Abstract
Platelets modulate vascular system integrity, and their loss is critical in haematological pathologies and after chemotherapy. Therefore, identification of molecules enhancing platelet production would be useful to counteract thrombocytopenia. We have previously shown that 2-arachidonoylglycerol (2-AG) acts as a true agonist of platelets, as well as it commits erythroid precursors toward the megakaryocytic lineage. Against this background, we sought to further interrogate the role of 2-AG in megakaryocyte/platelet physiology by investigating terminal differentiation, and subsequent thrombopoiesis. To this end, we used MEG-01 cells, a human megakaryoblastic cell line able to produce in vitro platelet-like particles.
2-AG increased the number of cells showing ruffled surface and enhanced surface expression of specific megakaryocyte/platelet surface antigens, typical hallmarks of terminal megakaryocytic differentiation and platelet production. Changes in cytoskeleton modeling also occurred in differentiated megakaryocytes and blebbing platelets. 2-AG acted by binding to CB1 and CB2 receptors, because specific antagonists reverted its effect. Platelets were split off from megakaryocytes and were functional: they contained the platelet-specific surface markers CD61 and CD49, whose levels increased following stimulation with a natural agonist like collagen. Given the importance of 2-AG for driving megakaryopoiesis and thrombopoiesis, not surprisingly we found that its hydrolytic enzymes were tightly controlled by classical inducers of megakaryocyte differentiation.
In conclusion 2-AG, by triggering megakaryocyte maturation and platelet release, may have clinical efficacy to counteract thrombocytopenia-related diseases.
Keywords: cluster of differentiation, cytoskeleton, Differentiation, endocannabinoid system, haematopoietic cells, megakaryocytes, platelets
Abbreviations
- 2-AG
2-arachidonoylglycerol
- AEA
anandamide
- APC
allophycocyanin
- CB1
type-1 cannabinoid receptor
- CB2
type-2 cannabinoid receptor
- CD
cluster of differentiation
- DAGL
diacylglycerol lipase
- eCB
endocannabinoid
- FAAH
fatty acid amide hydrolase
- FITC
fluorescein isothiocyanate
- HEL
human erythroleukemia
- MAGL
monoacylglycerol lipase
- PE
phycoerythrin
- TPA
12-O-tetradecanoylphorbol-13-acetate
Introduction
Platelets are cytoplasmic fragments released from megakaryocytes within bone marrow, which play a crucial role in haemostasis, by controlling thrombus formation and vascular tone, as well as by releasing soluble molecules needed for leukocyte-leukocyte and leukocyte-endothelium interactions.1,2 Platelets are also receiving growing attention in senescence, as platelet-derived growth factor has been reported to induce both senescence and cellular transformation in human fibroblasts;3 given that senescence plays a key role during oncogenesis and inflammation,3,4 the role of these anucleated cells is starting to be further investigated. Loss of platelets, due either to decreased survival in the periphery or to reduced production, can occur in several diseases, including thrombocytopenic purpura, acute leukemia, aplastic anaemia, multiple myeloma, HELLP (hemolysis, elevated liver enzymes, low platelets) and Scott (a rare bleeding disorder) syndromes.5-12 Thrombocytopenia is also a detrimental side effect of chemo- and radio-therapies, often resulting in bleeding episodes, chemotherapy dose reductions or re-scheduling.13-15 Up to date, the most effective approaches for treating the life-threatening complications of thrombocytopenia are platelet transfusion and supplementation with cytokines or thrombopoietic agents.16-19 Nonetheless, their use is limited by side effects, costs and availability of blood donors. Therefore, more efficacious treatments to raise platelet count are needed.
Endocannabinoids (eCBs) are lipid mediators, which exert their biological action by binding to type-1 (CB1) and type-2 (CB2) cannabinoid receptors, both localized to different extents in the central nervous system and peripheral tissues.20,21 To date, the most biologically active eCBs, N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG), together with other plant-derived CBs, are receiving growing attention due to their ability to regulate biological events, including cell death and differentiation.22-25
To date, both AEA and 2-AG can be listed among regulators of megakaryocyte/platelet functions, although they are endowed with distinct biological activity. AEA is a co-agonist of classical aggregating agents,26 and extends platelet survival by inhibiting pro-apoptotic cascades.27 Conversely, 2-AG is a true platelet agonist,28 and is an active megakaryopoietic agent.29 We have previously demonstrated that 2-AG (but not AEA) drives human erythroleukemia (HEL) cells toward megakaryocytic differentiation: physiological concentrations of 2-AG up-modulate the expression of megakaryocyte/platelet surface antigens, while down-modulating the expression of markers of erythroid phenotype.29 Although 2-AG has been proven to play a role in haematopoietic lineage determination, we could not investigate its role on terminal steps of the differentiation program, because HEL cells are not competent for platelet shedding.
Against this background, here we went further to interrogate the role of 2-AG on megakaryopoiesis and thrombopoiesis, exploiting human megakaryocytic MEG-01 cells that are able to produce platelet-like particles, structurally and functionally similar to freshly isolated human platelets, and commonly used to study megakaryocyte biology.30,31
Results
2-AG promotes terminal megakaryocytic differentiation
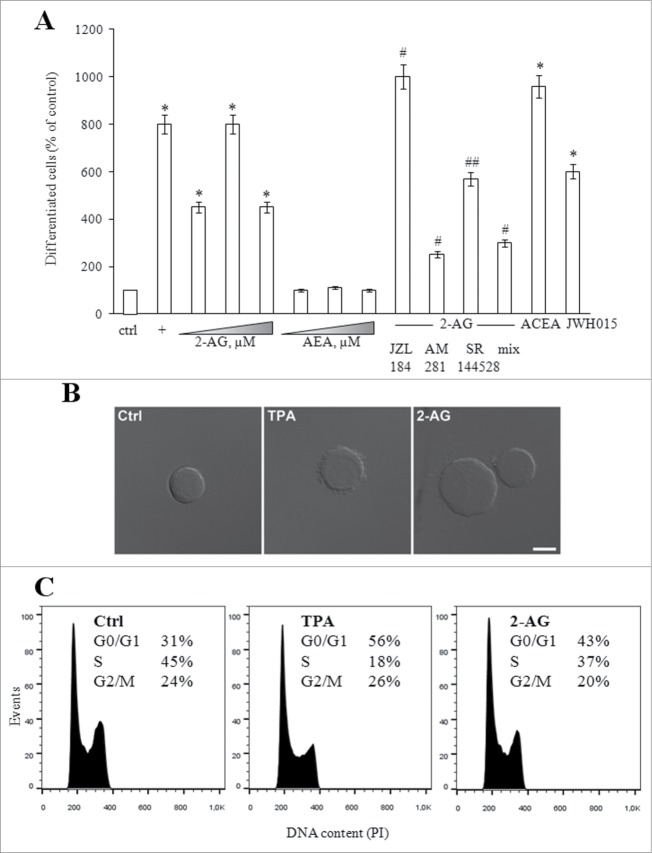
To evaluate the effect of eCBs on megakaryopoiesis, proliferating MEG-01 cells were grown for 24 hours in the presence of increasing concentrations (0.1–10 μM) of 2-AG or AEA, as well as of 10 nM TPA, a known inducer of megakaryocytic differentiation.31 Much alike TPA, 2-AG induced morphological changes associated with terminal differentiation: in the presence of 2-AG, cells increased adherence and showed irregular surface membrane with enhanced beaded extensions (Figs. 1A and B). The effect was dose-dependent, being already evident at 0.1 μM, and reaching a peak at 1 μM 2-AG (Fig. 1A). Conversely, AEA did not exert any effect (Fig. 1A).
Figure 1.
(See previous page). Effect of eCBs on mekagaryocyte differentiation. (A) MEG-01 cells were left untreated (ctrl) or treated with increasing concentrations (0.1-10 μM) of 2-AG or AEA, or with 10 nM TPA (+), or with 0.1 μM ACEA (CB1 agonist) or JWH015 (CB2 agonist); cells were also incubated with 1 μM 2-AG, after pre-incubation with 0.1 μM JZL184, 0.1 μM AM281 or 0.1 μM SR144528 [the last 2 compounds used alone or in combination (mix)]. After 24 hours, the percentage of differentiated cells was counted by an inverted microscope. Values are reported as percentage of control, set to 100% (absolute value = 10.0 ± 0.1% of differentiated cells). * P < 0.001 vs ctrl; # p < 0.01 and ## P< 0.05 vs 2-AG-treated cells. (B) Differential interference contrast micrographs of cells left untreated (ctrl) or treated with 10 nM TPA or 1μM 2-AG for 24 hours. Scale bar, 10 μm. (C) Cell-cycle analysis performed on cells treated as in (B); percentages of cells in G0/G1, S and G2/M phases are given in each panel.
To check whether the pro-differentiating effect of 2-AG could be mediated by its hydrolysis product arachidonic acid, we pre-treated cells with JZL184, a selective inhibitor of the 2-AG-degrading enzyme MAGL.32 Inhibition of MAGL led to a significant enhancement of differentiation, confirming the role of intact 2-AG itself (Fig. 1A). Cells were also pre-incubated with AM281 or SR144528 (specific antagonists of CB1 and CB2 receptors, respectively).33 Both compounds reduced the number of differentiated cells (Fig. 1A), with AM281 being more effective. Neither AM281 nor SR144528 affected cell morphology, when incubated alone (data not shown). Remarkably, the effects of both antagonists were not additive (Fig. 1A), speaking in favor of the main engagement of CB1 receptor subtype in 2-AG-dependent megakaryopoiesis. Additional proofs were obtained through incubation with ACEA (a CB1 agonist)34 and JWH015 (a CB2 agonist),35 which both increased the number of differentiated cells (Fig. 1A). Again, the effect was more evident in the presence of the CB1 agonist. Altogether, these data suggest that CB1 receptor, and to a lesser extent CB2 receptor, mediate 2-AG-dependent megakaryopoiesis.
Cell cycle was also analyzed. Although less potently than TPA, 2-AG significantly increased the portion of MEG-01 cells in the G0/G1 phase, while decreasing the percentage of cells in the S phase (Fig. 1C), thus suggesting that 2-AG lowered the proliferation rate and increased differentiation.
2-AG induces cytoskeleton remodelling in differentiating megakaryocytes
Microtubule and actin filaments play a crucial role in pro-platelet elongation and branching, respectively.36,37 This process depends on elaboration of a dense and highly organized array of cytoskeletal polymers, thus we investigated 2-AG-triggered morphological shape changes by confocal microscopy, after F-actin and tubulin staining. On the basis of membrane morphology, cells were categorized in 3 sub-populations, representing different stages of maturation (see also Fig. 1B):37 cells with smooth edges (undifferentiated and immature megakaryocytes), cells with membrane blebbing (rough cells) and cells extending cytoplasmic processes (spinous cells). The majority of control cells (77±10 %) displayed an undifferentiated phenotype, with a low percentage of rough (14±2 %) and spinous (9±2 %) cells (Fig. 2). TPA induced MEG-01 cells to become flat with the appearance of polylobulated nuclei and bleb-like structures in the plasma membrane (59±8 %) (Fig. 2) and cells exhibiting the spinous-phenotype represented 7±2 % of total cells. By contrast, cells cultured with 2-AG mostly assumed the spinous phenotype (52±8 %), characterized by marked cytoskeletal reorganization with formation of a circumferential band of microtubules just below the plasma membrane, loss of the centrosome, and accumulation of F-actin bundles in spiky filopodia-like protrusions (Fig. 2). The frequency of 2-AG-treated cells displaying membrane blebbing was below 10±3 %. Pre-incubation with AM281 partially reversed the effects of 2-AG, while SR144528 had only a small, yet not significant, effect (Fig. 2), suggesting that 2-AG triggered cytoskeletal re-organization mainly occurred via CB1 receptor.
Figure 2.
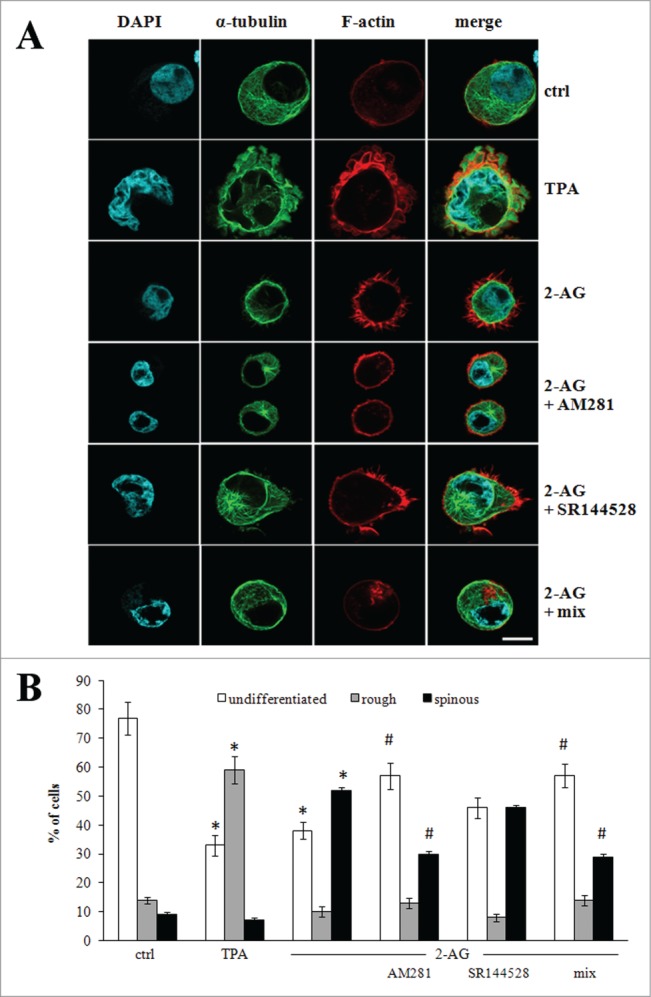
Cytoskeleton reorganization of MEG-01 cells. (A) Representative confocal microscopy images of cells left untreated (ctrl) or treated with 10 nM TPA or 1 μM 2-AG, the last being used alone or after pre-treatment with 0.1 μM AM281 or SR144528, incubated alone or in combination (mix). Cyan: nuclei stained with 4’,6-diamidino-2-phenylindole (DAPI). Green: α-tubulin. Red: F-actin. The last panel on the right is merging of the 3 fluorescence signals. Scale bar, 10 μm. Note the smooth surface in undifferentiated cell, the ruffled surface (rough cell) in the presence of TPA, and the thin cytoplasmic projections (spinous cell) in the presence of 2-AG. (B) Percentage of undifferentiated (white bars), rough (gray bars) and spinous (black bars) cells visualized in MEG-01 cells treated as in (A). Data refer to n = 27 cells analyzed in 3 independent experiments. * P< 0.001 vs the corresponding bar in ctrl cells; # P< 0.01 vs the corresponding bar in 2-AG-treated cells.
2-AG enhances expression of platelet-related markers
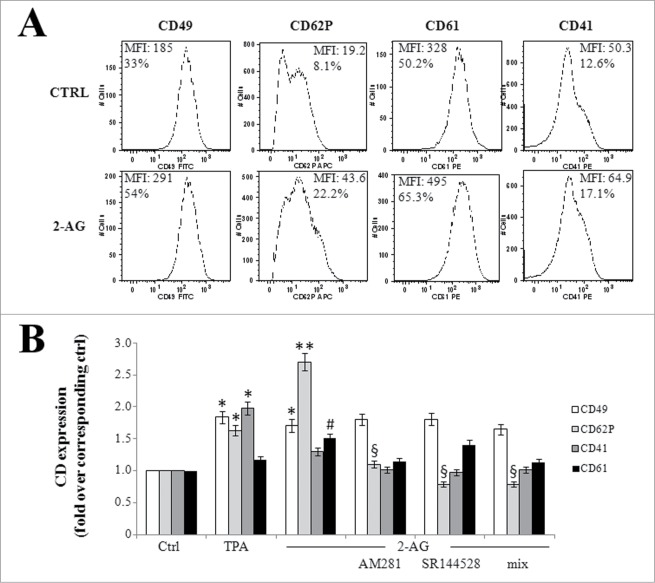
Next, we evaluated the expression of specific megakaryocyte/platelet surface antigens. Like TPA, 2-AG slightly increased (1.4 fold) the percentage of cells positive for α (CD41) and β (CD61) chains of fibrinogen receptor,38 and doubled surface expression of the very late antigen α 2 chain (CD49) of the collagen receptor (Fig. 3).39 Interestingly, the major effect was seen with expression of P-selectin (CD62P), a membrane glycoprotein exposed on megakaryocytes and activated platelets,40 that increased 3 fold over untreated cells (Fig. 3) and that we have already shown to be sensitive to 2-AG.41 Similarly to what we observed in endothelial cells,41 the positive effect on P-selectin exposure depended on activation of both CB receptors, as shown by AM281 and SR144528 treatment (Fig. 3). Conversely, the 2 antagonists seemed to have no effect on 2-AG-triggered modulation of other surface markers (Fig. 3), suggesting a mechanism of action specific for each molecule.
Figure 3.
Expression of megakaryocyte/platelet surface antigens. (A) Cells were left untreated (ctrl) or treated with 1 μM 2-AG for 24 hours, before staining with specific CD antibodies. The antibodies used were: FITC-conjugated CD49, APC-conjugated CD62P, PE-conjugated CD61, and PE-conjugated CD41. Mean fluorescent intensity (MFI) and percentage of positive cells are given for each panel. (B) Cells were left untreated (ctrl) or treated with 10 nM TPA, or with 1 μM 2-AG, used alone or after pre-incubation with 0.1 μM AM281 or SR144528 [the last 2 drugs used alone or in combination (mix)]. After 24 hours, immunophenotyping was performed by FACS analysis. Values are reported as fold over control, set to 1. * P < 0.01, ** P < 0.001 and # P < 0.001 vs the corresponding bar in ctrl cells; § P < 0.01 vs the corresponding bar in 2-AG-treated cells.
2-AG enhances production of functional platelet-like particles
We went further insight by evaluating the effect of 2-AG on the early stages of thrombopoiesis. To this aim, we evaluated production and release of platelet-like particles from mature megakaryocytes incubated for 24 hours with 2-AG. MEG-01 cells stimulated either with TPA or 2-AG significantly increased (about 10 fold) the number of platelet-like particles split off from megakaryocytes into the culture medium (Table 1). The use of specific agonists (ACEA and JWH015) and antagonist (AM281 and SR144528) confirmed, once again, the involvement of both CB receptors (data not shown). Next, we investigated whether platelets released from MEG-01 cells upon 2-AG stimulus were active and responsive to classical activators. To this end, isolated platelet-like particles were incubated with collagen, able to promote platelet activation and aggregation, and expression of specific surface markers was analyzed by FACS. Incidentally, platelets from both TPA- and 2-AG-treated MEG-01 cells seemed to be partially activated under basal conditions, maybe because of the prolonged exposure to the compounds. Nonetheless, collagen was able to increase granularity of platelet-like particles, as well as expression of fibrinogen and collagen receptors (Table 1), that were indicative of platelet activation because they play a key role during aggregation.38-40
Table 1.
Immuno-phenotypic characteristics of MEG-01-derived platelets.
| Platelets from control cells | Platelets from TPA-treated cells | Platelets from 2-AG-treated cells | Platelets from TPA-treated cells plus collagen | Platelets from 2-AG-treated cells plus collagen | |
|---|---|---|---|---|---|
| Parameter | |||||
| Number | 1 | 10.0 ± 0.8* | 9.2 ± 0.5* | - | - |
| Side scatter (SSC)a | 1 | 1.8 ± 0.1** | 1.8 ± 0.05** | 2.1 ± 0.1§ | 2.2 ± 0.1§ |
| Forward scatter (FSC)a | 1 | 0.6 ± 0.05 | 0.8 ± 0.02 | 0.7 ± 0.05§ | 1.0 ± 0.2§ |
| CD49a | 1 | 2.2 ± 0.1# | 3.3 ± 0.1# | 3.5 ± 0.1§ | 4.0 ± 0.1§ |
| CD61 a | 1 | 3.13 ± 0.1# | 2.1 ± 0.1# | 4.1 ± 0.1§ | 2.7 ± 0.1§ |
Platelet activation was achieved by treatment with 10 μg/ml collagen and analyzed by FACS. Results are expressed as fold over untreated MEG-01-derived platelets, set to 1. a Mean fluorescent intensity. * P < 0.001 vs control; ** P < 0.05 vs control; # P< 0.01 vs control; § P< 0.05 vs the corresponding control in basal conditions.
Megakaryocytes self-regulate 2-AG metabolism during differentiation
Given the importance of 2-AG for driving megakaryopoiesis and thrombopoiesis, we investigated whether megakaryocyte maturation per se modulates 2-AG tone. To this aim, we analyzed the activity of the major enzymes involved in 2-AG metabolism. We found that, when compared to proliferating cells, TPA-differentiated cells showed a significantly increased activity of both MAGL (1.6 fold) and FAAH (2.8 fold), which are responsible for 2-AG hydrolysis; under the same experimental conditions, no changes in the activity of DAGL, the 2-AG-biosynthesizing enzyme, was observed (Fig. 4A). Differentiated megakaryocytes regulated 2-AG activity also by acting at the level of its receptors; indeed, reduced expression of both CB1 and CB2 receptors on plasma membrane occurred during differentiation (Fig. 4B), confirming the ability of megakaryocytes to self-regulate the endogenous tone of 2-AG. This finding suggested a specific regulation of CB receptors along differentiation, as immature megakaryoblasts showed the highest expression,29 while platelets showed the lowest expression of these eCB-binding proteins.42
Figure 4.
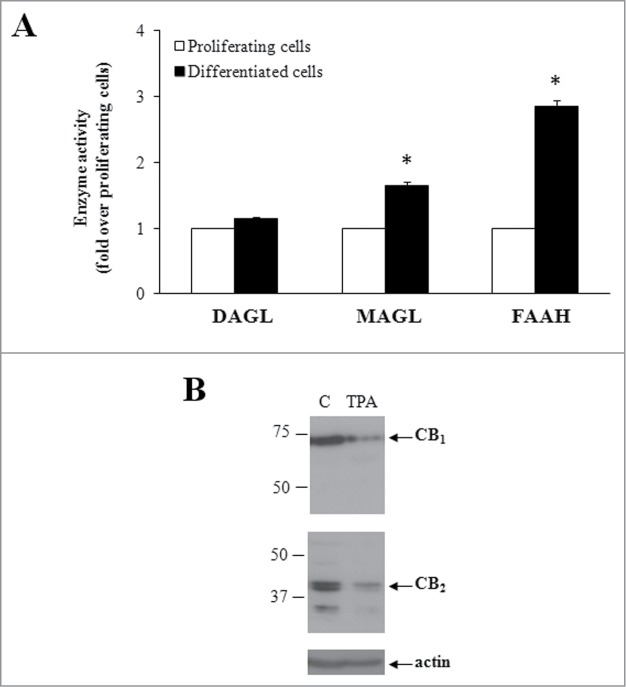
Modulation of the eCB system during differentiation. (A) Activity of 2-AG synthesising (DAGL) and degrading (MAGL, FAAH) enzymes, in proliferating (white bars) and TPA-differentiated (black bars) cells. Values are reported as fold over proliferating cells (absolute values: MAGL 69.1±9 .3; DAGL 8.1±0 .3; FAAH 28.5±1 .5 pmol/min/mg protein). * P < 0.05 vs proliferating cells. (B) Expression of CB receptors in proliferating (C) and TPA-differentiated cells by Western blotting. Molecular weights (kDa) are shown on the left-hand side. Gels are representative of 4 independent experiments.
Discussion
Megakaryocyte differentiation is composed of several consecutive stages, including formation of megakaryocytic progenitors, maturation of megakaryocytes, cell apoptosis, and production of platelets.43-47 Cytological changes and modulation of surface receptor expression distinguish each step, making these features suitable markers for every stage of maturation.48-50 In recent years, scientific progress has led to the production of functional platelets in vitro, in order to overcome problems related to platelet transfusion. However, large-scale production for clinical use is far from being achieved, because the number of platelets obtained in vitro is much lower than that in vivo, and manufactured platelets appear activated in the absence of agonists. Therefore, there is the need to define endogenously produced, regulatory factors essential for promoting platelet biogenesis (as well as to extend their lifespan), in order to develop novel strategies that generate functional platelets in adequate amounts.
By using an in vitro model, we provided evidence that 2-AG may be one of these modulators, as it is able to stimulate megakaryocyte maturation and enhance platelet production. The megakaryoblastic cell line MEG-01, established from the bone marrow of a patient with chronic myelogenous leukemia,30,31 represents a useful model for the study of human megakaryopoiesis. MEG-01 cells treated with 2-AG underwent differentiation, characterized by loss of rounded morphology and gaining of a spinous phenotype with extended cytoplasmic protrusions, and by expression of surface markers specific for late stages of megakaryocyte maturation. These findings are in agreement with our previous data on the bi-potent erythroleukemia HEL cell line, showing that 2-AG was a specific signal for the megakaryocytic phenotype.29
2-AG not only stimulated MEG-01 maturation, but it also had a platelet production-enhancing effect. Indeed, platelet-like particles could be recovered from the culture medium of 2-AG-treated MEG-01 cells, with a yield that was 8–10 times higher than the spontaneous one. These particles expressed platelet-specific receptors on their plasma membrane, and appeared to be functional: in response to classical agonists, including collagen, they raised the surface levels of specific markers important for blood coagulation, changed shape and became activated.
The platelet-enhancing effect of 2-AG, together with the pro-survival effect of AEA on platelets27 may have potential clinical significance in thrombocytopenias and myelosuppression triggered by radio- or chemo-therapy. The main advantage of 2-AG over classical thrombopoietic compounds resides in the fact that it is an endogenous lipid, whose tone can be finely-tuned by regulation of its synthesising and degrading enzymes. In this context, it is noteworthy that eCBs can be counted among factors that govern haematopoietic stem cell biology. In bone marrow, CB receptors are highly expressed in haematopoietic stem cells,51-53 and stromal cells release significant amounts of eCBs that modulate differentiation and migration, alone or in synergy with classical growth factors.51,52 Therefore, it is tempting to speculate that pharmacological alteration of 2-AG tone in specialized niches of bone marrow would affect self-renewal and differentiation of haematopoietic cells, as well as lineage commitment.54 In addition, owing to the specific effect of 2-AG on megakaryocyte precursors, it would be helpful to manage bone marrow failure and blood cell loss occurring in several pathological conditions. The flip side could be that 2-AG is a true platelet agonist, so its levels (as well as its acute vs chronic stimulation) should be kept tightly under control to avoid unwanted platelet activation, and to reduce some side-effects of 2-AG signaling at both central and peripheral levels. Indeed, activated platelets accumulate in the brain following injury and release factors that protect neurons from cell death, but the same anti-apoptotic cascade stimulated by activated platelets also provided chemo-resistance to several tumors.55 Moreover, it has recently been reported that prostaglandin E2 glyceryl ester (a COX-2-derived 2-AG metabolite) exacerbated excitotoxic damage,56 while epoxyeicosatrienoic acids (cytochrome P450 epoxygenase metabolites of arachidonic acid) protect from mitochondrial dysfunction and cell death.57 The dual function of 2-AG should be taken into account, especially considering that platelet hyperactivation is related to aging, inflammation and cancer.58,59 Age-related decline in cardiovascular function (and, therefore, susceptibility to thrombotic and inflammatory disorders) is often associated to increased levels of reactive oxygen species and oxidative stress;60,61 since aged platelets display increased NADPH oxidase expression and hydrogen peroxide generation, platelet hyperactivity may contribute to this phenomenon.62 Aging and activated platelets also modulate lineage-specific development, as well as the senescence program itself, thus promoting cell transformation and playing a role in the early progression to malignancy;63-66 in particular, by shedding microRNA-containing microvesicles, activated platelets may have clinical relevance in promoting tumor growth and spread,67 and potentially could be used as biomarkers, as circulating microRNA-containing microvesicles may differ according to cancer stage.68 Noticeably, mature megakaryocytes drop down 2-AG levels in order to ensure a correct homeostasis of eCB effects.29
Although promising for the chance to broaden the field of investigation, the thrombopoietic activity of 2-AG will have to be assessed under authentic in vivo conditions, in order to better understand the molecular mechanisms underlying megakaryopoiesis and thrombopoiesis.
Materials and Methods
Reagents
Chemicals were of the purest analytical grade. AEA, 2-AG, AM281, 12-O-tetradecanoylphorbol-13-acetate (TPA) and collagen type I were from Sigma Chemical Co. ACEA and JWH015, SR144528 and JZL184 were from Alexis Corporation.
Cell cultures
Human megakaryoblastic MEG-01 cells (ATCC) were grown in DMEM:F12 (1:1) medium (Invitrogen) supplemented with 2 mM L-glutamine and 20% heat-inactivated foetal bovine serum, at 37°C in a humidified atmosphere of 5% CO2. Differentiation was achieved by culturing cells in medium containing 10 nM TPA (positive control)69 or increasing concentrations of the tested compounds, for the indicated periods of time.
Western blotting
Membrane fractions (20 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electroblotted onto polyvinylidene difluoride membranes, incubated with primary antibodies and detected with chemiluminescence, as described.27
Enzymatic activities
Diacylglycerol lipase (DAGL) activity was evaluated following the release of [14C]2-AG from [14C]diacylglycerol, by thin layer chromatography and scintillation counting.70 Fatty acid amide hydrolase (FAAH) activity was evaluated measuring the release of [14C]ethanolamine from [14C]AEA, by scintillation counting.71 Monoacylglycerol lipase (MAGL) activity was assayed by measuring the release of [3H]glycerol from [3H]2-oleoyl-glycerol, by scintillation counting.72
Platelet isolation
Platelet-like particles were isolated from differentiated MEG-01 cells by sequential centrifugation, as reported.30 Briefly, cells were pelletted at 150 x g for 10 min and the resulting supernatant was centrifuged again at 500 x g for 15 min to remove all contaminant cells. Platelets were further centrifuged at 1000 x g for 15 min and resuspended in Tyrode's buffer (100 mM Hepes, 1.3 M NaCl, 29 mM KCl, 120 mM NaHCO3, pH 7.4), containing 1/10 (v/v) ACD (112 mM glucose, 130 mM citric acid, 152 mM sodium citrate) and 2 mM glucose and counted by an inverted microscope.
Flow cytometry
Immunophenotyping of cells was analyzed by flow cytometry in a FACSCanto instrument (Beckton Dickinson). Briefly, MEG-01 cells or platelet-like particles were fixed in 4% paraformaldehyde and stained with appropriate cluster of differentiation (CD) antibodies. The antibodies used were: phycoerythrin (PE)-conjugated CD61, fluorescein isothiocyanate (FITC)-conjugated CD49, PE-conjugated CD41 and allophycocyanin (APC)-conjugated CD62P (Beckton Dickinson). Platelets were also assessed for functionality, after treatment with 10 μg/mL collagen type I for 15 min at room temperature.
The cell cycle was evaluated by propidium iodide (50 μg/ml) staining, after prior incubation with 13 kunits/ml RNase A, as described.73
For each analysis, 10 thousand events were acquired and analyzed using the Flowjo software (TreeStar).
Confocal microscopy
MEG-01 cells, fixed on cover-glasses with 3% paraformaldehyde plus 4% sucrose for 20 min at room temperature, were blocked for 30 min with 5% bovine serum albumin in phosphate buffered saline, containing 0.1% saponin, before staining with primary anti-α-tubulin antibody (1:200; Sigma) for 1 hour. Then, cells were incubated with Alexa Fluor 488-conjugated secondary antibody (1:1000; Invitrogen) and Alexa Fluor 568-phalloidin (1:100; Invitrogen) for an additional 1 hour. Cellular localization of F-actin and α-tubulin was visualized by means of a Zeiss LSM400 confocal microscope, and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/). Differential interference contrast images were collected using a 40.0×1.25 objective.
Statistical analysis
All values are expressed as means ± SEM of at least 3 independent experiments, each performed in triplicate. The Student's unpaired t test or one-way ANOVA (followed by Bonferroni post-hoc analysis) were used to analyze experimental data by means of the InStat 3 program (GraphPAD). Significant differences were accepted at p < 0 .05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This investigation was supported by grants from Italian Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN 2009 to LA, PRIN 2010 to MM).
References
- 1. Jurk K, Kehrel BE. Platelets: physiology and biochemistry. Semin Thromb Hemost 2005; 31:381-92; PMID:16149014 [DOI] [PubMed] [Google Scholar]
- 2. Wagner DD, Burger PC. Platelets in Inflammation and Thrombosis. Arterioscler Thromb Vasc Biol 2003; 23:2131-7; PMID:14500287 [DOI] [PubMed] [Google Scholar]
- 3. Vindrieux D, Gras B, Garcia-Belinchon M, Mourah S, Lebbe C, Augert A, Bernard D. Platelet-derived growth factor B induces senescence and transformation in normal human fibroblasts. Aging 2013; 5:531-8; PMID:23934686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coleman PR, Chang G, Hutas G, Grimshaw M, Vadas MA, Gamble JR. Age-associated stresses induce an anti-inflammatory senescent phenotype in endothelial cells. Aging 2013; 5:913-24; PMID:24334613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feng X, Scheinberg P, Samsel L, Rios O, Chen J, McCoy JP. Jr, Ghanima W, Bussel JB, Young NS. Decreased plasma cytokines are associated with low platelet counts in aplastic anemia and immune thrombocytopenic purpura. J Thromb Haemost 2012; 10:1616-23; PMID:22537155; http://dx.doi.org/ 10.1111/j.1538-7836.2012.04757.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kwaan HC, Huyck T. Thromboembolic and bleeding complications in acute leukemia. Expert Rev Hematol 2010; 3:719-30; PMID:21091148 [DOI] [PubMed] [Google Scholar]
- 7. Lieu YK, Reddy EP. Impaired adult myeloid progenitor CMP and GMP cell function in conditional c-myb-knockout mice. Cell Cycle 2012; 11:3504-12; PMID:22918254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amodio N, Bellizzi D, Leotta M, Raimondi L, Biamonte L, D'Aquila P, Di Martino MT, Calimeri T, Rossi M, Lionetti M, et al. miR-29b induces SOCS-1 expression by promoter demethylation and negatively regulates migration of multiple myeloma and endothelial cells Cell Cycle 2013; 12:3650-62; PMID:24091729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walters DK, Arendt BK, Jelinek DF. CD147 regulates the expression of MCT1 and lactate export in multiple myeloma cells. Cell Cycle 2013; 12:3175-83; PMID:24013424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. John K, Wielgosz S, Schulze-Osthoff K, Bantel H, Hass R. Increased plasma levels of CK-18 as potential cell death biomarker in patients with HELLP syndrome. Cell Death Dis 2013; 4:e886; PMID:24157880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harper MT, Poole AW. Chloride channels are necessary for full platelet phosphatidylserine exposure and procoagulant activity. Cell Death Dis 2013; 4: e969; PMID:24357800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kmit A, van Kruchten R, Ousingsawat J, Mattheij NJ, Senden-Gijsbers B, Heemskerk JW, Schreiber R, Bevers EM, Kunzelmann K. Calcium-activated and apoptotic phospholipid scrambling induced by Ano6 can occur independently of Ano6 ion currents. Cell Death Dis 2013; 4:e611; PMID:23618909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baldo BA, Pham NH. Adverse reactions to targeted and non-targeted chemotherapeutic drugs with emphasis on hypersensitivity responses and the invasive metastatic switch. Cancer Metastasis Rev 2013; 32:723-61; PMID:24043487; http://dx.doi.org/ 10.1007/s10555-013-9447-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen W, Wu Q, Mo L, Nassi M. Intra-arterial chemotherapy is not superior to intravenous chemotherapy for malignant gliomas: a systematic review and meta-analysis. Eur Neurol 2013; 70:124-32; PMID:23859844 [DOI] [PubMed] [Google Scholar]
- 15. Dy GK, Adjei AA. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin 2013; 63:249-79; PMID:23716430; 10.3322/caac.21184 [DOI] [PubMed] [Google Scholar]
- 16. de Graaf CA, Metcalf D. Thrombopoietin and hematopoietic stem cells. Cell Cycle 2011; 10:1582-9; PMID:21478671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Demeter J, Istenes I, Fodor A, Paksi M, Dombi P, Valasinyószki E, Csomor J, Matolcsy A, Nagy ZG. Efficacy of romiplostim in the treatment of chemotherapy induced thrombocytopenia (CIT) in a patient with mantle cell lymphoma. Pathol Oncol Res 2011; 17:141-3; PMID:20628840; http://dx.doi.org/ 10.1007/s12253-010-9276-4 [DOI] [PubMed] [Google Scholar]
- 18. Thiagarajan P, Afshar-Kharghan V. Platelet transfusion therapy. Hematol Oncol Clin North Am 2013; 27:629-43; PMID:23714315; http://dx.doi.org/ 10.1016/j.hoc.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 19. Wu S, Zhang Y, Xu L, Dai Y, Teng Y, Ma S, Ho SH, Kim JM, Yu SS, Kim S, et al. Multicenter, randomized study of genetically modified recombinant human interleukin-11 to prevent chemotherapy-induced thrombocytopenia in cancer patients receiving chemotherapy. Support Care Cancer 2012; 20:1875-84; PMID:22041866; http://dx.doi.org/ 10.1007/s00520-011-1290-x [DOI] [PubMed] [Google Scholar]
- 20. Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, et al. Identification and functional characterization of brain stem cannabinoid CB2 receptors. Science 2005; 310:329-32; PMID:16224028 [DOI] [PubMed] [Google Scholar]
- 21. Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and CB1 cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signalling. Neuroscience 2003; 119:481-96; PMID:12770562 [DOI] [PubMed] [Google Scholar]
- 22. Dando I, Donadelli M, Costanzo C, Dalla Pozza E, D'Alessandro A, Zolla L, Palmieri M. Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis 2013; 4:e664; PMID:23764845; http://dx.doi.org/ 10.1038/cddis.2013.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rimmerman N, Ben-Hail D, Porat Z, Juknat A, Kozela E, Daniels MP, Connelly PS, Leishman E, Bradshaw HB, Shoshan-Barmatz V, et al. Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: a novel mechanism for cannabinoid-induced cell death, Cell Death Dis 2013; 4:e949; PMID:24309936; http://dx.doi.org/ 10.1038/cddis.2013.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vara D, Morell C, Rodríguez-Henche N, Diaz-Laviada I. Involvement of PPARγ in the antitumoral action of cannabinoids on hepatocellular carcinoma. Cell Death Dis 2013; 4:e618; PMID:23640460; http://dx.doi.org/ 10.1038/cddis.2013.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mecha M, Torrao AS, Mestre L, Carrillo-Salinas FJ, Mechoulam R, Guaza C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis 2012; 3:e331; PMID:22739983; http://dx.doi.org/ 10.1038/cddis.2012.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maccarrone M, Del Principe D, Finazzi Agrò A. Endocannabinoids: New physiological (co-) agonists of human platelets. Thromb Haemost 2002; 88:165-6; PMID:12152663 [PubMed] [Google Scholar]
- 27. Catani MV, Gasperi V, Evangelista D, Finazzi Agrò A, Avigliano L, Maccarrone M. Anandamide extends platelets survival through CB(1)-dependent Akt signalling. Cell Mol Life Sci 2010; 67:601-10; PMID:19936621; http://dx.doi.org/ 10.1007/s00018-009-0198-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malorni W, Bari M, Straface E, Battista N, Matarrese P, Finazzi-Agrò A, Del Principe D, Maccarrone M. Morphological evidence that 2-arachidonoylglycerol is a true agonist of human platelets. Thromb Haemost 2004; 92:1159-61; PMID:15543349 [PubMed] [Google Scholar]
- 29. Catani MV, Fezza F, Baldassarri S, Gasperi V, Bertoni A, Pasquariello N, Finazzi-Agrò A, Sinigaglia F, Avigliano L, Maccarrone M. Expression of the endocannabinoid system in the bi-potential HEL cell line: commitment to the megakaryoblastic lineage by 2-arachidonoylglycerol. J Mol Med 2009; 87:65-74; PMID:18820887; http://dx.doi.org/ 10.1007/s00109-008-0406-3 [DOI] [PubMed] [Google Scholar]
- 30. O'Brien JJ, Spinelli SL, Tober J, Blumberg N, Francis CW, Taubman MB, Palis J, Seweryniak KE, Gertz JM, Phipps RP. 15-deoxy-delta12,14-PGJ2 enhances platelet production from megakaryocytes. Blood 2008; 112:4051-60; PMID:18755987; http://dx.doi.org/ 10.1182/blood-2008-05-158535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takeuchi K, Satoh M, Kuno H, Yoshida T, Kondo H, Takeuchi M. Platelet-like particle formation in the human megakaryoblastic leukaemia cell lines, MEG-01 and MEG-01s. Br J Haematol 1998; 100:436-44; PMID:9488640 [DOI] [PubMed] [Google Scholar]
- 32. Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol 2009; 5:37-44; PMID:19029917; http://dx.doi.org/ 10.1038/nchembio.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 2010; 62:588-631; PMID:21079038; http://dx.doi.org/ 10.1124/pr.110.003004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J Pharmacol Exp Ther 1999; 289:1427-33; PMID:10336536 [PubMed] [Google Scholar]
- 35. Griffin G, Fernando SR, Ross RA, McKay NG, Ashford ML, Shire D, Huffman JW, Yu S, Lainton JA, Pertwee RG. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur J Pharmacol 1997; 339:53-61; PMID:9450616 [DOI] [PubMed] [Google Scholar]
- 36. Italiano JE, Jr, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol 1999; 147:1299-312; PMID:10601342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Machlus KR, Italiano JE. The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol 2013; 201:785-96; PMID:23751492; http://dx.doi.org/ 10.1083/jcb.201304054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Breton-Gorius J, Vainchenker W. Expression of platelet proteins during the in vitro and in vivo differentiation of megakaryocytes and morphological aspects of their maturation. Sem Hematol 1986; 23:43-67; PMID:3003921 [PubMed] [Google Scholar]
- 39. Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood 2003; 102:449-61; PMID:12649139 [DOI] [PubMed] [Google Scholar]
- 40. Berman CL, Yeo EL, Wencel-Drake JD, Furie BC, Ginsberg MH, Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest 1986; 78:130-7; PMID:2941452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gasperi V, Evangelista D, Chiurchiù V, Florenzano F, Savini I, Oddi S, Avigliano L, Catani MV, Maccarrone M. 2-Arachidonoylglycerol modulates human endothelial cell/leukocyte interactions by controlling selectin expression through CB1 and CB2 receptors. Int J Biochem Cell Biol 2014; 51:79-88; http://dx.doi.org/ 10.1016/j.biocel.2014.03.028 [DOI] [PubMed] [Google Scholar]
- 42. Catani MV, Gasperi V, Catanzaro G, Baldassarri S, Bertoni A, Sinigaglia F, Avigliano L, Maccarrone M. Human platelets express authentic CB₁ and CB₂ receptors. Curr Neurovasc Res 2010; 7:311-8; PMID:20854251 [DOI] [PubMed] [Google Scholar]
- 43. Abd-Elrahman I, Deutsch V, Pick M, Kay S, Neuman T, Perlman R, Ben-Yehuda D. Differential regulation of the apoptotic machinery during megakaryocyte differentiation and platelet production by inhibitor of apoptosis protein Livin. Cell Death Dis 2013; 4:e937; PMID:24287698; http://dx.doi.org/ 10.1038/cddis.2013.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen S, Su Y, Wang J. ROS-mediated platelet generation: a microenvironment-dependent manner for megakaryocyte proliferation, differentiation, and maturation. Cell Death Dis 2013; 4:e722; PMID:23846224; http://dx.doi.org/ 10.1038/cddis.2013.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lordier L, Pan J, Naim V, Jalil A, Badirou I, Rameau P, Larghero J, Debili N, Rosselli F, Vainchenker W, et al. Presence of a defect in karyokinesis during megakaryocyte endomitosis. Cell Cycle 2012; 11:4385-9; PMID:23159853; http://dx.doi.org/ 10.4161/cc.22712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rukoyatkina N, Mindukshev I, Walter U, Gambaryan S. Dual role of the p38 MAPK/cPLA2 pathway in the regulation of platelet apoptosis induced by ABT-737 and strong platelet agonists. Cell Death Dis 2013; 4:e931; PMID:24263105; http://dx.doi.org/ 10.1038/cddis.2013.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kodama T, Hikita H, Kawaguchi T, Shigekawa M, Shimizu S, Hayashi Y, Li W, Miyagi T, Hosui A, Tatsumi T, et al. Mcl-1 and Bcl-xL regulate Bak/Bax-dependent apoptosis of the megakaryocytic lineage at multistages. Cell Death Differ 2012; 19:1856-69; PMID:22790873; http://dx.doi.org/ 10.1038/cdd.2012.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fernández-Morales B, Pavón L, Calés C. CDC6 expression is regulated by lineage-specific transcription factor GATA1. Cell Cycle 2012; 11:3055-66; PMID:22871742; http://dx.doi.org/ 10.4161/cc.21471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Besancenot R, Roos-Weil D, Tonetti C, Abdelouahab H, Lacout C, Pasquier F, Willekens C, Rameau P, Lecluse Y, Micol JB, et al. JAK2 and MPL protein levels determine TPO-induced megakaryocyte proliferation versus differentiation. Blood 2014. pii: blood-2014-03-559815; PMID:25143485; http://dx.doi.org/ 10.1182/blood-2014-03-559815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smith BW, Murphy GJ. Stem cells, megakaryocytes, and platelets. Curr Opin Hematol 2014; 21:430-7; PMID:25023469; http://dx.doi.org/ 10.1097/MOH.0000000000000064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Patinkin D, Milman G, Breuer A, Fride E, Mechoulam R. Endocannabinoids as positive or negative factors in hematopoietic cell migration and differentiation. Eur J Pharmacol 2008; 595:1-6; PMID:18778813; http://dx.doi.org/ 10.1016/j.ejphar.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 52. Jiang S, Fu Y, Avraham HK. Regulation of hematopoietic stem cell trafficking and mobilization by the endocannabinoid system. Transfusion 2011; 51: 65S-71S; PMID:22074629; http://dx.doi.org/ 10.1111/j.1537-2995.2011.03368.x [DOI] [PubMed] [Google Scholar]
- 53. Jiang S, Alberich-Jorda M, Zagozdzon R, Parmar K, Fu Y, Mauch P, Banu N, Makriyannis A, Tenen DG, Avraham S, et al. Cannabinoid receptor 2 and its agonists mediate hematopoiesis and hematopoietic stem and progenitor cell mobilization. Blood 2011; 117:827-38; PMID:21063029; http://dx.doi.org/ 10.1182/blood-2010-01-265082 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54. Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, Bari M, Guzmán M, Maccarrone M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res 2013; 52:633-50; PMID:24076098; http://dx.doi.org/ 10.1016/j.plipres.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 55. Au AE, Sashindranath M, Borg RJ, Kleifeld O, Andrews RK, Gardiner EE, Medcalf RL, Samson AL. Activated platelets rescue apoptotic cells via paracrine activation of EGFR and DNA-dependent protein kinase. Cell Death Dis 2014; 5: e1410; PMID:25210793; http://dx.doi.org/ 10.1038/cddis.2014.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Valdeolivas S, Pazos MR, Bisogno T, Piscitelli F, Iannotti FA, Allarà M, Sagredo O, Di Marzo V, Fernández-Ruiz J. The inhibition of 2-arachidonoyl-glycerol (2-AG) biosynthesis, rather than enhancing striatal damage, protects striatal neurons from malonate-induced death: a potential role of cyclooxygenase-2-dependent metabolism of 2-AG. Cell Death Dis 2013; 4:e862; PMID:24136226; http://dx.doi.org/ 10.1038/cddis.2013.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Samokhvalov V, Alsaleh N, El-Sikhry HE, Jamieson KL, Chen CB, Lopaschuk DG, Carter C, Light PE, Manne R, Falck JR, et al. Epoxyeicosatrienoic acids protect cardiac cells during starvation by modulating an autophagic response. Cell Death Dis 2013; 4:e885; PMID:24157879; http://dx.doi.org/ 10.1038/cddis.2013.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mohebali D, Kaplan D, Carlisle M, Supiano MA, Rondina MT. Alterations in platelet function during aging: clinical correlations with thromboinflammatory disease in older adults. J Am Geriatr Soc 2014; 62:529-35; PMID:24512275; http://dx.doi.org/ 10.1111/jgs.12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dovizio M, Alberti S, Guillem-Llobat P, Patrignani P. Role of platelets in inflammation and cancer: novel therapeutic strategies. Basic Clin Pharmacol Toxicol 2014; 114:118-27; PMID:24118902; http://dx.doi.org/ 10.1111/bcpt.12156 [DOI] [PubMed] [Google Scholar]
- 60. Maynard S, Keijzers G, Gram M, Desler C, Bendix L, Budtz-Jørgensen E, Molbo D, Croteau DL, Osler M, Stevnsner T, et al. Relationships between human vitality and mitochondrial respiratory parameters, reactive oxygen species production and dNTP levels in peripheral blood mononuclear cells. Aging 2013; 5:850-64; PMID:24304678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shafique E, Choy WC, Liu Y, Feng J, Cordeiro B, Lyra A, Arafah M, Yassin-Kassab A, Zanetti AV, Clements RT, et al. Oxidative stress improves coronary endothelial function through activation of the pro-survival kinase AMPK. Aging 2013; 5:515-30; PMID:24018842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dayal S, Wilson KM, Motto DG, Miller FJ. Jr, Chauhan AK, Lentz SR. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation 2013; 127:1308-16; PMID:23426106; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.112.000966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tang Q, Koh LK, Jiang D, Schwarz H. CD137 ligand reverse signaling skews hematopoiesis towards myelopoiesis during aging. Aging 2013; 5:643-52; PMID:23945137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gautam S, Kirschnek S, Gentle IE, Kopiniok C, Henneke P, Häcker H, Malleret L, Belaaouaj A, Häcker G. Survival and differentiation defects contribute to neutropenia in glucose-6-phosphatase-β (G6PC3) deficiency in a model of mouse neutrophil granulocyte differentiation. Cell Death Differ 2013; 20:1068-79; PMID:23686134; http://dx.doi.org/ 10.1038/cdd.2013.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vasina EM, Cauwenberghs S, Staudt M, Feijge MA, Weber C, Koenen RR, Heemskerk JW. Aging- and activation-induced platelet microparticles suppress apoptosis in monocytic cells and differentially signal to proinflammatory mediator release. Am J Blood Res 2013; 3:107-23; PMID:23675563 [PMC free article] [PubMed] [Google Scholar]
- 66. Peche LY, Scolz M, Ladelfa MF, Monte M, Schneider C. MageA2 restrains cellular senescence by targeting the function of PMLIV/p53 axis at the PML-NBs. Cell Death Differ 2012; 19:926-36; PMID:22117195; http://dx.doi.org/ 10.1038/cdd.2011.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mezouar S, Mege D, Darbousset R, Farge D, Debourdeau P, Dignat-George F, Panicot-Dubois L, Dubois C. Involvement of platelet-derived microparticles in tumor progression and thrombosis. Semin Oncol 2014; 41:346-58; PMID:25023350; http://dx.doi.org/ 10.1053/j.seminoncol.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 68. Antonov AV, Knight RA, Melino G, Barlev NA, Tsvetkov PO. MIRUMIR: an online tool to test microRNAs as biomarkers to predict survival in cancer using multiple clinical data sets. Cell Death Differ 2013; 20:367; PMID:23175189; http://dx.doi.org/ 10.1038/cdd.2012.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ogura M, Morishima Y, Okumura M, Hotta T, Takamoto S, Ohno R, Hirabayashi N, Nagura H, Saito H. Functional and morphological differentiation induction of a human megakaryoblastic leukemia cell line (MEG-01) by phorbol diesters. Blood 1988; 72:49-60; PMID:2455575 [PubMed] [Google Scholar]
- 70. Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid knockout in the brain. J Cell Biol 2003; 163:463-8; PMID:14610053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gasperi V, Ceci R, Tantimonaco M, Talamonti E, Battista N, Parisi A, Florio R, Sabatini S, Rossi A, Maccarrone M. The fatty acid amide hydrolase in lymphocytes from sedentary and active subjects. Med Sci Sports Exerc 2014; 46:24-32; PMID:23793235; http://dx.doi.org/ 10.1249/MSS.0b013e3182a10ce6 [DOI] [PubMed] [Google Scholar]
- 72. Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA 2002; 99:10819-24; PMID:12136125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Savini I, Arnone R, Catani MV, Avigliano L. Origanum vulgare induces apoptosis in human colon cancer caco2 cells. Nutr Cancer 2009; 61:381-9; PMID:19373612; http://dx.doi.org/ 10.1080/01635580802582769 [DOI] [PubMed] [Google Scholar]