytiotrophoblast in human placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R287–R294. doi: 10.1152/ajpregu.2000.279.1.R287. [DOI] [PubMed] [Google Scholar]
- 22.Bowers G.N., Jr McComb R.B. A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase. Clin. Chem. 1966;12:70–89. [PubMed] [Google Scholar]
- 23.Jones H.N., Jansson T., Powell T.L. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am. J. Physiol. Cell Physiol. 2009;297:C1228–C1235. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- 24.Mader S., Lee H., Pause A., Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol. Cell. Biol. 1995;15:4990–4997. doi: 10.1128/mcb.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnelly N., Gorman A.M., Gupta S., Samali A. The eIF2alpha kinases: their structures and functions. Cell Mol. Life Sci. 2013;70:3493–3511. doi: 10.1007/s00018-012-1252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thoreen C.C., Chantranupong L., Keys H.R., Wang T., Gray N.S., Sabatini D.M. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alessi D.R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 28.Sarbassov D.D., Ali S.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 29.Lang F., Strutz-Seebohm N., Seebohm G., Lang U.E. Significance of SGK1 in the regulation of neuronal function. J. Physiol. 2010;588:3349–3354. doi: 10.1113/jphysiol.2010.190926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansson N., Rosario F.J., Gaccioli F., Lager S., Jones H.N., Roos S., Jansson T., Powell T.L. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J. Clin. Endocrinol. Metab. 2013;98:105–113. doi: 10.1210/jc.2012-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosario F.J., Jansson N., Kanai Y., Prasad P.D., Powell T.L., Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology. 2011;152:1119–1129. doi: 10.1210/en.2010-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mando C., Tabano S., Pileri P., Colapietro P., Marino M.A., Avagliano L., Doi P., Bulfamante G., Miozzo M., Cetin I. SNAT2 expression and regulation in human growth-restricted placentas. Pediatr. Res. 2013;74:104–110. doi: 10.1038/pr.2013.83. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal B.B., Kunnumakkara A.B., Harikumar K.B., Gupta S.R., Tharakan S.T., Koca C., Dey S., Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann. N.Y. Acad. Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lager S., Aye I.L., Gaccioli F., Ramirez V.I., Jansson T., Powell T.L. Labor inhibits placental mechanistic target of rapamycin complex 1 signaling. Placenta. 2014;35:1007–1012. doi: 10.1016/j.placenta.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jansson N., Pettersson J., Haafiz A., Ericsson A., Palmberg I., Tranberg M., Ganapathy V., Powell T.L., Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J. Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]