Abstract
Polyploid decidual cells are specifically differentiated cells during mouse uterine decidualization. However, little is known about the regulatory mechanism and physiological significance of polyploidization in pregnancy. Here we report a novel role of E2F8 in the polyploidization of decidual cells in mice. E2F8 is highly expressed in decidual cells and regulated by progesterone through HB-EGF/EGFR/ERK/STAT3 signaling pathway. E2F8 transcriptionally suppresses CDK1, thus triggering the polyploidization of decidual cells. E2F8-mediated polyploidization is a response to stresses which are accompanied by decidualization. Interestingly, polyploidization is not detected during human decidualization with the down-regulation of E2F8, indicating differential expression of E2F8 may lead to the difference of decidual cell polyploidization between mice and humans.
Keywords: E2F8, decidualization, polyploidization
Abbreviations
- CDK1
cyclin-dependent kinase 1
- CPT
Camptothecin
- E2
estrogen
- MKC
megakaryocyte
- PA
Purvalanol A
- P4
progesterone
- TGC
Trophoblast giant cell.
Introduction
Polyploidization has been reported in several animal tissues, including human ones.1 Polyploidization of endometrial stromal cells, one of the representative characteristics during uterine decidualization, displays a number of features including supporting embryo development, mitochondrial activity, pregnancy maintenance, and placentation.2,3 Defects during the peri-implantation period, including decidualization, are especially clinically relevant to early pregnancy loss.4 Several studies found that compromised decidualization always carries aberrant polyploidization of decidual cells. Female mice lacking death effector domain-containing protein (DEDD) are infertile with defective polyploidization of stromal cells.3 Hoxa-10 knockout mice display severely compromised decidualization with reduced polyploidy and cyclin D3 expression, while over-expression of cyclin D3 enhances decidualization partially through improving the polyploidization of stromal cells.5 Deletion of bone morphogenetic protein receptor type 2 results in mid-gestation abnormalities in decidualization with decreased polyploid stromal cells.6 Polyploid decidual cells shows a noteworthy gene expression profile when compared to the non-polyploid decidual cells, in which most of the up-regulated genes are primarily involved in ATP binding, metabolic process and mitochondria activity, while the down-regulated genes are associated with apoptosis and immune processes.2 The regulatory mechanism and physiological significance of polyploid decidual cells are worth of further study to gain a better understanding of the maternal-embryonic interaction.
Recently, knockout mouse models revealed a remarkable role of E2F transcription factors in regulating cell cycle progress, including mitosis, polyploidization, cell proliferation and apoptosis.7-9 The E2F family of transcription factors consists of 8 members that are synergistically involved in cell cycle regulation. E2F1-3 belong to transcriptional activator and participate in normal cell cycle transitions.10,11 E2F4 and E2F5 are commonly associated with transcriptional repression and linked to cell quiescence.12 E2F6 functions to polycomb-mediated gene regulation.13 E2F7 and E2F8, the latest identified atypical E2F transcription factors, serve as transcriptional repressors and display multifunctional roles. Noteworthy, E2F7 and E2F8 possess 2 DNA-binding domains (DBD), which are different from one DBD in typical E2Fs, and bind the consensus E2F-binding sites of target genes.14,15 Several studies have associated atypical E2Fs in cell proliferation, differentiation and apoptosis, embryo development, angiogenesis, polyploidization of hepatocytes and trophoblast giant cells (TGC) and tumorigenesis.7,9,16-23 However, the expression, regulation and function of atypical E2Fs in mouse uterus during early pregnancy are still unknown.
This work aimed to study the regulatory mechanism of polyploidization during decidualization with special emphasis on whether atypical E2Fs is involved in decidual cell polyploidization and the physiological role of polyploid decidual cells. We demonstrated that in response to decidualization, E2F8, rather than E2F7, is highly expressed in decidual cells and regulated by progesterone through HB-EGF/EGFR/ERK/STAT3 signaling pathway, which mediates endoreplication of decidual cells through transcriptional repression on cyclin-dependent kinase 1 (CDK1), a master cyclin-dependent kinase in G2/M phase. DNA damage and oxidative stress are concomitant metabolic alterations during decidualization and lead to the activation of apoptosis. Our data showed that the polyploidization of decidual cells is, at least partly, a response to DNA damage and oxidative stress. E2F8 knockdown will compromise polyploidization and the anti-apoptotic effect under the stress condition. However, the polyploidization of stromal cells is not essential for human uterine decidualization.
Results
Polyploidization of stromal cell during mouse decidualization
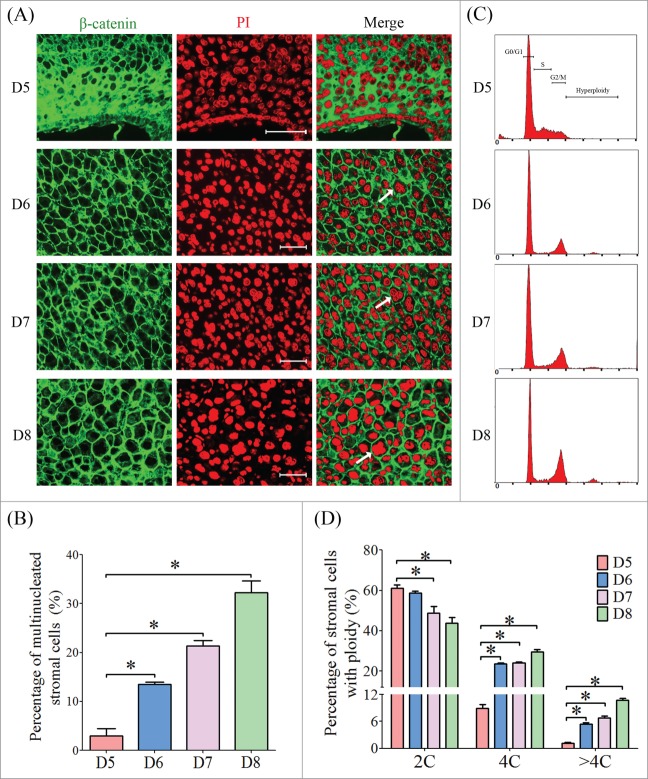
To determine the onset of mouse stromal cell polyploidization, we inspected the cell morphology of the sections from implantation sites from days 5 to 8 labeled with PI and β-catenin.9 Stromal cells surrounding the embryos on day 5 displayed as mono-nucleated cells, while the stromal cells were dramatically transformed into multinucleated cell, and most of them were presented as bi-nucleated on days 6 to 8 (Fig. 1A). The proportion of multinucleated cells was gradually increased as the progression of decidualization (Fig. 1B). Flow cytometry analysis showed that stromal cells on day 5 were predominately in G1 and S phases, whereas most of the stromal cells on days 6 to 8 were arrested in G2/M phase, containing a high proportion of tetraploid (4C) and hyperploid cells (>4C) (Fig. 1C, D). Most of the multinucleated cells were found at the decidual zone localized at the anti-mesometrium (Fig. 2A–C).
Figure 1.
The polyploidization of stromal cell during early pregnancy. (A) Inspection of ploidy in decidual cells by immunofluorescence. The cell membrane was shown by anti-β-catenin and nuclei were stained with PI. (B) The percentages of multinucleated cells at implantation sites from days 5 to 8 of pregnancy. (C) Flow cytometry analysis of PtdIns-stained decidual cells isolated from days 5 to 8 of pregnancy. (D) The percentages of cells containing 2C, 4C and >4C based on flow cytometry. Bar, 100 μm. * P < 0.05.
Figure 2.
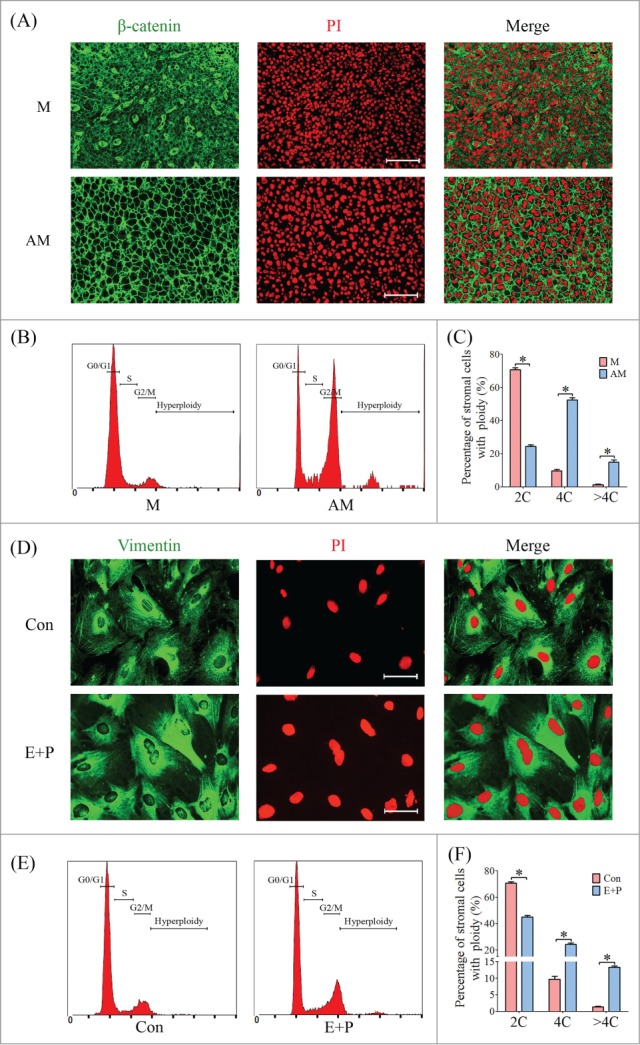
The polyploidization of stromal cells on day 8 of pregnancy and under in vitro decidualization. (A) Inspection of ploidy in decidual cells by immunofluorescence. The cell membrane and nucleus were stained by anti-β-catenin and PI, respectively. M, mesometrium; AM, anti- mesometrium. (B) Flow cytometry analysis on cell cycle distribution of PtdIns-stained decidual cells isolated from day 8 of pregnancy. (C) The percentages of cells containing 2C, 4C and >4C. (D) Inspection of ploidy in cultured stromal cells by immunofluorescence. The cell cytoplasm and nucleus were stained by anti-Vimentin and PI, respectively. Con, control group; E+P, in vitro decidualization. (E) Flow cytometry analysis on cell cycle distribution of PtdIns-stained stromal cells. (F) Percentage of cells containing 2C, 4C and >4C. * P < 0.05, bars, 100 μm.
To further analyze the polyploidization process during decidualization, mouse stromal cells were isolated and induced for in vitro decidualization. Some of the decidualized cells displayed as bi-nucleated or tri-nucleated cells, whereas the cells in control group were primarily mono-nucleated cells (Fig. 2D). Flow cytometry revealed the percentage of 4C and >4C was increased among the decidualized cells compared to control (Fig. 2E, F).
Atypical E2F transcription factors are induced during decidualization
Due to the remarkable role of atypical E2Fs in the polyploidization of hepatocytes and TGC,7 we assumed that E2F7 and E2F8 may be involved in mouse decidualization. Expression of E2f7 and E2f8 mRNA was undetectable in mouse uterus from days 1 to 4 of pregnancy. From days 5 to 8, E2f7 and E2f8 mRNA signals were gradually spread and distributed in the whole decidual zone (Fig. 3A). Compared with inter-implantation sites, the mRNA and protein levels of E2F7 and E2F8 were strongly upregulated at implantation sites from days 5 to 8 (Fig. 3B–D). Under artificial decidualization, E2f7 expression was weakly detected in the luminal epithelium in control uterine horn and in the decidual cells in oil-induced uterine horn. E2f8 expression was negative in uninjected control horn, but strongly expressed in decidual cells under artificial decidualization (Fig. 4A). Compared to control, the levels of both E2F7 and E2F8 mRNA and protein were significantly up-regulated in deciduoma (decidua formed under artificial decidualization) (Fig. 4B, C). Under in vitro decidualization, E2F7 mRNA level was only significantly increased at 72 h, and E2F7 protein level was just slightly upregulated compared to control. However, E2F8 expression was remarkably induced by in vitro decidualization (Fig. 4D, E).
Figure 3.
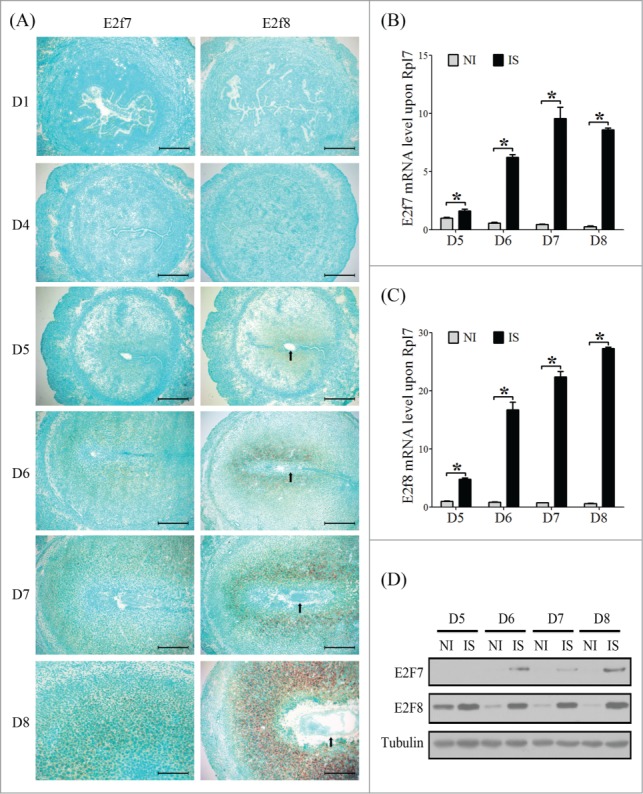
Atypical E2Fs expression during early pregnancy. (A) The mRNA localization of E2f7 and E2f8 in mouse uterus during early pregnancy was detected by in situ hybridization. Real-time PCR was performed to quantify the mRNA level of E2f7 (B) and E2f8 (C) in mouse uterus from day 5 to day 8 (NI, inter-implantation site; IS, implantation sites). (D) Western blot of E2F7 and E2F8 protein in mouse uterus from day 5 to day 8. Tubulin was used as internal reference. Arrows, embryo. * P < 0.05, bar, 300 μm.
Figure 4.
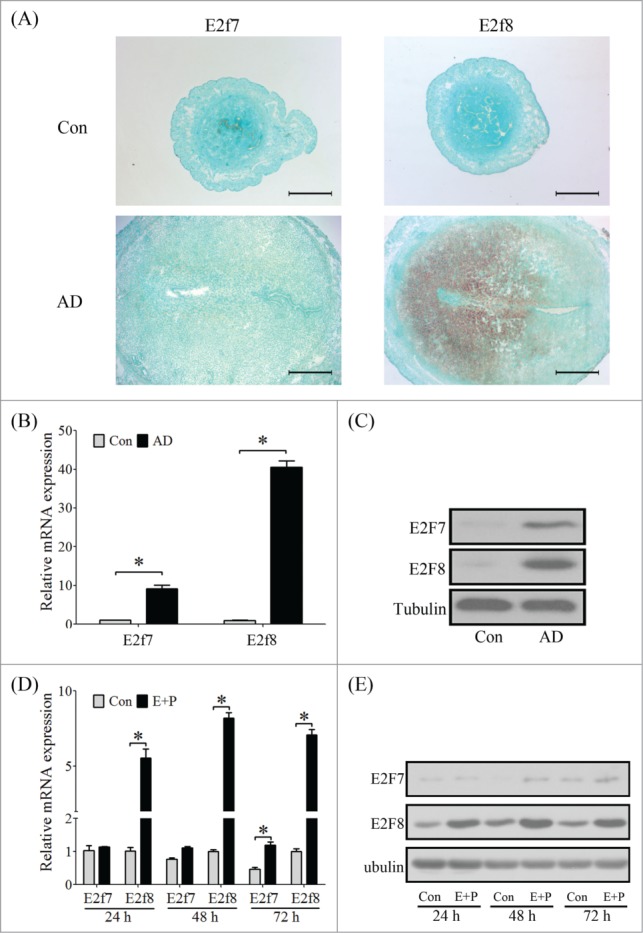
Atypical E2Fs expression under in vivo and in vitro decidualization. (A) The mRNA localization of E2f7 and E2f8 in mouse uterus under artificial decidualization was detected by in situ hybridization (AD, artificial decidualization). (B) Real-time PCR was performed to quantify the mRNA level of E2f7 and E2f8 in deciduoma. (C) Western blot of E2F7 and E2F8 proteins in deciduoma. (D) Real-time PCR was performed to quantify the mRNA levels of E2f7 and E2f8 in stromal cells under in vitro decidualization for 24 h, 48 h, and 72 h, respectively. (E) Western blot of E2F7 and E2F8 protein in stromal cells under in vitro decidualization. Tubulin was used as internal reference. * P < 0.05, bars, 300 μm.
E2F8 silence obstructs the polyploidization of decidual cells
Based on the expression pattern of atypical E2Fs during decidualization, we next confirmed the role of atypical E2Fs in decidual cell polyploidization. Knockdown of E2f7 enhanced the level of E2f8 (Fig. 5A). However, knockdown of E2f8 didn't increase the level of E2f7 (Fig. 5B), indicating that E2F8 could compensate for E2F7. Therefore, siRNAs targeted for E2f7 and E2f8 were transfected individually or collectively into stromal cells, and then performed in vitro decidualization. The polyploidization wasn't interrupted by knockdown of E2f7 alone, possibly due to the increased level of E2f8 for compensating E2F7. However, knockdown of E2f8 alone or a combination of E2f7 and E2f8 remarkably reduced the proportion of multinucleated cells (Fig. 5C, E). Cell cycle analysis further proved that the proportion of polyploid stromal cells (4C and >4C) was notably reduced by knockdown of E2f8 alone or a combination of E2f7 and E2f8 (Fig. 5D, F).
Figure 5.
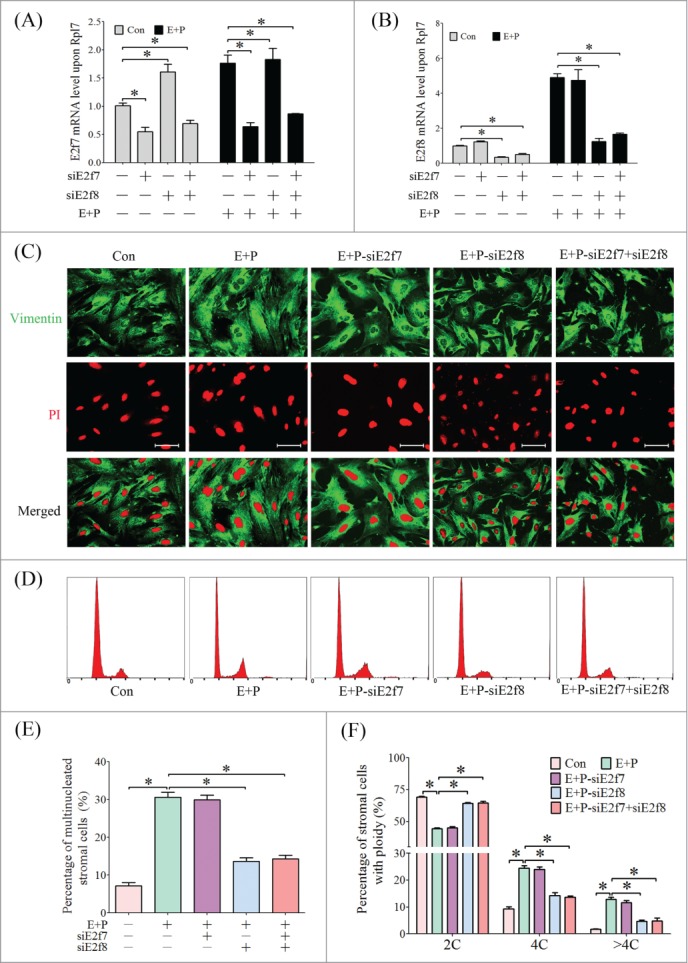
Effect of atypical E2Fs on polyploidization of stromal cells. (A) E2F7 expression after siRNA transfection. (B) E2f8 expression after siRNA transfection. (C) Multiple nucleated cells shown by immunofluorescence with anti-Vimentin. (D) Flow cytometry of stromal cells under in vitro decidualization and siRNA treatments. (E) The percentages of multi-nucleated cells under in vitro decidualization and siRNA treatments based on immunofluorescence. (F) The percentages of cells containing 2C, 4C and >4C based on flow cytometry. *P < 0.05, bar, 100 μm.
Regulation of ovarian steroids on atypical E2Fs expression
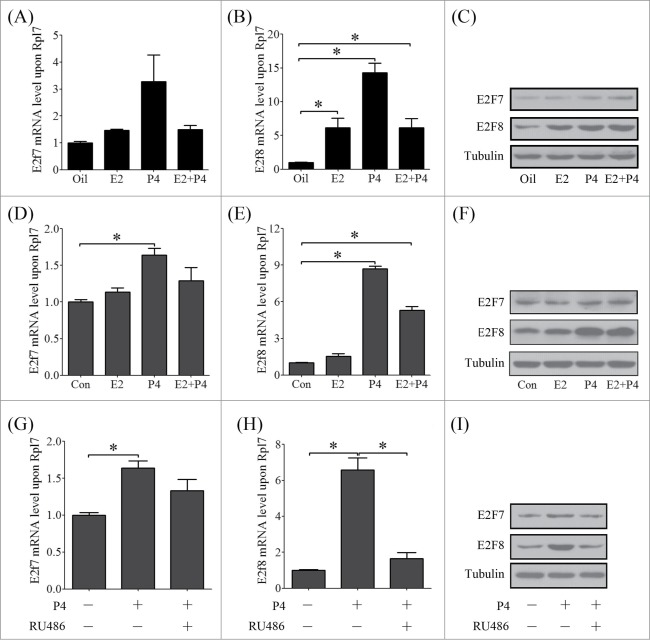
The mutual effects between estrogen and progesterone precisely regulate each step during pregnancy.24 Ovariectomized mice were used to elucidate whether atypical E2Fs expression is regulated by estrogen (E2) and progesterone (P4). The results showed that E2 or P4 had no effects on E2F7 expression, but significantly induced the expression of E2F8 (Fig. 6A–C). In cultured mouse stromal cells, E2F7 expression was significantly induced by P4, but less than 2 folds (Fig. 6D). However, E2F8 expression was stimulated around 9 folds by P4 (Fig. 6 E, F). P4 regulates stromal cell decidualization through progesterone receptor (PR) and PR knockout mice display compromised decidualization and obviously with the absence of polyploidy.24 When stromal cells were treated with RU486, a progesterone antagonist, RU486 could abrogate the induction of P4 on E2F8 (Fig. 6G–I).
Figure 6.
The regulation of ovarian steroids on atypical E2Fs. Real-time PCR was performed to investigate the regulation of estrogen and progesterone on E2f7 (A) and E2f8 (B) in mouse uterus (E2, Estradiol-17 β; P4, progesterone). (C)The protein levels of E2F7 and E2F8 in mouse uteri treated with E2, P4 or a combination of E2 and P4. The mRNA level of E2f7 (D) and E2f8 (E) in stromal cell treated with estrogen and progesterone. (F) The protein levels of E2F7 and E2F8 in stromal cells treated with E2, P4 or a combination of E2 and P4. The mRNA level of E2f7 (G) and E2f8 (H) in stromal cell treated with progesterone and RU486. (I) The protein levels of E2F7 and E2F8 in stromal cells treated with P4 or P4 plus RU486.
HB-EGF/EGFR/ERK/STAT3 signal pathway regulates E2F8 expression in stromal cells
Since HB-EGF has been shown to be regulated by progesterone during decidualization and tightly related to the polyploidization of decidual cells,25,26 we assumed that HB-EGF may be involved in mediating the regulation of E2F8 expression in mouse uterus during decidualization. Compared with control group, the stromal cells treated with HB-EGF showed an increased proportion of tetraploid (Fig. 7A, B). HB-EGF treatment could induce the phosphorylation of EGFR, ERK, STAT3 and simultaneously the expression of E2F8 (Fig. 7C, D).
Figure 7.
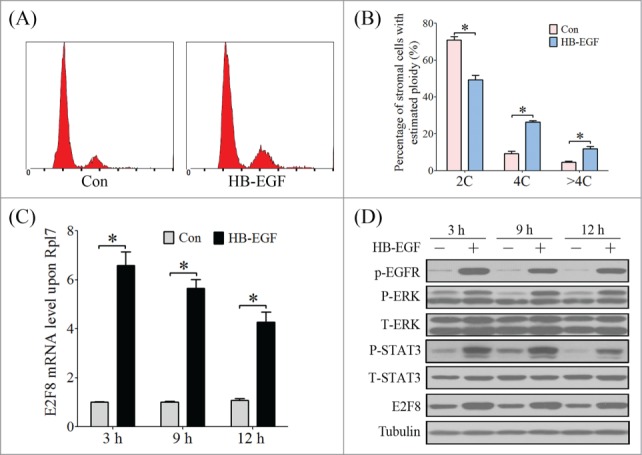
The effect of HB-EGF on stromal cell polyploidization and E2F8 expression. (A) Cell cycle distribution analyzed by flow cytometry of PI-stained stromal cells under in vitro decidualization with HB-EGF. (B) Percentage of cells in 2C, 4C and >4C based on flow cytometry. (C) The mRNA level of E2f8 in stromal cells after HB-EGF treatment for 3 h, 6 h and 9 h, respectively. (D) Western blot of P-EGFR, P-ERK, ERK, P-STAT3, STAT3 and E2F8 in stromal cells after HB-EGF treatment.
To address whether EGFR/ERK/STAT3 pathway is involved in HB-EGF-induced E2F8 expression, stromal cells were treated with EGFR inhibitor, ERK inhibitor and STAT3 inhibitor, respectively. Real time PCR and Western blot showed that HB-EGF-induced E2F8 activation was attenuated by the inhibitor for EGFR, ERK or STAT3 (Fig. 8A–F). Promoter analysis showed there is a STAT3 binding site on the promoter of E2F8 at the distance of −325 bp from TSS (Fig. 8G). The luciferase activity of E2f8 promoter containing the STAT3 binding sites (full length) was significantly increased by progesterone, but progesterone had no effects on the luciferase activity of E2f8 promoter after STAT3 binding site was deleted (Fig. 8H).
Figure 8.
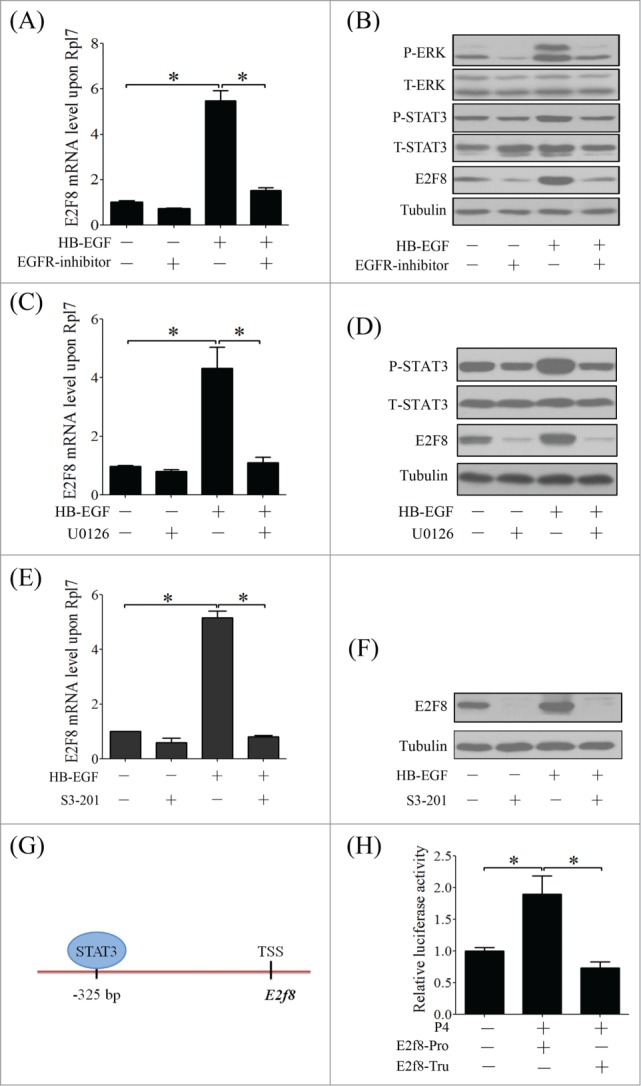
The regulatory network of HB-EGF/EGFR/ERK/STAT3 signal pathway in polyploidization and E2F8 expression. (A) The effect of EGFR inhibitor on mRNA level of E2f8 in stromal cells after HB-EGF. (B) Western blot of P-ERK, ERK, P-STAT3, STAT3 and E2F8 in stromal cells after HB-EGF and EGFR inhibitor treatment. (C) The effect of U0126 on mRNA level of E2f8 in stromal cells after HB-EGF treatment. (D) Western blot of P-STAT3, STAT3 and E2F8 in stromal cells after HB-EGF and U0126 treatment. The effect of STAT3 inhibitor (S3–201) on E2f8 mRNA (E) and protein level (F) after HB-EGF treatment. (G) The STAT3 binding site in the promoter of E2f8. (H) The relative luciferase activity of E2f8 promoter in pGL3 plasmid under P4 treatment. E2f8-Pro, E2F8 promoter with the STAT3 binding sites (full length); E2f8-Tru, E2F8 promoter without the STAT3 binding sites (truncated). Tubulin was used as internal reference. * P < 0.05.
Embryo induced E2F8 expression and stromal cell polyploidization
Delayed implantation model was used to examine whether E2F8 expression is dependent on active embryos. The mRNA signal of E2f8 was negative in mouse uterus when embryo implantation was delayed by ovariectomy, while E2F8 was strongly expressed in the subluminal stromal cells after estrogen activation and embryo implantation (Fig. 9A). To further examine effects of embryos on E2F8 expression, we co-cultured uterine stromal cells with intravital fluorochrome-stained embryos. Compared to control, E2F8 expression of stromal cells was significantly stimulated by co-culturing with blastocysts (Fig. 9B). Simultaneously, immunofluorescence results showed that a part of the stromal cells became polyploidy around the invaded embryo (Fig. 9C).
Figure 9.
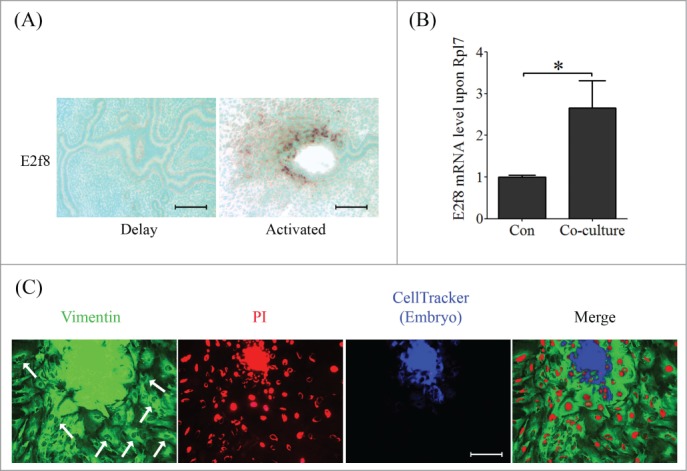
Embryo induced E2F8 expression and polyploidization. (A) The mRNA localization of E2f8 in delayed and activated mouse uterus. (B) Real-time PCR was performed to quantify the mRNA level of E2F8 in stromal cells co-cultured with embryo. (C) CellTracker stained embryo (blue signal) was co-cultured with stromal cells. The cell outline and nucleus were stained by Vimentin (green signal) and PtdIns (red signal), respectively. Arrow, polyploid stromal cells. * P < 0.05, bars, 100 μm.
E2F8 mediates endoreplication and polyploidization by transcriptionally repressing on CDK1
Next we explored the regulatory mechanism of E2F8 in mediating polyploidization of decidual cell. Endoreplication refers to a specific biological process that the cells duplicate their genome multiple times without undergoing cell division, that is, M phase or cytokinesis is subdued.27 CDK1 is thought to be activated by A-type cyclins at the end of interphase to facilitate the onset of mitosis.28 Real time PCR and western blot showed that CDK1 expression was gradually repressed during decidualization in vivo and in vitro, indicating that decidual cells may become into polyploidy by favoring endoreplication (Fig. 10A–D). To confirm the role of CDK1 in polyploidization conversion, the stromal cells were treated with Purvalanol A (PA), a specific inhibitor for CDK1.29 Immunofluorescence and flow cytometry verified an increased percentage of polyploid stromal cells (4C and >4C) by inhibiting CDK1 (Fig. 10E–G). Therefore, the suppression of CDK1 may directly result in cytokinesis failure and polyploidy formation.
Atypical E2Fs are known as transcriptional repressors and regulate many of cell cycle-related genes by binding to their promoters.8 Real time PCR and western blot results showed that knockdown of E2f8, or both of E2f7 and E2f8, could prominently enhance the expression of CDK1 in both mRNA and protein levels, but knockdown of E2f7 had no obvious effects on CDK1 expression (Fig. 10H, I), indicating that E2F8 is the master regulator in mouse uterus. These results suggested that E2F8 may regulate the expression of CDK1 as a transcriptional repressor.
Figure 10.
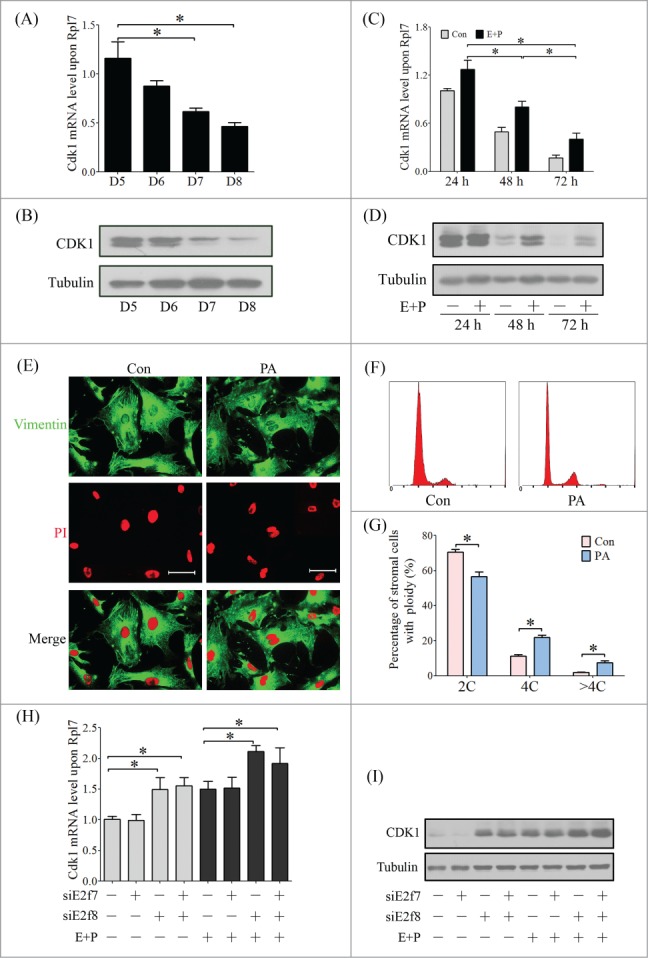
CDK1 expression during decidualization and polyploidization. The Cdk1 mRNA level (A) and protein level (B) in implantation sites from days 5 to 8 of pregnancy. The Cdk1 mRNA level (C) and protein level (D) in stromal cells under in vitro decidualization. (E) Inspection of ploidy in stromal cells treated with CDK1 inhibitor (Purvalanol A, PA) by immunofluorescence. (F) Cell cycle distribution analyzed by flow cytometry of PI-stained cells treated with PA. (G) Percentage of cells in 2C, 4C and >4C based on flow cytometry. Effect of E2f7 and E2f8 knockdown on mRNA level (H) and protein level (I) of CDK1 in stromal cells. *P < 0.05, bar, 100 μm.
E2F8 is a response to oxidative stress and DNA damage during decidualization
Uterine decidualization commonly accompanies with extensive stromal cell proliferation and differentiation, and remarkable metabolic alteration, to establish a favorable environment for pregnancy.30 However, several inevitable deleterious metabolites or damage stresses, including reactive oxide species (ROS) and DNA damage, will lead to adverse impact on embryo development and pregnancy.31 SOD activity assay confirmed that the antioxidant system was boosted in decidual cells during in vivo and in vitro decidualization (Fig. 11A, B). E2F8 expression was induced by treating stromal cells with 50 μM H2O2 or 10 μM paraquat for the induction of oxidative stress (Fig. 11C, D).32 p-H2A.X, a representative marker of DNA damage,33 was highly expressed at implantation sites during decidualization compared to inter-implantation sites (Fig. 11E). UV irradiation and Camptothecin (CPT, the topoisomerase I inhibitor) were used to induce DNA damage in stromal cells,33 E2F8 expression was up-regulated by CPT or UV irradiation (Fig. 11F–H). However, E2F8 expression was inhibited when these cells were treated with the higher concentration of H2O2, paraquat or CPT, or a longer time period of UV irradiation, suggesting that the over-dose stresses may be beyond the anti-stress capacity of stromal cells and will induce cell apoptosis. Therefore, our data suggested that E2F8 expression is a response to oxidative stress and DNA damage.
Figure 11.
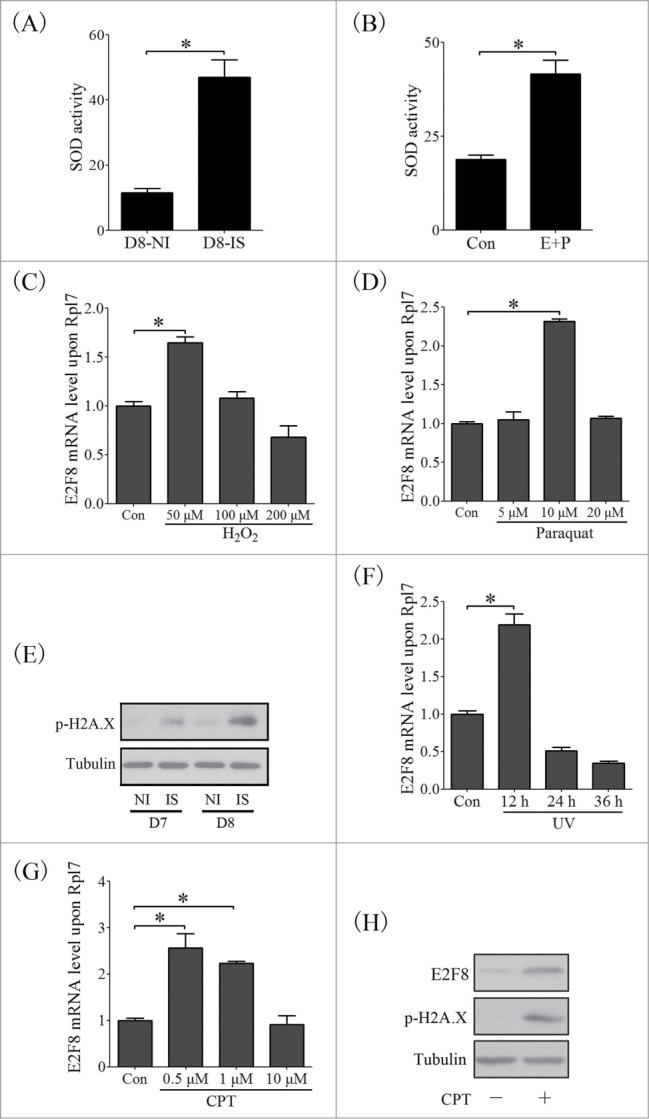
kE2F8 is induced by oxidative stress and DNA damage during decidualization. (A) The SOD activity in decidua tissues on day 8 of pregnancy. (B) The SOD activity in stromal cells under in vitro decidualization. The mRNA level of E2f8 in stromal cells treated with H2O2 (C) and Paraquat (D) for the induction of oxidative stress. (E) Western blot of p-H2A.X in mouse uterus from day 7 and 8 of pregnancy. The mRNA of E2f8 in stromal cells treated with UV (F) and CPT (G). (H) Western blot of E2F8 and p-H2A.X in stromal cells treated with CPT.
E2F8-mediated polyploidization enhances the stress-resistance of decidual cells
Our data showed that E2F8 expression is a response to oxidative stress and DNA damage. The next question is whether E2F8-mediated polyploidization functions in providing reserve capacity for confronting stress and damage. Paraquat and CPT were used to induce oxidative stress and DNA damage, respectively.32,33 Immunofluorescence images showed that a part of the stromal cells switched to polyploidy under the stress conditions even without the induction of decidualization. However, knockdown of E2f8 inhibited the polyploidization of these stress-induced cells (Fig. 12A–F). Next we investigated whether E2F8 downregulation had any adverse impact on decidualization and cellular function. Dtprp is a marker for mouse decidualization.33 Knockdown of E2f8 failed to reveal any significant effects on Dtprp level under normal in vitro decidualization. However, for stress-treated stromal cells, knockdown of E2f8 led to a reduced expression of Dtprp (Fig. 12G, H). We then knockdowned the expression of E2F8 in stromal cells, exposed the cells to oxidative stress and DNA damage, and examined the expression of apoptosis marker, cleaved PARP, in stromal cells. Western blot analysis revealed no obvious apoptosis in CPT or paraquat-treated decidualized stromal cells, whereas knockdown of E2f8 led to an increased level of cleaved PARP in paraquat or CPT-treated stromal cells (Fig. 12I, J), suggesting that the increased polyploidy may facilitate cell resistance to stress-induced apoptosis.
Figure 12.
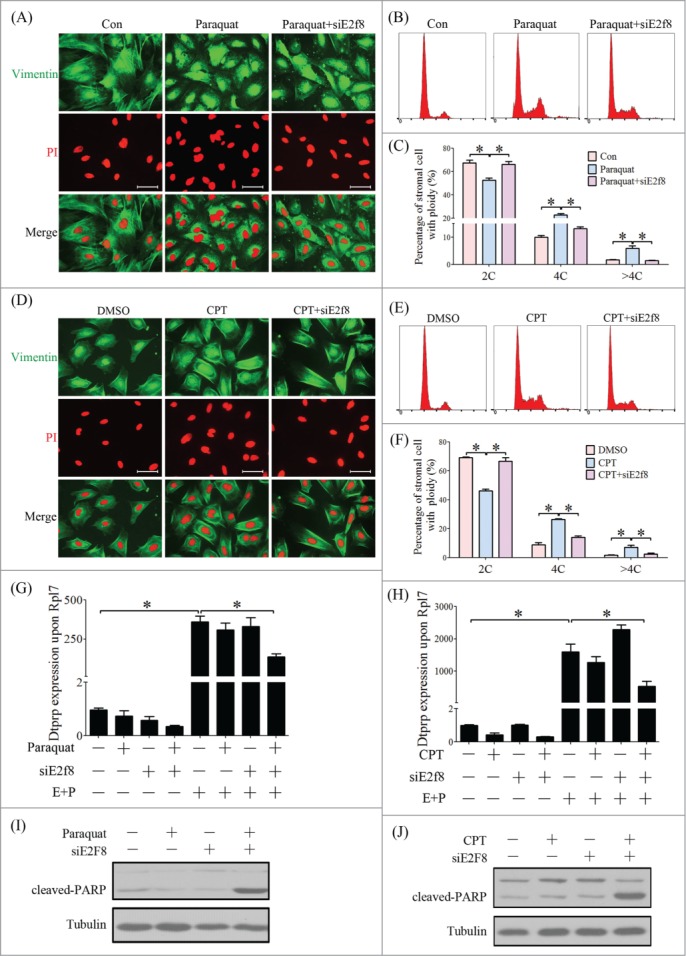
E2F8-mediated polyploidy is adaptive to oxidative stress and DNA damage during decidualization. (A) Inspection of ploidy of stromal cells treated with Paraquat and E2f8 knockdown by immunofluorescence. (B) Cell cycle distribution analyzed by flow cytometry of PtdIns-stained cells treated with Paraquat and E2f8 knockdown. (C) Percentage of cells in 2C, 4C and >4C based on flow cytometry. (D) Inspection of ploidy of stromal cells treated with CPT and E2f8 knockdown by immunofluorescence. (E) Cell cycle distribution analyzed by flow cytometry of PI-stained cells treated with CPT and E2f8 knockdown. (F) Percentage of cells in 2C, 4C and >4C based on flow cytometry. Effect of E2f8 knockdown on Dtprp mRNA level in stromal cells treated with Paraquat (G) and CPT (H). Effects of E2f8 knockdown on cleaved-PARP protein level in stromal cells treated with Paraquat (I) and CPT (J). * P < 0.05, bar, 100 μm.
Atypical E2Fs expression and polyploidization in human endometrial stromal cells during decidualization
After we showed an important role of E2F8 in the polyploidization of mouse endometrial stromal cells during decidualization, we extended our focus on the polyploidization and the involvement of atypical E2Fs during human decidualization. Due to the spontaneous decidualization in humans,24 we examined the polyploid of human endometrium during secretory phase and the decidual tissues during 6 to 9 weeks of pregnancy. Beyond our expectation, we found that the stromal cells in secretory phase or decidual cells were mainly mono-nucleated cells. The proportions of multi-nucleated cells were 1.1% and 3.7%, respectively (Fig. 13A, B).
Figure 13.
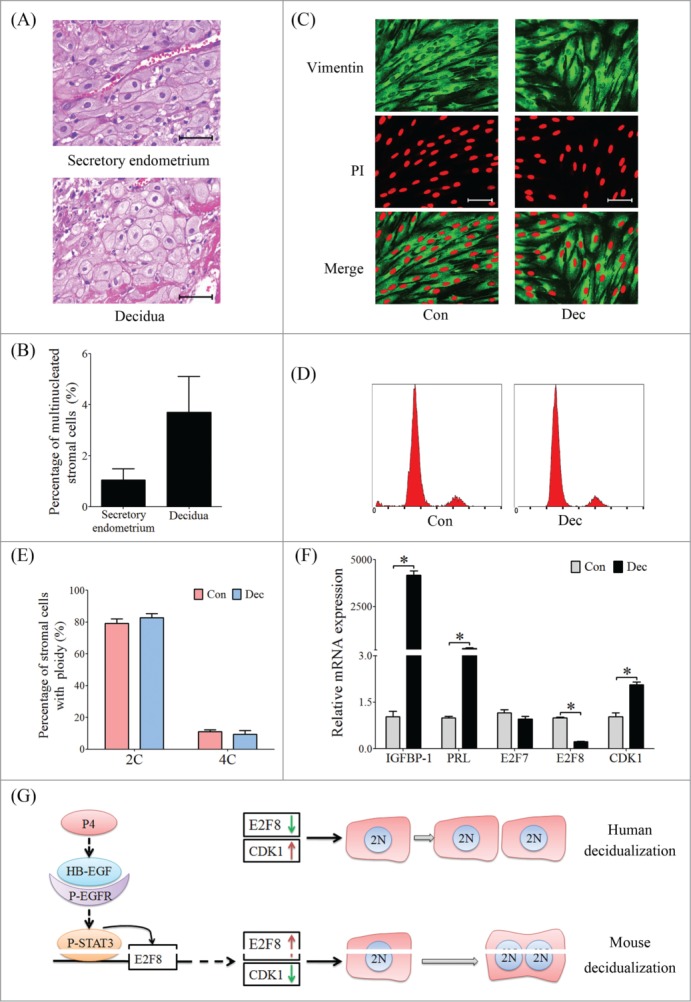
Atypical E2Fs expression and polyploidization during human decidualization. (A) Histological sections of human endometrium from secretory phase and decidual tissue from early pregnancy, respectively. (B) The percentages of multinucleated cells counted from 10 randomly selected areas in human endometrium and decidua, respectively. (C) Immunofluorescence of human stromal cells under in vitro decidualization. The cell outline and nucleus were stained by anti-Vimentin and PtdIns, respectively. Con, control group; Dec, in vitro decidualization. (D) Flow cytometry of PI-stained stromal cells. (E) The percentages of cells containing 2C and 4C based on flow cytometry. (F) Real-time PCR analysis for the mRNA levels of IGFBP-1, PRL, E2F7, E2F8 and CDK1 in human stromal cells under in vitro decidualization for 8 days. (G) Schematic presentation of differential expression of E2F8 may lead to the difference of decidual cell polyploidization between mice and humans. * P < 0.05, bar, 100 μm.
To further confirm whether the polyploidization process occurs in human endometrial stromal cells, we induced human stromal cells for in vitro decidualization for 8 days. Cells were labeled with PtdIns and Vimentin to distinguish cell border (Fig. 13C). Human stromal cells displayed as mono-nucleated in both of control and decidual groups. Flow cytometry analysis revealed that the proportion of tetraploid (4C) in decidual stromal cells was similar to control group (Fig. 13D, E). Next we detected the expression level of atypical E2Fs in human stromal cells under in vitro decidualization. IGFBP-1 and PRL, the reliable markers of decidualization in humans, were significantly induced during decidualization. There was no significant change for E2F7 mRNA level between control and decidual group. However, the mRNA level of E2F8 was significantly repressed in decidual stromal cells. On the contrary, CDK1 expression was upregulated in decidual stromal cells (Fig. 13F). On the basis of the above results we considered the hypothesis that differential expression of E2F8 and CDK1 may lead to stromal cell polyploidization diversity between mice and humans (Fig. 13G).
Discussion
E2F8 expression in mouse uterus is associated with the polyploidization of decidual cells
In the present study, we showed that plentiful polyploid decidual cells are detected during decidualization, and considerably distributed in anti-mesometrium and the junctional region between mesometrium and anti-mesometrium. Similar to hepatocytes, the polyploid decidual cells are basically composed of tetraploids or bi-nucleates (4C), which differ from the hyperploidy in TGC or megakaryocytes (MKC) that the ploidy could reach to 1000 C.34 Flow cytometry showed that most of decidualized stromal cells are arrested in G2/M phase, indicating that M phase blocking or cytokinesis failure may contribute to stromal cell polyploidization. Similarly, cytokinesis failure is also observed in bi-nucleated hepatocytes.35
E2F7 and E2F8, 2 atypical members in E2F family, are essential for the polyploidization of hepatocytes and TGC.7,9 Based on our information, this is the first study to focus on the expression of atypical E2Fs in mouse uterus during early pregnancy. E2F7 and E2F8 are negative in mouse uteri from days 1 to 4. E2F8 is up-regulated at implantation sites from days 5 to 8, and induced by artificial decidualization and in vitro decidualization. More importantly, E2F8 expression is mainly localized at anti-mesometrial side and the junctional region between mesometrium and anti-mesometrium, which is consistent to the distribution of polyploid decidual cells. However, E2F7 expression in mouse uterus is not conspicuous as E2F8. Knockdown of E2f8, instead of E2f7, results in decreased proportion of polyploid decidual cells, suggesting E2F8 may be involved in decidual cell polyploidization. Therefore, we focused on E2F8 for further study.
Progesterone activates the expression of E2F8 through HB-EGF/EGFR/ERK/STAT3 signal pathway
Our study showed E2F8 expression in mouse uterine stromal cells is regulated by progesterone both in vivo and in vitro, which is abrogated by PR antagonist, suggesting progesterone may regulate E2F8 through PR. PR knockout mice also display compromised decidualization and obviously with the absence of polyploidy.36 The results presented here found stromal cells cultured with HB-EGF produced an increased number of polyploid cells. HB-EGF is an early molecular marker of embryo-uterine cross-talk during implantation and regulated by progesterone. Maternal deficiency of HB-EGF displays a compromised pregnancy outcome.25 Beads carrying HB-EGF were very potent in inducing decidualization and stromal cell polyploidy.26 We wonder whether HB-EGF is the key intermediary between progesterone and E2F8. Our results showed that E2F8 is activated in stromal cells by HB-EGF treatment, as well as the phosphorylation of EGF receptor (EGFR), ERK and STAT3. EGFR, served as the preferred receptor ligand for HB-EGF and activated during early pregnancy, triggering a multitude of signaling and transcriptional events.37 In uterine stromal cells, HB-EGF induced E2F8 expression is blocked by EGFR inhibitor. Uterine E2f8 expression in Egfr knockout mice is downregulated after deciduogenic stimuli when compared to wild type.38 ERK1/2 signal pathway is a classic downstream of HB-EGF and also involved in decidualization,39 ERK1/2 signal pathway inhibitor restrained the expression of E2F8 in HB-EGF treated stromal cell. STAT3 inhibitor also suppresses the expression of E2F8 in stromal cells. Moreover, we found a STAT3 binding site on the promoter of E2f8, indicating the transcriptional regulation of STAT3 on E2F8. STAT3 had been tightly linked to implantation and decidualization in mice and humans.40,41 Conditional ablation of Stat3 in mouse uterus results in embryo implantation failure. Although there is no special indications that whether the polyploidization of stromal cells is existed, uterine Stat3 knockout are defective in hormonally induced decidual reaction, clearly with the absence of stromal cell polyploidization.40 In summary, our results illustrate that E2F8 expression in stromal cells is regulated by progesterone through activating HB-EGF/EGFR/ERK/STAT3 signal pathway.
E2F8 initiates the endoreplication of mouse decidual cells
Our results suggest that E2F8 is involved in polyploidization of decidual cells. Previous studies also found that E2F8 participates in the polyploidization of hepatocytes and TGC through favoring endocycle or endoreplication.7 In endocycling, cells largely avoid mitosis and the duplicated chromatids remain structurally associated within one nucleus. In contrast, endoreplication or endomitosis refers to a process that the cells undergo a part of mitosis but fail to execute telophase or cytokinesis. Therefore, the duplicated chromosomes produced by endoreplication may be packed into separated nucleus, thus forming multinucleated cells.1,27,34,42 Our results showed that most of the polyploid decidual cells are manifested as binucleated and arrested in G2/M phase, suggesting that endoreplication and M phase arrest may be the main mechanisms involved in decidual cell polyploidization. CDK1, also known as cell division control protein 2, controls the onset of mitotic phase.43 Our studies showed the expression of CDK1 is suppressed in stromal cells during decidualization, which is coincided with G2/M phase arrest in polyploid stromal cells. Inhibiting the activity of CDK1 will induce the formation of polyploid stromal cells. CDK1 deficiency in hepatocytes results the activation of re-replication and increased polyploidy.44 These results indicate depressed CDK1 activity may responsible for endoreplication conversion in decidual cells.
Here we present E2F8, known as a transcriptional repressor, is able to bind to the consensus E2F recognition sites and repress the expression of cell cycle-related E2F targets. Our results showed a negative correlation between CDK1 and E2F8. Chip assay also verified that E2F8 is enriched on the promoter of Cdk1.7 Transcriptome analysis demonstrated the chromosome segregation, mitosis and cytokinesis related genes are upregulated in E2f8 knockout mice, including Cyclin A2 and Cyclin B1.9 These results suggest that E2F8 initiates the endoreplication through repressing the mitosis and cytokinesis.
Polyploid decidual cells are adaptive to decidualization
Here we present that mouse uterine stromal cells spontaneously convert to polyploidy by oxidative stress or DNA damage treatments without the induction of E2 and P4. Simultaneously, E2F8 is a response to oxidative stress and DNA damage, suggesting E2F8 is associated with stress-induced polyploidization of stromal cells. It has been recognized that decidualized stromal cells are endowed with anti-apoptotic capacity to oxidative stress.45,46 However, the anti-apoptosis mechanism of decidual cells remains obscure. The results presented here reveal an increased proportion of polyploid stromal cells during mouse decidualization. It remains to determine whether these extra chromosomes are functioning in providing reserve capacity for confronting stress and damage? Our results showed that knockdown of E2F8 in stromal cells leads to reduced polyploidy and increased apoptosis when the cells expose to oxidative stress and DNA damage, suggesting that the increased polyploidy may facilitate cell resistance to stress-induced apoptosis. During cell cycle transition, E2F7 and E2F8 should restrain E2F1 activity throughout the S and G2 phases, otherwise the cells may favor E2F1 induced apoptosis; Except cell cycle regulation, E2F7 and E2F8 were induced in cells treated with DNA-damaging agents, transcriptionally repressing E2F1 expression and inhibiting E2F1-dependent apoptosis.19 Mice that are doubly deficient for E2F7 and E2F8 failed to survive on E11.5, displaying widespread apoptosis without defects in proliferation, and the high level of E2F1 and p53, while inhibiting p53 and E2F1 could rescue cell apoptosis.20 Taken together, these results demonstrate E2F8 mediated polyploidy status could increase the cellular resistance to stresses and endow the decidual cells with pro-survival strategies by inhibiting pro-apoptotic pathways.
Materials and Methods
Animals and treatments
All experiments were approved by the University Animal Ethics Committee and performed according to South China Agricultural University institutional and national guidelines. Adult CD1 mice were housed in a controlled environment with a 14 h light: 10 h dark cycle. Female mice were mated with fertile or vasectomized males to induce pregnancy or pseudopregnancy (day 1 is the day of vaginal plug). From days 1 to 4, pregnancy was confirmed by flushing the embryos from the oviducts or uteri. The implantation sites on day 5 were visualized through intravenous injection of 0.1 ml of 1% Chicago blue dye (Sigma-Aldrich Inc., St. Louis, MO, USA) in saline. Artificial decidualization, delayed implantation, and steroid hormonal treatment models were performed as previously described.33
Immunofluorescence
The immunofluorescence was performed as previously described.17 Briefly, formalin-fixed tissues were embedded in paraffin and cut into 4 μm sections. After deparaffinization and dehydration, sections were boiled in 10 mM citrate buffer for 15 min and cooled to room temperature. The sections were blocked by 5% donkey serum (Jackson ImmunoResearch, Westgrove, Pennsylvania) for 1 h at 37°C. Immunofluorescent cell membrane staining was done by using of anti-β-catenin (Cell signaling technology, Beverly, MA), anti-Vimentin (Cell signaling technology) as first antibody, and anti-rabbit Alexa Fluor 488 (Jackson ImmunoResearch, 1:200) as second antibody, the nucleus were stained with propidium iodide (PI, Sigma). The sections were inspected by Leica fluorescence microscope.
Isolation of decidual cells in vivo
Decidual cells from day 5 to day 8 were isolated as described previously.2 Briefly, deciduoma tissues were separated from the muscle and minced by scissors, then placed in HBSS containing 0.25% collagenase I (Invitrogen, Carlsbad, CA) and 0.1% hyaluronidase (Sigma) for 45 min at 37°C. The enzyme digested tissue suspension was then passed through different size of gauge needles in sequence to form a single cell suspension.
Flow cytometry
Freshly prepared uterine decidual cell suspensions were collected in PBS and fixed in 70% cold ethanol overnight at 4°C. Cells were washed by PBS and treated with RNase for 40 min at 37°C, and then stained with PtdIns on ice. Following filtration through nylon mesh to remove the cell clumps, the cell suspensions were analyzed on flow cytometry immediately. The cell cycles were analyzed by calculating the proportion of G0/G1, S and G2/M phases from DNA histogram data.
Isolation of mouse uterine stromal cells and treatments
Primary mouse uterine stromal cells were enzymatic isolated on day 4 of pregnancy and cultured with DMEM/F12 (Sigma) containing 10% charcoal-treated FBS (Biological Industries, Israel). As previously described,33 the stromal cells were treated with 10 nM of estradiol-17 β (Sigma) plus 1 μM of progesterone (Sigma) to induce in vitro decidualization. Both estradiol-17 β and progesterone were dissolved in ethanol.
For steroid hormonal treatments in vitro, stromal cells were treated with 10 nM of estradiol-17 β, 1 μM of progesterone or a combination of estradiol-17 β and progesterone, respectively. For further studies, stromal cells were treated with RU486 (Sigma), HB-EGF (R&D Systems, Minneapolis, MN), EGFR inhibitor (Calbiochem, San Diego, CA), U0126 (Cell signaling Technology), S3I-201 (Calbiochem), Purvalanol A (PA, Sigma), Camptothecin (CPT, Alexis Biochemicals, Swiss), and UV irradiation, respectively.
For co-culture model of embryo and uterine stromal cells, the mouse blastocyst were collected at day 4 of pregnancy and stained with CellTracker (Invitrogen) for 30 min at 37°C, then transferred them onto the confluent monolayer of uterine stromal cells in DMEM/F12 containing 10% cFBS for 48 h.
In situ hybridization
Total RNAs from mouse uterus on day 8 of pregnancy were reverse-transcribed and each hybridization probe template was amplified with the corresponding primers (Table 1). pGEM-T vector plasmid (Promega, Madison, WI) was used for cloning the amplified fragment. Digoxigenin-labeled antisense or sense cRNA probes were transcribed in vitro by using digoxigenin RNA labeling kit (Roche Applied Science).
Table 1.
Primers used in this study
| Gene name | Primer sequences | Accession number | Size (bp) | Application |
|---|---|---|---|---|
| E2f7 | TTTCTAGCTCGCTATCCG CAGGGTGACAATCTTGGT | NM_178609 | 465 | In situ hybridization |
| E2f7 | TGTCAGCCCTCACTCAACACTGCTCTGCCTTTACCA | NM_178609 | 122 | Real-time PCR |
| E2f8 | CCCTAGCCCATTGTCATCCGCTTTCAGGTCCTCTTT | NM_001013368 | 361 | In situ hybridization |
| E2f8 | GTGCTTCGTAGAACTCCCTGGCAATGTCATACAGCCTCCT | NM_001013368 | 228 | Real-time PCR |
| E2f8 | CCGCTCGAGACACTAAATGTATGAGGAATGCCAAGCTTAAAGGTGGTGTTCTCTCC | NM_001013368 | 2725 | Overexpression |
| E2f8 | TTACACGCGTTCGATCTCGGATAAATGGTTGCAAGCTTCAGCCAGACAGCATCTCA | NM_001013368 | Promoter analysis | |
| E2f8 | CCGCTCGAGTCGATCTCGGATAAATGGTATGCAAGCTTCGCTCACCTGTCAATCCTC | NM_001013368 | Promoter analysis | |
| Cdk1 | TTTAGGTTTGTTGTAAAGC | NM_007659 | 129 | Real-time PCR |
| TAAGAGAGGACAGGAGATT | ||||
| Rpl7 | GCAGATGTACCGCACTGAGATTCACCTTTGGGCTTACTCCATTGATA | M29016 | 129 | Real-time PCR |
| Dtprp | AGCCAGAAATCACTGCCACTTGATCCATGCACCCATAAAA | NM_010088 | 119 | Real-time PCR |
| E2F7 | CCCAAGAGCATCCAACGCTGGCAAAGCGGCAGGTTA | NM_203394.2 | 168 | Real-time PCR |
| E2F8 | GCCCATCCTATGCCATCTACGGTATCATTGGCTGCCTTCT | NM_024680.3 | 158 | Real-time PCR |
| CDK1 | ACCATACCCATTGACTAACATATAACCTGGAATCCTGC | NM_001786.4 | 251 | Real-time PCR |
| GAPDH | GAAGGTGAAGGTCGGAGTGATGGCAACAATATCCACTT | BC023632 | 94 | Real-time PCR |
| IGFBP-1 | CCAAACTGCAACAAGAATGGTAGACGCACCAGCAGAG | NM_000596 | 87 | Real-time PCR |
| PRL | AAGCTGTAGAGATTGAGGAGCATCAGGATGAACCTGGCTGACTA | NM_000948 | 76 | Real-time PCR |
In situ hybridization was performed as previously described.33 Briefly, frozen sections (10 μm) were mounted on 3-aminopropyltriethooxysilane (Sigma)-treated slides and fixed in 4% paraformaldehyde solution in PBS. Hybridization was performed at 55°C for 16 h. Then, the sections were incubated with sheep anti-digoxigenin antibody conjugated to alkaline phosphatase (1:5000, Roche). The signal was visualized with the buffer containing 0.4 mM 5-bromo-4-chloro-3-indolyl phosphate and 0.4 mM nitrobluetetrazolium. Endogenous alkaline phosphatase activity was inhibited with 2 mM levamisole (Sigma). The sections were counterstained with 1% methyl green. The positive signal of in situ hybridization was visualized as a dark brown color.
RNA extraction and real-time PCR
Total RNAs from mouse tissues and cultured cells were extracted by a Trizol Kit (Sigma) and reverse-transcribed into cDNAs with the PrimeScript reverse transcriptase reagent kit (TaKaRa Bio Inc.., Tokyo, Japan). For real-time PCR, cDNA was amplified using a SYBR Premix Ex Taq kit (TaKaRa; DRR041S) on the BIORAD-CFX96™ Real-Time System (BioRad) according to the manufacturer's recommendations. All reactions were run in triplicate. The corresponding primer sequences used for real-time PCR were listed in Table 1.
Western blot analysis
The uterine tissues were homogenized in lysis buffer (50 mM Tris-HCl, pH 7.5, 0.1% SDS, 150 mM NaCl, 1% Triton X-100, and 0.25% sodium deoxycholate), supplemented with Protease Inhibitor Cocktail (Roche) on ice, cultured cells were scratched and collected with lysis buffer. Protein lysates were separated by SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Membranes were incubated with primary antibodies as follows: anti-E2F7 (Abcam, Cambridge, UK), anti-E2F8 (Antibodies-Online, Atlanta, USA), anti-Tubulin (Cell Signaling Technology, CST), anti-P-EGFR (CST), anti-P-ERK1/2 (CST), anti-ERK1/2 (CST), anti-p-H2A.X (CST), anti-P-STAT3 (CST), anti-STAT3 (CST), anti-CDK1 (CST), anti-cleaved-PARP (CST).
Transfection and luciferase assay
siRNAs targeted to E2f7 and E2f8 were transfected into uterine stromal cells using Lipofectamine 2000 (Invitrogen) according to the instructions. For luciferase reporter assay, the promoter sequence of mouse E2f8 was amplified by PCR from mouse genomic DNA. The corresponding primer sequences were listed in Table 1. The amplified products were digested by restriction enzymes and inserted into the pGL3-basic vector (Promega) upstream from the start codon of luciferase. E2f8-pGL3 was co-transfected with pRL-TK plasmid containing Renilla luciferase using Lipofectamine 2000 for 6 h.
Superoxide dismutase (SOD) activity assay
Decidual tissues were rinsed with PBS to remove red blood cells and clots, then homogenize the tissues in 1 ml of 20 mM HEPES buffer (pH 7.2) containing 1 mM EGTA, 210 mM mannitol and 70 mM sucrose. Cultured cells were collected by using a rubber policeman and sonicated in HEPES buffer. After centrifugation, the supernatants were collected for SOD activity detection according to the instruction of SOD assay kit (Jiancheng Bioengineering Institute, Nanjing).
Patients and endometrial sample collection
Female patients who received endocervical curettage or induced abortion operation at Renmin Hospital of Wuhan University were recruited in this study. The endometrial samples were collected during endometrium biopsy examination on days 21 to 24 (mid-secretory phase) of menstrual cycle. The decidual tissues were collected under abortion operation during 6 to 9 weeks of pregnancy. The samples were fixed in formalin and analyzed by hematoxylin-eosin staining as previously described.7 The study was approved by the Ethics Committee of Renmin Hospital of Wuhan University.
Culture and treatments of human endometrial stromal cells
Human endometrial stromal cell line (hESC, ATCC®CRL-4003™) was obtained from ATCC and cultured as described previously.47 Human stromal cells were cultured in DMEM/F12 with 2% charcoal stripped fetal calf serum, 1% antibiotics and puromycin. Cells were induced for in vitro decidualization with 10 nM estradiol-17 β (Sigma), 1 μM medroxy-progesterone acetate (MPA, Sigma) and 0.5 mM 8-Br-cAMP (Sigma) for 8 days. The media were replenished every 48 h. Prolactin (PRL) and insulin like growth factor binding protein-1 (IGFBP-1) were used as makers for in vitro decidualization.
Statistical analysis
Data are presented as the mean ± standard deviation, unless indicated otherwise. Equal variance was tested by F-test. Differences between 2 groups were compared using a t-test in normal distributed groups. Otherwise, t-test assuming unequal variance was used. In all cases, difference were considered significant at the level of P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Basic Research Program of China (2011CB944402 and 2013CB910803) and National Natural Science Foundation of China (31271602, 31471397 and 31272263).
References
- 1.Pandit SK, Westendorp B, de Bruin A. Physiological significance of polyploidization in mammalian cells. Trends Cell Biol 2013; 23: 556-66; PMID:23849927; http://dx.doi.org/ 10.1016/j.tcb.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 2.Ma X, Gao F, Rusie A, Hemingway J, Ostmann AB, Sroga JM, Jegga AG, Das SK. Decidual cell polyploidization necessitates mitochondrial activity. PLoS One 2011; 6: e26774; PMID:22046353; http://dx.doi.org/ 10.1371/journal.pone.0026774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mori M, Kitazume M, Ose R, Kurokawa J, Koga K, Osuga Y, Arai S, Miyazaki T. Death effector domain-containing protein (DEDD) is required for uterine decidualization during early pregnancy in mice. J Clin Invest 2011; 121: 318-27; PMID:21135503; http://dx.doi.org/ 10.1172/JCI44723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med 2013; 11: 154; PMID:23803387; http://dx.doi.org/ 10.1186/1741-7015-11-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sroga JM, Gao F, Ma X, Das SK. Overexpression of cyclin D3 improves decidualization defects in Hoxa-10(−/−) mice. Endocrinology 2012; 153: 5575-86; PMID:23008516; http://dx.doi.org/ 10.1210/en.2012-1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagashima T, Li Q, Clementi C, Lydon JP, DeMayo FJ, Matzuk MM. BMPR2 is required for postimplantation uterine function and pregnancy maintenance. J Clin Invest 2013; 123: 2539-50; PMID:23676498; http://dx.doi.org/ 10.1172/JCI65710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen HZ, Ouseph MM, Li J, Pecot T, Chokshi V, Kent L, Bae S, Byrne M, Duran C, Comstock G, et al.. Canonical and atypical E2Fs regulate the mammalian endocycle. Nat Cell Biol 2012; 14: 1192-202; PMID:23064266; http://dx.doi.org/ 10.1038/ncb2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lammens T, Li J, Leone G, De Veylder L. Atypical E2Fs: new players in the E2F transcription factor family. Trends Cell Biol 2009; 19: 111-8; PMID:19201609; http://dx.doi.org/ 10.1016/j.tcb.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandit SK, Westendorp B, Nantasanti S, van Liere E, Tooten PC, Cornelissen PW, Toussaint MJ, Lamers WH, de Bruin A. E2F8 is essential for polyploidization in mammalian cells. Nat Cell Biol 2012; 14: 1181-91; PMID:23064264; http://dx.doi.org/ 10.1038/ncb2585 [DOI] [PubMed] [Google Scholar]
- 10.Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science 1992; 258: 424-9; PMID:1411535; http://dx.doi.org/ 10.1126/science.1411535 [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ, et al.. The E2F1-3 transcription factors are essential for cellular proliferation. Nature 2001; 414: 457-62; PMID:11719808; http://dx.doi.org/ 10.1038/35106593 [DOI] [PubMed] [Google Scholar]
- 12.Beshiri ML, Holmes KB, Richter WF, Hess S, Islam AB, Yan Q, Plante L, Litovchick L, Gevry N, Lopez-Bigas N, et al.. Coordinated repression of cell cycle genes by KDM5A and E2F4 during differentiation. Proc Natl Acad Sci U S A 2012; 109: 18499-504; PMID:23093672; http://dx.doi.org/ 10.1073/pnas.1216724109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westendorp B, Major JL, Nader M, Salih M, Leenen FH, Tuana BS. The E2F6 repressor activates gene expression in myocardium resulting in dilated cardiomyopathy. FASEB J 2012; 26: 2569-79; PMID:22403008; http://dx.doi.org/ 10.1096/fj.11-203174 [DOI] [PubMed] [Google Scholar]
- 14.Maiti B, Li J, de Bruin A, Gordon F, Timmers C, Opavsky R, Patil K, Tuttle J, Cleghorn W, Leone G. Cloning and characterization of mouse E2F8, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem 2005; 280: 18211-20; PMID:15722552; http://dx.doi.org/ 10.1074/jbc.M501410200 [DOI] [PubMed] [Google Scholar]
- 15.de Bruin A, Maiti B, Jakoi L, Timmers C, Buerki R, Leone G. Identification and characterization of E2F7, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem 2003; 278: 42041-9; PMID:12893818; http://dx.doi.org/ 10.1074/jbc.M308105200 [DOI] [PubMed] [Google Scholar]
- 16.Endo-Munoz L, Dahler A, Teakle N, Rickwood D, Hazar-Rethinam M, Abdul-Jabbar I, Sommerville S, Dickinson I, Kaur P, Paquet-Fifield S, et al.. E2F7 can regulate proliferation, differentiation, and apoptotic responses in human keratinocytes: implications for cutaneous squamous cell carcinoma formation. Cancer Res 2009; 69: 1800-8; PMID:19223542; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2725 [DOI] [PubMed] [Google Scholar]
- 17.Deng Q, Wang Q, Zong WY, Zheng DL, Wen YX, Wang KS, Teng XM, Zhang X, Huang J, Han ZG. E2F8 contributes to human hepatocellular carcinoma via regulating cell proliferation. Cancer Res 2010; 70: 782-91; PMID:20068156; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3082 [DOI] [PubMed] [Google Scholar]
- 18.Aksoy O, Chicas A, Zeng T, Zhao Z, McCurrach M, Wang X, Lowe SW. The atypical E2F family member E2F7 couples the p53 and RB pathways during cellular senescence. Genes Dev 2012; 26: 1546-57; PMID:22802529; http://dx.doi.org/ 10.1101/gad.196238.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zalmas LP, Zhao X, Graham AL, Fisher R, Reilly C, Coutts AS, La Thangue NB. DNA-damage response control of E2F7 and E2F8. EMBO Rep 2008; 9: 252-9; PMID:18202719; http://dx.doi.org/ 10.1038/sj.embor.7401158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Ran C, Li E, Gordon F, Comstock G, Siddiqui H, Cleghorn W, Chen HZ, Kornacker K, Liu CG, et al.. Synergistic function of E2F7 and E2F8 is essential for cell survival and embryonic development. Dev Cell 2008; 14: 62-75; PMID:18194653; http://dx.doi.org/ 10.1016/j.devcel.2007.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouseph MM, Li J, Chen HZ, Pecot T, Wenzel P, Thompson JC, Comstock G, Chokshi V, Byrne M, Forde B, et al.. Atypical E2F repressors and activators coordinate placental development. Dev Cell 2012; 22: 849-62; PMID:22516201; http://dx.doi.org/ 10.1016/j.devcel.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu T, Ghazaryan S, Sy C, Wiedmeyer C, Chang V, Wu L. Concomitant inactivation of Rb and E2f8 in hematopoietic stem cells synergizes to induce severe anemia. Blood 2012; 119: 4532-42; PMID:22422820; http://dx.doi.org/ 10.1182/blood-2011-10-388231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weijts BG, Bakker WJ, Cornelissen PW, Liang KH, Schaftenaar FH, Westendorp B, de Wolf CA, Paciejewska M, Scheele CL, Kent L, et al.. E2F7 and E2F8 promote angiogenesis through transcriptional activation of VEGFA in cooperation with HIF1. EMBO J 2012; 31: 3871-84; PMID:22903062; http://dx.doi.org/ 10.1038/emboj.2012.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Lin H, Kong S, Wang S, Wang H, Armant DR. Physiological and molecular determinants of embryo implantation. Mol Aspects Med 2013; 34: 939-80; PMID:23290997; http://dx.doi.org/ 10.1016/j.mam.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci U S A 2007; 104: 18315-20; PMID:17986609; http://dx.doi.org/ 10.1073/pnas.0707909104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol 2004; 265: 181-95; PMID:14697362; http://dx.doi.org/ 10.1016/j.ydbio.2003.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sher N, Von Stetina JR, Bell GW, Matsuura S, Ravid K, Orr-Weaver TL. Fundamental differences in endoreplication in mammals and Drosophila revealed by analysis of endocycling and endomitotic cells. Proc Natl Acad Sci U S A 2013; 110: 9368-73; PMID:23613587; http://dx.doi.org/ 10.1073/pnas.1304889110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009; 9: 153-66; PMID:19238148; http://dx.doi.org/ 10.1038/nrc2602 [DOI] [PubMed] [Google Scholar]
- 29.Goga A, Yang D, Tward AD, Morgan DO, Bishop JM. Inhibition of CDK1 as a potential therapy for tumors over-expressing MYC. Nat Med 2007; 13: 820-7; PMID:17589519; http://dx.doi.org/ 10.1038/nm1606 [DOI] [PubMed] [Google Scholar]
- 30.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 2012; 18: 1754-67; PMID:23223073; http://dx.doi.org/ 10.1038/nm.3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005; 3: 28; PMID:16018814; http://dx.doi.org/ 10.1186/1477-7827-3-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirota Y, Acar N, Tranguch S, Burnum KE, Xie H, Kodama A, Osuga Y, Ustunel I, Friedman DB, Caprioli RM, et al.. Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress. Proc Natl Acad Sci U S A 2010; 107: 15577-82; PMID:20713718; http://dx.doi.org/ 10.1073/pnas.1009324107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei W, Feng XH, Deng WB, Ni H, Zhang ZR, Jia B, Yang XL, Wang TS, Liu JL, Su RW, et al.. Progesterone and DNA damage encourage uterine cell proliferation and decidualization through up-regulating ribonucleotide reductase 2 expression during early pregnancy in mice. J Biol Chem 2012; 287: 15174-92; PMID:22403396; http://dx.doi.org/ 10.1074/jbc.M111.308023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ullah Z, Lee CY, Lilly MA, DePamphilis ML. Developmentally programmed endoreduplication in animals. Cell Cycle 2009; 8: 1501-9; PMID:19372757; http://dx.doi.org/ 10.4161/cc.8.10.8325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celton-Morizur S, Merlen G, Couton D, Margall-Ducos G, Desdouets C. The insulin/Akt pathway controls a specific cell division program that leads to generation of binucleated tetraploid liver cells in rodents. J Clin Invest 2009; 119: 1880-7; PMID:19603546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pawar S, Hantak AM, Bagchi IC, Bagchi MK. Minireview: steroid-regulated paracrine mechanisms controlling implantation. Mol Endocrinol 2014; 28: 1408-22; PMID:25051170; http://dx.doi.org/ 10.1210/me.2014-1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang D, Zhang J, Jiang X, Li X, Wang Y, Ma J, Jiang H. Heparin-binding epidermal growth factor-like growth factor: a hepatic stellate cell proliferation inducer via ErbB receptors. J Gastroenterol Hepatol 2014; 29: 623-32; PMID:24303948; http://dx.doi.org/ 10.1111/jgh.12412 [DOI] [PubMed] [Google Scholar]
- 38.Large MJ, Wetendorf M, Lanz RB, Hartig SM, Creighton CJ, Mancini MA, Kovanci E, Lee KF, Threadgill DW, Lydon JP, et al.. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet 2014; 10: e1004451; PMID:24945252; http://dx.doi.org/ 10.1371/journal.pgen.1004451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CH, Kim TH, Lee JH, Oh SJ, Yoo JY, Kwon HS, Kim YI, Ferguson SD, Ahn JY, Ku BJ, et al.. Extracellular signal-regulated kinase 1/2 signaling pathway is required for endometrial decidualization in mice and human. PLoS One 2013; 0: e75282; http://dx.doi.org/ 10.1371/journal.pone.0075282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, Ku BJ, Lydon JP, Jeong JW. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB J 2013; 27: 2553-63; PMID:23531596; http://dx.doi.org/ 10.1096/fj.12-225664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shuya LL, Menkhorst EM, Yap J, Li P, Lane N, Dimitriadis E. Leukemia inhibitory factor enhances endometrial stromal cell decidualization in humans and mice. PLoS One 2011; 6: e25288; PMID:21966484; http://dx.doi.org/ 10.1371/journal.pone.0025288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox DT, Duronio RJ. Endoreplication and polyploidy: insights into development and disease. Development 2013; 140: 3-12; PMID:23222436; http://dx.doi.org/ 10.1242/dev.080531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santamaria D, Barriere C, Cerqueira A, Hunt S, Tardy C, Newton K, Caceres JF, Dubus P, Malumbres M, Barbacid M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature 2007; 448: 811-5; PMID:17700700; http://dx.doi.org/ 10.1038/nature06046 [DOI] [PubMed] [Google Scholar]
- 44.Diril MK, Ratnacaram CK, Padmakumar VC, Du T, Wasser M, Coppola V, Tessarollo L, Kaldis P. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc Natl Acad Sci U S A 2012; 109: 3826-31; PMID:22355113; http://dx.doi.org/ 10.1073/pnas.1115201109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kajihara T, Jones M, Fusi L, Takano M, Feroze-Zaidi F, Pirianov G, Mehmet H, Ishihara O, Higham JM, Lam EW, et al.. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol Endocrinol 2006; 20: 2444-55; PMID:16709600; http://dx.doi.org/ 10.1210/me.2006-0118 [DOI] [PubMed] [Google Scholar]
- 46.Zuo RJ, Zhao YC, Lei W, Wang TS, Wang BC, Yang ZM. Crystallin alphaB acts as a molecular guard in mouse decidualization: regulation and function during early pregnancy. FEBS Lett 2014; 588: 2944-51; PMID:24951838; http://dx.doi.org/ 10.1016/j.febslet.2014.05.045 [DOI] [PubMed] [Google Scholar]
- 47.Yuhki M, Kajitani T, Mizuno T, Aoki Y, Maruyama T. Establishment of an immortalized human endometrial stromal cell line with functional responses to ovarian stimuli. Reprod Biol Endocrinol 2011; 9: 104; PMID:21801462; http://dx.doi.org/ 10.1186/1477-7827-9-104 [DOI] [PMC free article] [PubMed] [Google Scholar]