Using whole-spine MR myelography in patients receiving diagnostic lumbar punctures, Wang et al. show that post-dural puncture headache is associated with more extensive and more rostral CSF leakages. Compared to other types of CSF leakage, periradicular leaks have a better spatial correlation with the dural defect introduced by lumbar puncture.
Keywords: cerebrospinal fluid leakage, magnetic resonance myelography, post-dural puncture headache, spontaneous intracranial hypotension
Using whole-spine MR myelography in patients receiving diagnostic lumbar punctures, Wang et al. show that post-dural puncture headache is associated with more extensive and more rostral CSF leakages. Compared to other types of CSF leakage, periradicular leaks have a better spatial correlation with the dural defect introduced by lumbar puncture.
Abstract
The spatial distribution and clinical correlation of cerebrospinal fluid leakage after lumbar puncture have not been determined. Adult in-patients receiving diagnostic lumbar punctures were recruited prospectively. Whole-spine heavily T2-weighted magnetic resonance myelography was carried out to characterize post-lumbar puncture spinal cerebrospinal fluid leakages. Maximum rostral migration was defined as the distance between the most rostral spinal segment with cerebrospinal fluid leakage and the level of lumbar puncture. Eighty patients (51 female/29 male, mean age 49.4 ± 13.3 years) completed the study, including 23 (28.8%) with post-dural puncture headache. Overall, 63.6% of periradicular leaks and 46.9% of epidural collections were within three vertebral segments of the level of lumbar puncture (T12–S1). Post-dural puncture headache was associated with more extensive and more rostral distributions of periradicular leaks (length 3.0 ± 2.5 versus 0.9 ± 1.9 segments, P = 0.001; maximum rostral migration 4.3 ± 4.7 versus 0.8 ± 1.7 segments, P = 0.002) and epidural collections (length 5.3 ± 6.1 versus 1.0 ± 2.1 segments, P = 0.003; maximum rostral migration 4.7 ± 6.7 versus 0.9 ± 2.4 segments, P = 0.015). In conclusion, post-dural puncture headache was associated with more extensive and more rostral distributions of periradicular leaks and epidural collections. Further, visualization of periradicular leaks was not restricted to the level of dural defect, although two-thirds remained within the neighbouring segments.
Introduction
Post-dural puncture headache (PDPH) occurs in up to one-third of patients receiving lumbar punctures (Kuntz et al., 1992; Strupp et al., 2001; Bezov et al., 2010a; van Oosterhout et al., 2013), and typically presents as an orthostatic headache that occurs within 5 days after dural puncture (Headache Classification Committee of the International Headache Society, 2004; Bezov et al., 2010a). CSF leakage has been considered the cause of PDPH as it is the most evident consequence of lumbar punctures. However, the association between PDPH and the amount or pattern of post-lumbar puncture CSF leakage has been inconclusive (Iqbal et al., 1995; Sakurai et al., 2012, 2013; Fakhran et al., 2014).
Findings of conventional T2-weighted spinal MRI in PDPH include spinal hygroma, and retrospinal focal fluid collection, and only occasionally, the spinal CSF leaks could be visualized directly (Iqbal et al., 1995; Vakharia et al., 1997; Yousry et al., 2001; Burtis et al., 2005). Heavily T2-weighted magnetic resonance myelography (MRM) is a non-invasive radiation-free imaging technique with excellent sensitivity for signals of water/CSF. It has been demonstrated to be comparable to computed tomographic myelography (CTM), the purported gold standard, in localizing spinal CSF leaks in spontaneous intracranial hypotension (Tsai et al., 2007, 2008; Wang et al., 2009). More importantly, acquisition of heavily T2-weighted MRM involves neither intravenous or intrathecal contrast administration nor radiation exposure. With the advent of heavily T2-weighted MRM, post-lumbar puncture CSF leakage could be localized and quantified systemically. Further, periradicular leaks, i.e. CSF leaks along the nerve roots, on heavily T2-weighted MRM or CTM have been used as targets for targeted epidural blood patches or even surgical intervention in the treatment of spontaneous intracranial hypotension (Schievink, 2008; Wang et al., 2009). However, a substantial proportion of patients with spontaneous intracranial hypotension did not have dural defects with active CSF leakage at surgery at the location of spinal CSF leaks identified on CTM (Cohen-Gadol et al., 2006). Whether the visualized periradicular leaks accurately co-localize with the actual dural defects is yet to be confirmed.
Subjects and methods
Study participants
This was a prospective study involving adult in-patients who received diagnostic lumbar punctures. Lumbar punctures were carried out using 22-gauge Quincke needles through the midline L3–4 interspace with a standard protocol (Ellenby et al., 2006). The amount of CSF collected was ∼5 cc, except for patients with suspected normal pressure hydrocephalus or increased intracranial pressure, whereby the amount of CSF drainage was around 20–30 cc. After the procedure, the patients were instructed to remain recumbent for 6 h, and were observed for 5 days for the development of PDPH. Heavily T2-weighted MRM was carried out immediately when the MRI machine was available. The inclusion and exclusion criteria are provided in the Supplementary material. The study protocol was approved by the Institutional Review Board of Taipei Veterans General Hospital and all participants gave written informed consent before entering the study.
Clinical assessment and management
The diagnosis of PDPH was made according to the ICHD-2 criteria (Headache Classification Committee of the International Headache Society, 2004). Lumbar epidural blood patches were performed when purely conservative measures failed (Bezov et al., 2010b).
Heavily T2-weighted magnetic resonance myelography
Whole-spine (C1–S5) axial heavily T2-weighted MRM was acquired after lumbar puncture on a 1.5 T superconducting system (Signa HDxt or Signa Excite, GE Medical Systems) as previously described (Wang et al., 2009). Heavily T2-weighted MRMs were interpreted by a senior neuroradiologist (J.-F.L.) blinded to the clinical conditions. Post-lumbar puncture CSF leakages were assessed with three measures: periradicular leaks (i.e. CSF leaks along the nerve roots), epidural collections, and retrospinal collections (Wang et al., 2009). Technical aspects of heavily T2-weighted MRM and further descriptions of the CSF leakages are provided in the Supplementary material. In addition to localization of the CSF leakages, the numbers of spinal segments with periradicular leaks and epidural collections visualized on heavily T2-weighted MRM were used as surrogates to semi-quantify post-lumbar puncture CSF leakage. Maximum rostral migration (RMax) was defined as the number of spinal segments between L3 and the most rostral segment with CSF leakage.
Statistical analysis
When comparing patients with and without PDPH, categorical data were analysed with the χ2 test, and the Student’s t-test was used for continuous variables. ANOVA was used for comparisons among (i) patients without PDPH; (ii) patients with PDPH but without epidural blood patch; and (iii) patients with PDPH and epidural blood patch; and post hoc analysis was performed using the least significant difference (LSD) method. Multivariate logistic regression modelling using the forward (Wald) method was carried out to determine factors predictive of PDPH, and their odds ratios (ORs) and 95% confidence intervals (CIs). For the multivariate analysis, factors with a P-value of < 0.05 in the univariate analyses were included, and adjustment for age and gender was made. All statistical analyses were carried out using PASW Statistics 18 for Windows (SPSS). All calculated P-values were two-tailed, and statistical significance was defined as P < 0.05.
Results
Study participants
During the study period (October 2010 to September 2013), 109 patients who received diagnostic lumbar puncture were screened, and 29 were excluded: MRI not available before discharge in 22, ineligible age in six, and withdrawal of consent in one. Eighty patients (51 female/29 male, age 49.4 ± 13.3 years, range 20–79) completed the study (Supplementary Table 1), and 23 (28.8%) developed PDPH. Six patients failed conservative treatment, and responded to single lumbar epidural blood patches.
Post-lumbar puncture spinal CSF leakage on heavily T2-weighted MRM
The mean interval between lumbar puncture and heavily T2-weighted MRM was 2.4 ± 2.1 days (range 0–12), and the cumulative percentages of patients receiving heavily T2-weighted MRM within 2, 3, 4 and 5 days were 71.3%, 81.3%, 88.8% and 92.5%, respectively. Overall, 40 patients (50.0%) had periradicular leaks, 33 (41.3%) had epidural collections, and 30 (37.5%) had retrospinal collections (Fig. 1 and Table 1). Patients with periradicular leaks (age 45.6 ± 12.3 versus 53.1 ± 13.4 years, P = 0.011) or epidural collections (age 45.7 ± 12.1 versus 52.0 ± 13.7 years, P = 0.037) were younger than those without, whereas gender distribution, opening or closing pressure at lumbar puncture, body weight and BMI were comparable (data not shown).
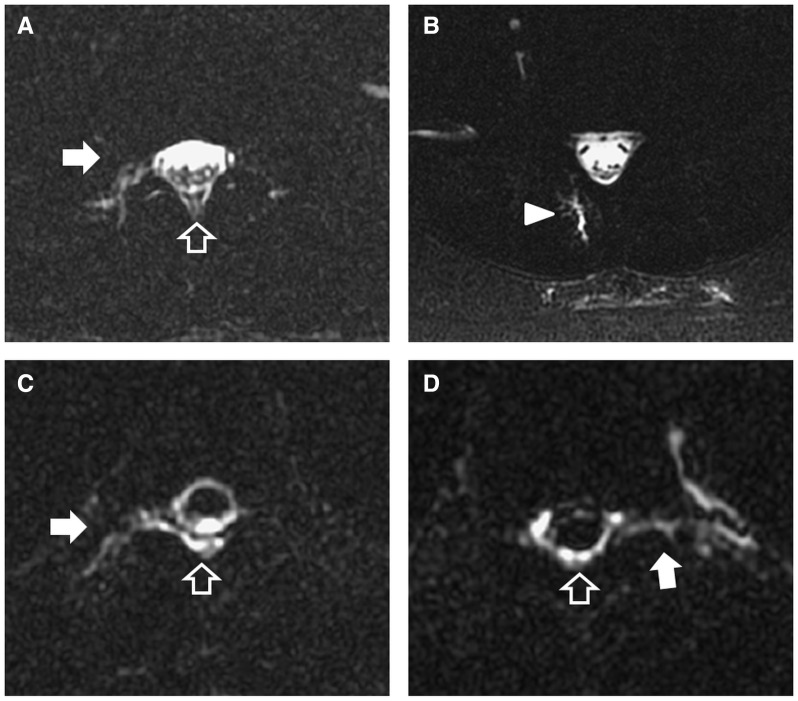
Figure 1.
Spinal CSF leakage in patients with post-dural puncture headache on heavily T2-weighted magnetic resonance myelography. Right periradicular leaks (filled arrows) and epidural collections (open arrow) at L2 (A) and L5 retrospinal collections (arrowhead; B) in a 43-year-old female patient. Right T5 periradicular leak (filled arrow; C) and left T10 periradicular leak (filled arrow; D) with epidural collections (open arrows) in a 40-year-old male patient (C and D).
Table 1.
Clinical characteristics and imaging findings in patients with and without PDPH
| All patients | PDPH (n = 23) | No PDPH (n = 57) | P-value* | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 49.4 ± 13.3 | 44.2 ± 12.8 | 51.4 ± 13.1 | 0.027 |
| Female patients (%) | 63.8 | 69.6 | 61.4 | 0.492 |
| Height (cm) | 163.5 ± 8.9 | 164.5 ± 9.5 | 163.0 ± 8.7 | 0.552 |
| Body weight (kg) | 65.2 ± 13.4 | 63.2 ± 12.6 | 66.0 ± 13.8 | 0.404 |
| Body-mass index | 24.3 ± 4.4 | 23.2 ± 3.3 | 24.7 ± 4.8 | 0.167 |
| Indication for lumbar puncture | ||||
| Suspected symptomatic headache | 50% | 52.2% | 49.1% | 0.805 |
| Pressure | ||||
| Opening pressure (mmH2O) | 160.3 ± 54.4 | 151.6 ± 40.0 | 162.9 ± 58.1 | 0.472 |
| Closing pressure (mmH2O) | 113.2 ± 42.0 | 112.3 ± 42.9 | 113.5 ± 42.1 | 0.923 |
| Any CSF leakage on HT2W MRM | 67.5% | 100% | 54.4% | <0.001 |
| Periradicular leaks | 50% | 95.7% | 31.6% | <0.001 |
| Length (seg) | 1.5 ± 2.3 | 3.0 ± 2.5 | 0.9 ± 1.9 | 0.001 |
| Cervical (%) | 1.3% | 4.3% | 0% | 0.113 |
| Thoracic (%) | 23.8% | 52.2% | 12.3% | <0.001 |
| RMax (seg) | 1.8 ± 3.3 | 4.3 ± 4.7 | 0.8 ± 1.7 | 0.002 |
| Epidural collections | 41.3% | 69.6% | 29.8% | 0.001 |
| Length (seg) | 2.2 ± 4.2 | 5.3 ± 6.1 | 1.0 ± 2.1 | 0.003 |
| Cervical (%) | 2.5 | 8.7 | 0 | 0.024 |
| Thoracic (%) | 20 | 34.8 | 14.8 | 0.036 |
| RMax (seg) | 2.0 ± 4.4 | 4.7 ± 6.7 | 0.9 ± 2.4 | 0.015 |
| Retrospinal collections | 37.5% | 52.2% | 31.6% | 0.085 |
HT2W = heavily T2-weighted; seg = segments; RMax = maximum rostral migration. Data are expressed as mean ± standard deviation or percentage. *Comparison between patients with and without PDPH.
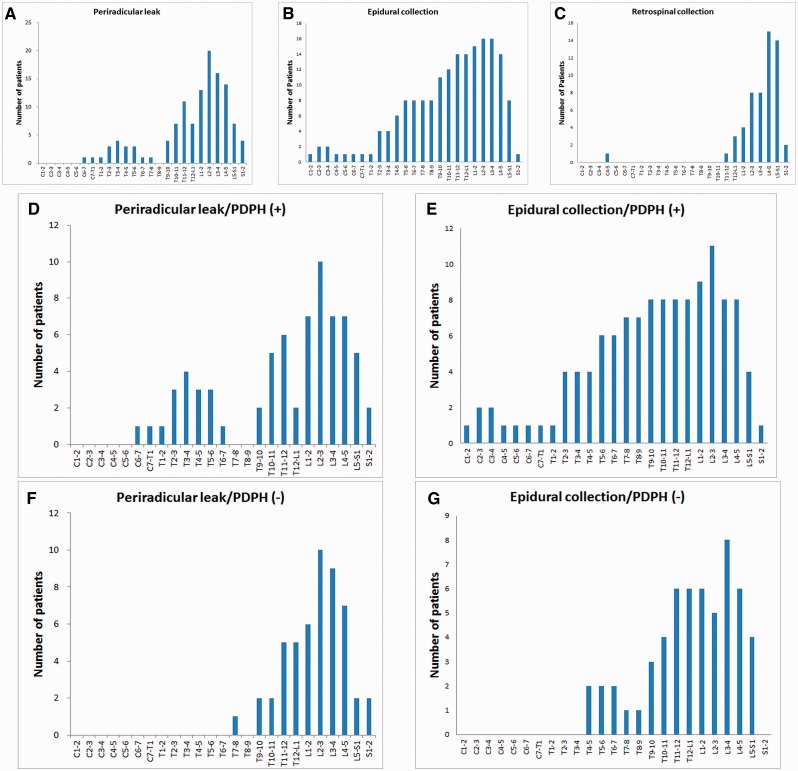
In patients with periradicular leaks, 65% had more than one spinal vertebral segment involved, whereas 84.8% of patients with epidural collections had multiple segment involvement. On average, periradicular leaks were visualized at 1.5 ± 2.3 segments, and the mean length of epidural collections were 2.2 ± 4.2 segments. The distribution of periradicular leaks centred around the upper lumbar region (85.1% between T9 and S2) (Fig. 2A–C). Epidural collections were most commonly seen between the mid-thoracic to the upper lumbar regions (85.9% between T5 and S1). Retrospinal collections mostly accumulated in the lower lumbar region (92.9% between T12 and S1). In total, 52 patients (65.0%) had evidence of spinal CSF leakages beyond L3–4, and 63.6% of the periradicular leaks and 46.9% of the epidural collections were seen between T12 and S1, i.e. within three segments of the lumbar puncture level.
Figure 2.
Distribution of spinal CSF leakages. In general, periradicular leaks were most commonly seen around the upper lumbar region (A), epidural collections were mainly distributed between middle thoracic to upper lumbar regions (B), and retrospinal collections were primarily seen in the lower lumbar region (C). When analysed separately, patients with post-dural puncture headache (D and E) had more rostral distributions of periradicular leaks (D and F) and epidural collections (E and G) than those without (F and G).
Comparisons between patients with and without PDPH
Patients with PDPH were younger than those without (age 44.2 ± 12.8 versus 51.4 ± 13.1, P = 0.027) (Table 1) and were more likely to have periradicular leaks (95.7% versus 31.6%, P < 0.001), and epidural collections (69.6% versus 29.8%, P < 0.001), but not retrospinal collections (52.2% versus 31.6%, P = 0.085). Compared to those without, patients with PDPH also had more extensive and more rostral distributions of periradicular leaks (length 3.0 ± 2.5 versus 0.9 ± 1.9 segments, P = 0.001; RMax 4.3 ± 4.7 versus 0.8 ± 1.7 segments, P = 0.002), and epidural collections (length 5.3 ± 6.1 versus 1.0 ± 2.1 segments, P = 0.003; RMax 4.7 ± 6.7 versus 0.9 ± 2.4 segments, P = 0.015) (Fig. 2D–F). Multivariate logistic regression analysis identified two factors associated with PDPH: the presence of periradicular leaks (OR 43.5, 95% CI 4.5–420.9, P = 0.001) and the length of epidural collections (OR 1.3 per segment, 95% CI 1.1–1.5, P = 0.011).
Epidural blood patches in patients with PDPH
The need for epidural blood patch was associated with longer and more rostral distributions of epidural collections (length 8.0 ± 4.2; RMax 7.7 ± 8.0 segments), as compared to patients without PDPH (length 1.0 ± 2.1, P < 0.001; RMax 0.9 ± 2.4 segments, P < 0.001), and patients with PDPH responding to conservative measures (length 4.4 ± 5.8, P = 0.037; RMax 3.6 ± 6.1 segments, P = 0.039) (Table 2). Further, 91.1% of patients who responded to conservative treatment did not have epidural collections above T9, whereas 95.8% of patients without epidural collections above T9 did not need epidural blood patches. By multivariate logistic regression analysis, only the length of epidural collections was predictive of epidural blood patch among all study participants (OR 1.2 per segment, 95% CI 1.1–1.4, P = 0.005).
Table 2.
Comparisons among patients without PDPH, with PDPH but not epidural blood patch, and with PDPH and epidural blood patches
| 1: PDPH(−) | 2: PDPH(+)/EBP(−) | 3: PDPH(+)/EBP(+) | P-value | |
|---|---|---|---|---|
| Periradicular leaks | ||||
| Length (seg) | 0.9 ± 1.9 | 2.6 ± 1.8 | 4.2 ± 3.8 | 1 versus 2 P = 0.003 |
| 2 versus 3 P = 0.122 | ||||
| 1 versus 3 P < 0.001 | ||||
| RMax (seg) | 0.8 ± 1.7 | 3.8 ± 4.4 | 5.5 ± 5.9 | 1 versus 2 P < 0.001 |
| 2 versus 3 P = 0.228 | ||||
| 1 versus 3 P < 0.001 | ||||
| Epidural collections | ||||
| Length (seg) | 1.0 ± 2.1 | 4.4 ± 5.8 | 8.0 ± 4.2 | 1 versus 2 P = 0.001 |
| 2 versus 3 P = 0.037 | ||||
| 1 versus 3 P < 0.001 | ||||
| RMax (seg) | 0.9 ± 2.4 | 3.6 ± 6.1 | 7.7 ± 8.0 | 1 versus 2 P = 0.017 |
| 2 versus 3 P = 0.039 | ||||
| 1 versus 3 P < 0.001 | ||||
RMax = maximum rostral migration; EBP = epidural blood patch.
Discussion
The findings suggest that the extent of CSF leakage on heavily T2-weighted MRM following diagnostic lumbar puncture parallels the risk of PDPH, as evidenced by higher detection rates and more extensive distributions of periradicular leaks and epidural collections. This is in line with Bier’s hypothesis that continued CSF leakage through the lumbar puncture site could be an important contributing factor for PDPH (Raskin, 1990). In fact, the risk of PDPH increases with a larger needle size, which could lead to larger dural defects and thus more likely persistent leakage following the procedure (Carson and Serpell, 1996). Nevertheless, not all patients with periradicular leaks or epidural collections developed PDPH. Although the data showed that younger individuals were more likely to have periradicular leaks and epidural collections, other factors that could modify the susceptibility to PDPH remain to be determined. For example, the speed of CSF extravasation could possibly play a role, and this could better be assessed with dynamic imaging techniques, such as dynamic CTM (Luetmer and Mokri, 2003).
Heavily T2-weighted MRM has a unique role in studying PDPH for its lack of radiation exposure, non-invasiveness and ability to delineate spinal CSF leakages. Ethical issues could arise for the use of invasive imaging techniques, such as CTM, radionuclide cisternography, or MRM with intrathecal gadolinium, i.e. gadolinium-enhanced magnetic resonance cisternography (Albayram et al., 2008). Dural punctures involved in these techniques could potentially aggravate CSF leakages and the associated symptoms, and would also lead to difficulty ascertaining the origin of the observed CSF leakages. In addition, the advantages of heavily T2-weighted MRM over conventional T2-weighted spinal MRIs were illustrated by its comparability to CTM in directly demonstrating spinal CSF leakages, especially periradicular leaks (Tsai et al., 2007, 2008; Wang et al., 2009). In contrast to the current findings, existing literature showed conflicting data regarding the correlation between the amount of CSF leakage and the risk of PDPH (Iqbal et al., 1995; Bezov et al., 2010a; Sakurai et al., 2013; Fakhran et al., 2014). Interestingly, the only large-scale study also identified epidural collections as a risk factor for PDPH in patients undergoing lumbar CTM (Fakhran et al., 2014), although the extent of epidural collections was not assessed. On the other hand, other studies were retrospective in design, had a small sample size, and more importantly, assessed only a limited proportion of the spinal regions (Iqbal et al., 1995; Sakurai et al., 2013). The whole-spine axial heavily T2-weighted MRM used in the present study provided a more comprehensive quantification of post-lumbar puncture spinal CSF leakages, and thus could be a more reliable measure.
Even though periradicular leaks on heavily T2-weighted MRM could be visualized beyond the level of lumbar puncture, 63.6% of the leaks were located within three segments. This could suggest that the majority of dural defects might be located in close proximity to the observed periradicular leaks. As the main bulk of epidural blood patches extends three to five segments from the injection sites (Beards et al., 1993; Vakharia et al., 1997), periradicular leaks on heavily T2-weighted MRM should be a reasonable guide for targeted epidural blood patches. On the other hand, as heavily T2-weighted MRM is radiographically comparable to CTM in localizing spinal CSF leaks (Wang et al., 2009), it is possible that periradicular leaks on heavily T2-weighted MRM or CTM may not be accurate enough as a guide for surgical intervention. In fact, only a minority of CSF leaks identified on preoperative CTM corresponded to dural defects with active CSF leakage at surgery in patients with spontaneous intracranial hypotension, even in those with a ‘leaking diverticulum’ on CTM (Cohen-Gadol et al., 2006). Moreover, it should be noted that a large proportion of ‘spinal CSF leaks’ detected by MRM with intrathecal gadolinium (Albayram et al., 2008) could overlap with post-lumbar puncture periradicular leaks. Caution should be exercised in the interpretation of ‘spinal CSF leaks’ on MRM with intrathecal gadolinium, as such leaks could result from the lumbar punctures for intrathecal gadolinium injection rather than from the spontaneous intracranial hypotension disease process.
Post-lumbar puncture epidural collections had a wider range of distribution than periradicular leaks. After extravasation from the dural rent resulting from lumbar puncture, CSF drainage could follow the direction of least resistance or gravity. The extravasated CSF could leave the spinal column via the neuroforamina immediately at the same level of dural defects or after traveling in the epidural space for a distance, which appears as periradicular leaks. On the other hand, following shrinkage of the thecal sac, accumulation of epidural collections (Fig. 2B) could reflect the anatomy of the spine, as the mid- to lower thoracic region is the dependent part when the patients lie supine. Although the clinical utility of epidural collections in localizing dural defects is limited, its distribution could be of some help in patient selection for epidural blood patches. Epidural blood patches have been the mainstay of treatment for PDPH, especially when conservative measures fail (Bezov et al., 2010b). Based on our results, the vast majority (95.8%) of patients with epidural collections not extending above T9 responded to conservative measures, and therefore prophylactic epidural blood patches may not be suitable for such patients.
The limitations are addressed as follows. First, the study population consisted of a large proportion of headache patients and middle-aged females, and whether such selection bias could have an impact on our results was uncertain. However, it was recently reported that PDPH was not more common among migraineurs (van Oosterhout et al., 2013), and suspected symptomatic headache was not a risk factor for PDPH in the present study. Second, lumbar puncture was carried out with 22-gauge Quincke needles. Whether the amount and distribution of spinal CSF leakage with other needle sizes or atraumatic needles could differ is yet to be determined. Third, there were variable intervals between lumbar puncture and heavily T2-weighted MRM, and it is not certain how this could alter the pattern of CSF leakages. Nevertheless, the clinical and imaging findings should be temporally correlated, as all of the PDPH patients received heavily T2-weighted MRMs while they were symptomatic. Fourth, the lumbar punctures in the present study were performed under the guidance of bony landmarks rather than fluoroscopy, and it is possible that some of the lumbar punctures might be carried out at levels other than L3–4. Last, the findings are based on the consequences of dural defects introduced by lumbar puncture. It remains to be determined to what extent the findings may apply to dural defects that occur spontaneously as in the case of spontaneous intracranial hypotension.
In conclusion, this study suggests that PDPH is associated with more extensive and more rostral spinal CSF leakages, and the presence of periradicular leaks and the length of epidural collections on heavily T2-weighted MRM could serve as useful indicators for PDPH. Visualization of spinal CSF leakages beyond the level of lumbar puncture was common. However, a substantial proportion of the visualized periradicular leaks remained in close proximity to the spinal level of dural defects. On the other hand, epidural collections had a more extensive distribution.
Funding
This study was supported in part by grants from the National Science Council (NSC) of Taiwan (NSC 102-2321-B-010-030, NSC 102-2314-B-075-054, 100-2314-B-010-018-MY3, and 99-2314-B-075-036-MY3), Taipei Veterans General Hospital (VGHUST102-G7-6-1, V102C-118, V102E9-001, V102B-039), NSC support for the Center for Dynamical Biomarkers and Translational Medicine, National Central University (NSC 101-2911-I-008-001, NSC 102-2911-I-008-001), the Brain Research Center, National Yang-Ming University, and a grant from the Ministry of Education (Aim for the Top University Plan). No additional external funding was received for this study.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- CTM
computed tomographic myelography
- MRM
magnetic resonance myelography
- PDPH
post-dural puncture headache
References
- Albayram S, Kilic F, Ozer H, Baghaki S, Kocer N, Islak C. Gadolinium-enhanced MR cisternography to evaluate dural leaks in intracranial hypotension syndrome. AJNR Am J Neuroradiol. 2008;29:116–21. doi: 10.3174/ajnr.A0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards SC, Jackson A, Griffiths AG, Horsman EL. Magnetic resonance imaging of extradural blood patches: appearances from 30 min to 18 h. Br J Anaesth. 1993;71:182–8. doi: 10.1093/bja/71.2.182. [DOI] [PubMed] [Google Scholar]
- Bezov D, Lipton RB, Ashina S. Post-dural puncture headache: part I diagnosis, epidemiology, etiology, and pathophysiology. Headache. 2010a;50:1144–52. doi: 10.1111/j.1526-4610.2010.01699.x. [DOI] [PubMed] [Google Scholar]
- Bezov D, Ashina S, Lipton R. Post-dural puncture headache: Part II–prevention, management, and prognosis. Headache. 2010b;50:1482–98. doi: 10.1111/j.1526-4610.2010.01758.x. [DOI] [PubMed] [Google Scholar]
- Burtis MT, Ulmer JL, Miller GA, Barboli AC, Koss SA, Brown WD. Intradural spinal vein enlargement in craniospinal hypotension. AJNR Am J Neuroradiol. 2005;26:34–8. [PMC free article] [PubMed] [Google Scholar]
- Carson D, Serpell M. Choosing the best needle for diagnostic lumbar puncture. Neurology. 1996;47:33–7. doi: 10.1212/wnl.47.1.33. [DOI] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Mokri B, Piepgras DG, Meyer FB, Atkinson JL. Surgical anatomy of dural defects in spontaneous spinal cerebrospinal fluid leaks. Neurosurgery. 2006;58(4 Suppl 2) doi: 10.1227/01.NEU.0000204712.16099.FB. ONS-238-45; discussion ONS-45. [DOI] [PubMed] [Google Scholar]
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Eng J Med. 2006;355:e12. doi: 10.1056/NEJMvcm054952. [DOI] [PubMed] [Google Scholar]
- Fakhran S, Palfey S, Thomas A, Schwarz S, Alhilali L. Incidental findings of CSF leakage in patients without spontaneous intracranial hypotension and development of post-dural puncture headache. Eur Radiol. 2014;24:827–33. doi: 10.1007/s00330-013-3070-0. [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24(Suppl 1): 9–160. [DOI] [PubMed]
- Iqbal J, Davis LE, Orrison WW., Jr An MRI study of lumbar puncture headaches. Headache. 1995;35:420–2. doi: 10.1111/j.1526-4610.1995.hed3507420.x. [DOI] [PubMed] [Google Scholar]
- Kuntz KM, Kokmen E, Stevens JC, Miller P, Offord KP, Ho MM. Post-lumbar puncture headaches: experience in 501 consecutive procedures. Neurology. 1992;42:1884–7. doi: 10.1212/wnl.42.10.1884. [DOI] [PubMed] [Google Scholar]
- Luetmer PH, Mokri B. Dynamic CT myelography: a technique for localizing high-flow spinal cerebrospinal fluid leaks. AJNR Am J Neuroradiol. 2003;24:1711–4. [PMC free article] [PubMed] [Google Scholar]
- Raskin NH. Lumbar puncture headache: a review. Headache. 1990;30:197–200. doi: 10.1111/j.1526-4610.1990.hed3004197.x. [DOI] [PubMed] [Google Scholar]
- Sakurai K, Matsukawa N, Okita K, Nishio M, Shimohira M, Ozawa Y, et al. Lumbar puncture-related cerebrospinal fluid leakage on magnetic resonance myelography: is it a clinically significant finding? BMC Anesthesiol. 2013;13:35. doi: 10.1186/1471-2253-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Nishio M, Yamada K, Shimohira M, Ozawa Y, Matsukawa N, et al. Comparison of the radioisotope cisternography findings of spontaneous intracranial hypotension and iatrogenic cerebrospinal fluid leakage focusing on chronological changes. Cephalalgia. 2012;32:1131–9. doi: 10.1177/0333102412459571. [DOI] [PubMed] [Google Scholar]
- Schievink WI. Spontaneous spinal cerebrospinal fluid leaks. Cephalalgia. 2008;28:1345–56. doi: 10.1111/j.1468-2982.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- Strupp M, Schueler O, Straube A, Von Stuckrad-Barre S, Brandt T. “Atraumatic” Sprotte needle reduces the incidence of post-lumbar puncture headaches. Neurology. 2001;57:2310–2. doi: 10.1212/wnl.57.12.2310. [DOI] [PubMed] [Google Scholar]
- Tsai PH, Fuh JL, Lirng JF, Wang SJ. Heavily T2-weighted MR myelography in patients with spontaneous intracranial hypotension: a case-control study. Cephalalgia. 2007;27:929–34. doi: 10.1111/j.1468-2982.2007.01376.x. [DOI] [PubMed] [Google Scholar]
- Tsai PH, Fuh JL, Lirng JF, Wang SJ. Comparisons between heavily T2-weighted MR and CT myelography studies in two patients with spontaneous intracranial hypotension. Cephalalgia. 2008;28:653–7. doi: 10.1111/j.1468-2982.2008.01562.x. [DOI] [PubMed] [Google Scholar]
- Vakharia SB, Thomas PS, Rosenbaum AE, Wasenko JJ, Fellows DG. Magnetic resonance imaging of cerebrospinal fluid leak and tamponade effect of blood patch in postdural puncture headache. Anesth Analg. 1997;84:585–90. doi: 10.1097/00000539-199703000-00022. [DOI] [PubMed] [Google Scholar]
- van Oosterhout WP, van der Plas AA, van Zwet EW, Zielman R, Ferrari MD, Terwindt GM. Postdural puncture headache in migraineurs and nonheadache subjects: a prospective study. Neurology. 2013;80:941–8. doi: 10.1212/WNL.0b013e3182840bf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Lirng JF, Fuh JL, Hseu SS, Wang SJ. Heavily T2-weighted MR myelography vs CT myelography in spontaneous intracranial hypotension. Neurology. 2009;73:1892–8. doi: 10.1212/WNL.0b013e3181c3fd99. [DOI] [PubMed] [Google Scholar]
- Yousry I, Forderreuther S, Moriggl B, Holtmannspotter M, Naidich TP, Straube A, et al. Cervical MR imaging in postural headache: MR signs and pathophysiological implications. AJNR Am J Neuroradiol. 2001;22:1239–50. [PMC free article] [PubMed] [Google Scholar]