Abstract
Certain anatomical and physiological aspects of the meninges and cerebrospinal fluid. By Lewis H. Weed (Department of Anatomy, Johns Hopkins University). Brain 1935; 58: 383–97. With The vascular factor in intracranial pressure and the maintenance of the cerebrospinal fluid circulation. By John E.A. Connell. St. Bartholomew’s Hospital. Brain 1943; 66: 204–28. With Lumbar puncture headache. By G.W. Pickering (From the Medical Clinic, St. Mary’s Hospital Medical School, London). Brain 1948; 71: 274–80
Sir George Pickering (1904–80) writes of
‘pain … more or less generalized over the calvarium … especially severe at the back of the head and [spreading] into the neck, shoulders or back … [with a] reaction to change of posture, its very striking exacerbation when the patient sits up, and its relief when the patient lies down … the headache [beginning] a few hours to a few days after lumbar puncture, and … usually [lasting] twenty-four hours or more’.
Cerebrospinal fluid had come a long way since Galen of Pergamon (130–200) declared the ventricles to be reservoirs for the animal spirits made in the brain that, mixed with air taken in via the cribriform plate, subserves all bodily motion and sensation, the detritus passing back through the cribriform plate if gaseous or via the pituitary fossa if phlegmatic. It followed that the seat of all mental activities was in the ventricles. From this emerged the medieval cell doctrine articulated by the Early Church Fathers in the 5th and 6th centuries AD: the first cell (our lateral ventricles) manages ‘common sense’; the second (our third) ‘judgment’ and ‘reasoning’ with ‘imagination’ straddling these two; and the third cell (our fourth ventricle) has to do with ‘memory’. With some variations of theme, nothing much changed for a millennium until Leonardo da Vinci (1452–1519) and, later, Andreas Vesalius (1514–64) took off the top of the head and had a look inside. Ideas on the ventricular structure were further developed by (Guilio Cesare) Aranzi (1530–89). Vesalius took mental functions out of the ventricles. He rejected many Galenic doctrines but left it that air drawn in during respiration and vital spirits delivered from the heart are converted within the ventricles into the animal spirits that work the organs of the senses and motion. Gradually thereafter, the brain became more prominent as the source of motion and mentation and the role of cerebrospinal fluid diminished in importance. It was left to (Domenico Felice Antonio) Cotugno (1736–1822), writing in De ischiade nervosa commentarius (1764), to describe with accuracy the distribution and circulation of cerebrospinal fluid; and, especially, to show that the ventricular and spinal fluids are continuous and derived from the blood stream. But embedded in a book on sciatica, not much attention was given to Cotugno’s work until François Magendie (1783–1955) perfected anatomical description of the structures that contain the cerebrospinal fluid [allowing the midline foramen that connects the fourth ventricle to the cisterna magna to be named after him, followed by those of (Hubert von) Luschka (1820–75)]; showing where the fluid lies within the meninges; where it comes from; and what it does and does not do. Finally, it was Magendie who coined the term ‘cerebrospinal fluid’. There were details yet to be resolved on how cerebrospinal fluid is elaborated by the choroid plexuses; the nature of the blood–cerebrospinal fluid barrier; and the removal of fluid through the cranial and spinal arachnoid villi. This was the work of Axel Key (1832–1901), Gustav Retzius (1842–1919), Walter Dandy (1886–1946) and Edwin Goldmann (1863–1913). Probably the most dedicated cerebrospinal fluid scientist of that era was Lewis Hill Weed (1886–1952) who addressed the issues of pressure and flow in the ventricular system, and the effects of respiration and the cardiac pulse.
But first it was necessary to have a means of studying the cerebropsinal fluid in life. We are indebted to Jos Frederiks and Peter Koehler for providing an account of the history of lumbar puncture (Frederiks JA, Koehler PJ. The first lumbar puncture. Journal of the History of Neuroscience 1997; 6: 147–53). They credit Heinrich Irenäus Quincke (1842–1922) with the introduction of this procedure rather than Walter Essex Wynter (1860–1945). Working at the Middlesex Hospital (now demolished), Wynter drained cerebrospinal fluid in four children with tuberculous meningitis using a lumbar incision and insertion of a tube to relieve pressure, but all four patients died (Wynter WE. Four cases of tubercular meningitis in which paracentesis of the theca vertebralis was perforated for the relief of fluid pressure. Lancet 1891; i: 981–2). The technique for needle lumbar puncture was introduced by Quinke later in 1891 (Die Lumbalpunction des Hydrocephalus. Berliner Klinische Wochenschrift 1891; 28: 929-33; 965–68). The procedure was not free from controversy. Arthur Wentworth (nk) an assistant professor at the Harvard Medical School, based at the Children’s Hospital in Boston, used lumbar puncture experimentally in normal neonates and older children in order to study the effects of manipulating changes in the pressure of cerebrospinal fluid arguing, not to the satisfaction of his critics, that if the procedure was harmless other than inducing a brief cry of momentary pain, it could be used well in advance of the moribund state that patients reached before anyone attempted to drain their cerebrospinal fluid (Wentworth AH. Some experimental work on lumbar puncture of the subarachnoid space. Boston Medical and Surgical Journal 1896; 135: 132–6; 156–61). It is thought likely that this disregard of the children’s welfare prevented Wentworth from becoming part of the faculty and professor of paediatrics at the newly established John Hopkins School of Medicine.
In his paper in Brain, based on a lecture given in London at the Second International Neurological Congress on 30 July 1930, Weed addresses the embryological origins of the three layers of meninges, accepting that they develop both from the mesoderm and neural crest. There follows a pulsatile element as fluid produced in the choroid plexuses forces open the developing subarachnoid space. But despite the long historical trail, evidently there are still some die-hards who insist that vascular tufts of the choroid plexus with their cylindrical epithelium are the organs of absorption rather than production of the cerebrospinal fluid. The fluid flows through the ventricular system to the basilar cisterns, slowly over the surface of the cerebral hemispheres, and ‘tardily’ down the spinal cord, gathering material derived from the perivascular channels, and—as Lewis Weed had himself suggested in 1914—then being absorbed into venous sinuses through the arachnoid villi. Opinions differ on whether the fluid is a filtrate or secretion. On this, Weed accepts the view of Louis Flexner (1902–96) that ultrafiltration under pressure exerted across the blood–cerebrospinal fluid barrier is not a sufficient explanation for its formation and that it is a secretion. As for absorption, his position is that this results from hydrostatic forces acting across the subarachnoid–venous barrier (∼15–35 mm in four-legged animals) and the balance of osmotic attractions (contributing 250–300 mm) exerted by colloids present in venous blood and spinal fluid. He has investigated osmotic gradients and hydrostatic pressure experimentally. Although the osmotic forces are constant, four-legged animals have rather low hydrostatic pressure gradients. Self-evidently, these alter with assumption of the standing posture. It follows that much has yet to be learned about pressure changes in the cerebrospinal fluid in humans. The Monro-Kellie doctrine [Alexander Monro (1733–1817); and George Kellie (1770–1829)] states that the volume of the intracranial contents is constant and therefore that the venous volume (and pressure) vary inversely with the arterial pulse; and (Sir) George Burrows (1801–87) has added volume and pressure of the cerebrospinal fluid into this equation as part of the doctrine of events contained within the ‘closed [cranial] box’. But it seems likely that this doctrine is excessively rigid. Changes in posture are to some extent accommodated by expansion and contraction of the venous vascular bed; by elasticity of the coverings and bony structures of the brain and the epidural space of the spinal meninges; and, perhaps, also ‘in the occipito-atlantoid ligament and the various intervertebral structures’. Since elasticity is evident with head-down or tail-down movement from the horizontal to vertical posture in mammals, it is not necessary to argue that adoption of the erect posture in man has involved entirely new mechanisms in order to accommodate change in hydrostatic pressure. But it does seem likely that there is a slight shift from cranial to spinal absorption with adoption of the standing posture which, under particular circumstances, may leave osmotic draw of fluid into the venous sinuses insufficient to overcome the reduction in hydrostatic force so that the outward passage of cerebrospinal fluid slows and may even reverse within the cranium, all absorption then occuring through caudal structures.
John O’Connell takes it as a given that the positive pressure of the cerebrospinal fluid reflects the balance between production and absorption. Forces acting on the fluid are hydrostatic and osmotic; equilibrium is dependent on the amount of arterial and venous blood in the ‘closed box’; and the fluid circulates slowly leading to regional variation in its constituents. Against that background, his interest is in further fluctuations that occur with the cardiac and respiratory rhythms that others have considered to impose no more than 2–4 mm and 5–10 mm fluctuation, respectively. [A recent research highlight in Nature (19 February 2015, page 277) draws attention to work in normal volunteers (published elsewhere) showing that breathing exerts a greater effect on the flow of cerebrospinal fluid than does the heartbeat, ‘an approach (sic) [that] could be used to study disorders that result in disruptions to the flow of the cerebrospinal fluid’: to which our response is nihil novi sub sole]. Mr O’Connell’s first point is that, with cessation of the circulation and of respiration, the cerebrospinal fluid pressure in cadavers is atmospheric. He has studied the lumbar and cisternal pressures in life with subjects in the horizontal position (Figs 1 and 2) and observed no more than 0.5–1 mm variation with changes in heartbeat and ∼2 mm with respiration. However, there appear to be much greater changes when ventricular pressure is measured—about 15 (5–50) mm for the cardiac and 35 (15–60) mm for the respiratory cycles, respectively—together resulting in fluctuations of 50 (20–110) mm in ventricular cerebrospinal fluid pressure with breathing and the heartbeat. But he is cautious because these fluctuations are to some extent an artefact of the method used for their measurement—the bore of the manometer differing for lumbar and ventricular puncture and proving influential in registering changes in pressure of the cerebrospinal fluid. Preferring the validity of the ventricular recordings, O’Connell concludes that ‘cerebrospinal fluid pressure far from being constant varies rhythmically and through an average of about 80 mm every three or four seconds’. As a neurosurgeon he has frequently observed the pulsating dura and intermittent spurt of cerebrospinal fluid if a large subarachnoid channel is inadvertently pricked, and in the lumbar spine at laminectomy. Using the term ‘venting’ rather than ‘elasticity’ O’Connell interprets the accommodation of these forces, and of changes in posture, as reciprocal oscillations in the size of the arterial and venous compartments. And he is careful to point out that, over and above the pulsatile changes, the vascular pressure contributes around 40 mm to the hydrostatic factor in maintaining the cerebrospinal fluid pressure, with the osmotic force exerting c. 150 mm. These are the factors that must account for any rapid changes in cerebrospinal fluid pressure since production and absorption are slow and only lead to marked alterations in pressure with obstruction or, conversely, artificial drainage and the use of intravenous hypertonic solutions. Applying Starling’s law of tissue fluid circulation, filtrate leaving from the arterial side of the capillary under hydrostatic force and re-entering under influence of the osmotic attraction of venous blood, fails to solve the problem of how the cerebrospinal fluid circulates through the subarachnoid space. O’Connell argues that there is an additional vascular factor whereby the amount of intracranial blood increases with the cardiac output, and with expiration, forcing cerebrospinal fluid into the basal cisterns; but since this is where intracranial pressure is itself highest, fluid then circulates over the cranial vault and down the spinal canal. Furthermore, since pressure is lowest in the sagittal sinus, this is where the pulsatile forces expand the arachnoid villi to produce the complex arachnoid granulations and pacchionian bodies where absorption mainly occurs (Fig. 3):
‘with every cardiac systole and every expiration the cerebral ventricles are compressed and fluid is expelled into the subarachnoid space [but this] is itself compressed [in the] basal cisternae. Cerebrospinal fluid is driven from them either into the spinal canal or towards the cranial vault’.
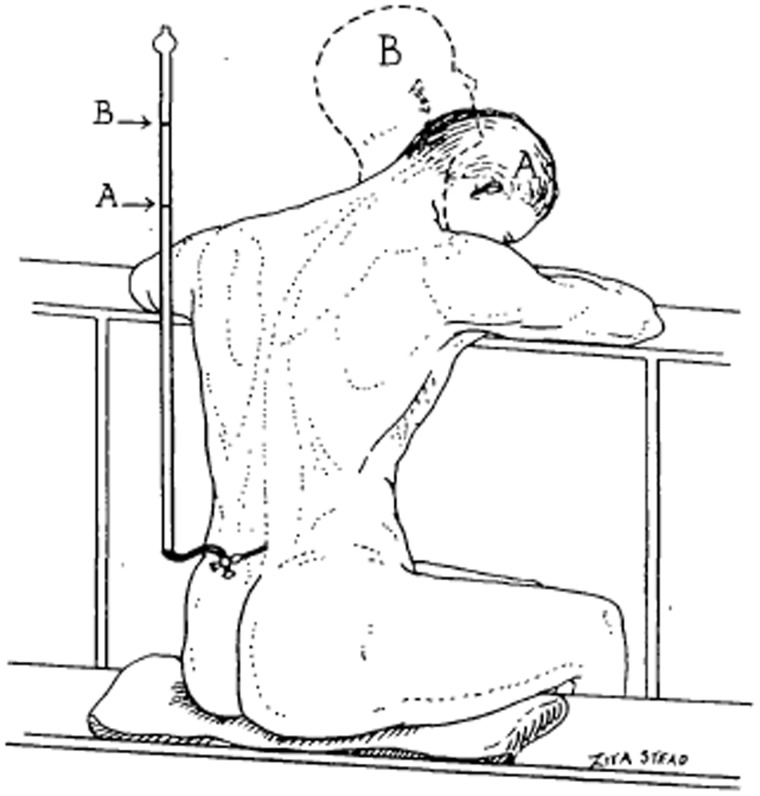
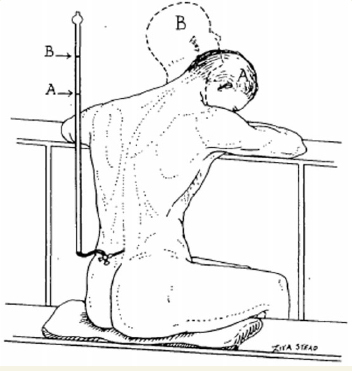
Figure 1.
Drawing to illustrate the rise in cerebrospinal fluid pressure which occurs when the flexed head (A) is extended (B). From O’Connell (1943).
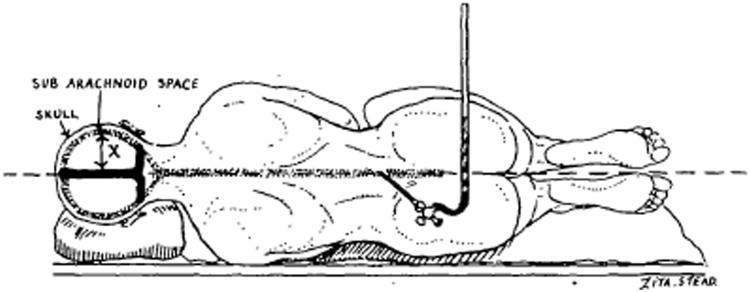
Figure 2.
Drawing to indicate that when lumbar puncture is done in the true horizontal position there is a column of cerebrospinal fluid (X) within the skull which is entirely above the horizontal plane. From O’Connell (1943).
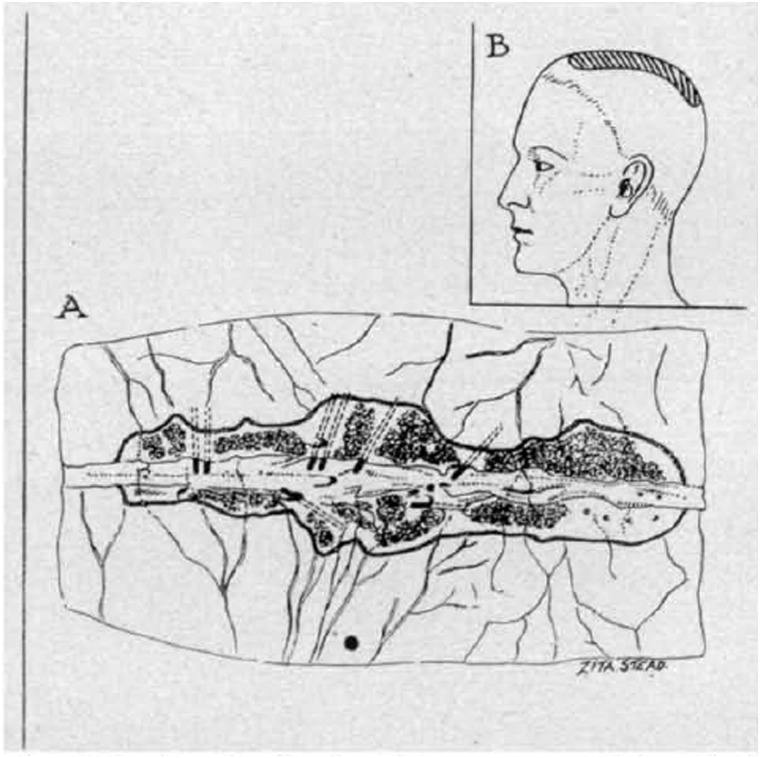
Figure 3.
(A) Drawing of a dissection of the superior sagittal sinus showing the single lateral lacuna on each side of it. The floor of each lacuna is carpeted by pacchionian granulations. (B) Diagram to indicate the usual position of the lacunae and pacchionian granulation (shaded area). From O’Connell (1943).
There may be some pulsatile reversal of these events with diastole and inspiration, perhaps with a valve-like shutting of the aqueduct of Sylvius and other narrow channels; but the net flow is out of the ventricles and towards the meningeal arachnoid villi, the spinal compartment acting to some extent as an expansile flood plain that takes up surpluses and deficiencies in the interests of keeping intracranial pressure, flow and volume relatively stable. It follows that the pressure dynamics and flow of cerebrospinal fluid explain the focal ventricular dilatations that occur after brain injury and tissue destruction; herniation of the brain through a defect of the skull, or fracture that fails to unite in children; and erosion of the vault through pulsatile pressure from internal hydrocephalus in the context of posterior fossa tumours.
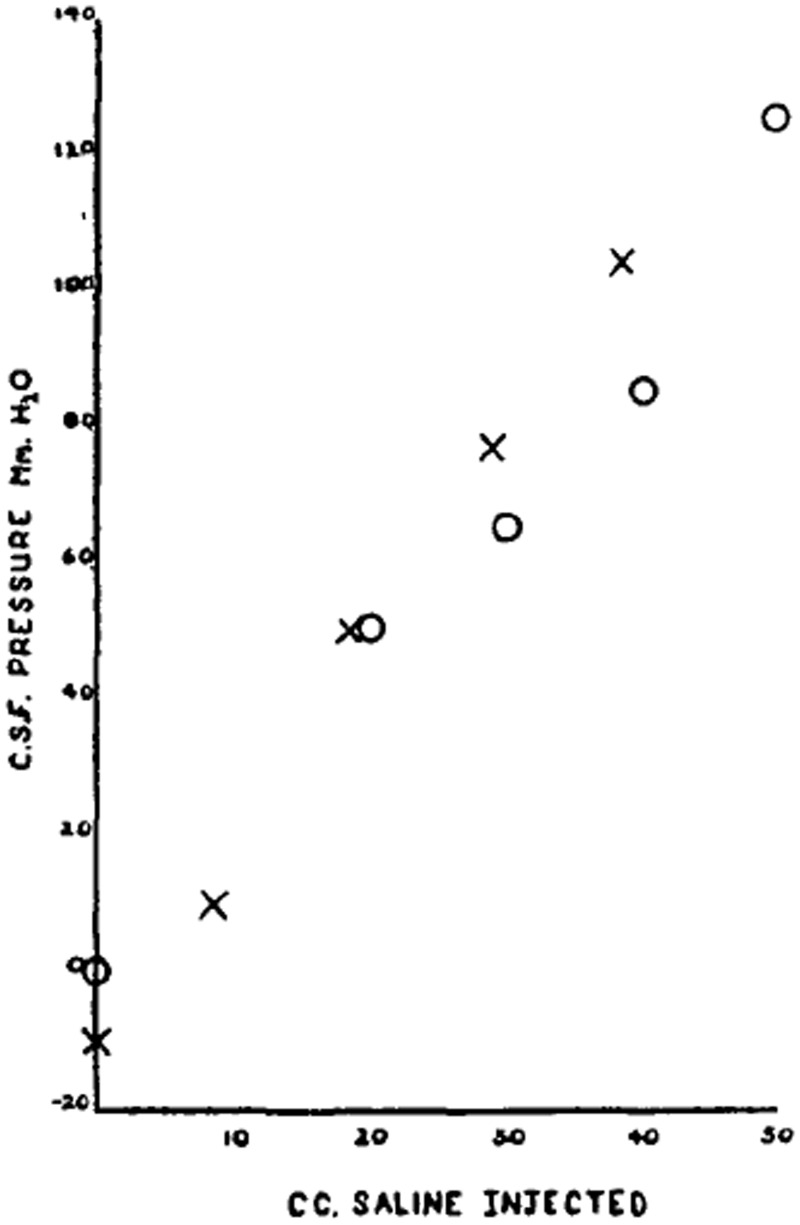
Against this background, the purpose of Pickering’s paper is straightforward. What is the mechanism of headache after lumbar puncture? Several authors have linked this to leakage of fluid through the hole made by the needle; and others have shown that the pressure is indeed low when measured during such headache; and can be treated by injecting fluid. Here he describes a small series of 11 cases, in seven of whom measurement and fluid replacement are applied. In cases of lumbar puncture headache, no fluid escapes on successfully penetrating the lumbar theca with the patient horizontal. The pressure when measured by attaching the needle to a saline filled tube is zero in six cases; and 80 mm cerebrospinal fluid in the seventh. Positive pressure can be restored by injecting around 30–50 cc of physiological saline (Fig. 4). With this manoeuvre, headache is abolished but only for 30–120 min before, presumably, continued leakage of fluid through the hole in the theca again lowers the pressure. The effect of posture on the pain is predictable and rapid. Sitting up leads to headache within 3–15 s which increases over the next minute, recovering within 2–3 min when the patient lies down. The pain increases with head movements in the upright position: ‘the source of the pain would seem to be in the tissue intervening between brain and skull, namely in the meninges, for it is here that the chief stress of such a manoeuvre would seem to fall’. In eight of ten cases tested, the headache also increases in intensity with digital compression of the jugular veins in the neck. But measurement of the cerebrospinal fluid pressure during jugular compression does not lead to an increase in pressure within the manometer tube. The pain is worse with pulsation of the heartbeat; and relieved by compression of the carotid artery low in the neck, rebounding at the first beat when the occlusion is released. And, apart from an illustration of the relationship between cerebrospinal fluid pressure and the volume of saline injected (Fig. 4), that’s it.
Figure 4.
Shows the relationship between c.s.f. pressure and volume of saline injected in two patients with lumbar puncture headache. Both patients were in the horizontal position and in each case about a minute was allowed for the pressure to level off. Crosses Case 2, 7 [November] 44, four days after original lumbar puncture. Circles case 7, 15 [November] 47, three days after original lumbar puncture. From Pickering (1948).
There follows a long discussion in which Pickering rehearses the literature on inducing headache by removing cerebrospinal fluid with the patient sitting, which—with his own observations—leads him to conclude that lumbar puncture headache is due to mechanical disturbance in the cranial cavity. Ideally, he would like to know that equally low pressures do not occur after lumbar puncture in individuals who do not experience headache. As for the example(s) of headache in the presence of normal cerebrospinal fluid pressure, Case 5 in his series, ‘I am not entirely satisfied that in such cases an element of meningeal inflammation can be excluded’. The condition results from losing 30–50 cc cerebrospinal fluid into the subarachnoid space, with compensatory expansion of the intracranial cerebral veins and elasticity of the spinal dura by the same amount. As a result, the intracranial structures shift with the brain moving towards the foramen magnum, as in examples of coning when lumbar puncture is carried out in the context of raised intracranial pressure, causing tension in anchored tissues including the tentorium and large basal arteries of the circle of Willis. Anything that encourages this movement, such as posture or compression of the jugular veins, will increase the pressure differential, and accentuate the shift and intensity of the headache. Reducing tension in the intracranial arteries by occluding the vessel in the neck should relieve the pain—as it does. In seeking to distinguish the relative contributions of gross movement of intracranial structures and stretching of the basal arteries, Pickering emphasizes the similarity between lumbar puncture headache and that seen with injection of histamine, which he had described in 1933–4 but without really understanding the mechanism at the time:
‘when I originally made this observation [on histamine headache], I could not explain it. I now attribute it to summation of two effects, arterial dilatation and displacement of the brain, each stretching the pain sensitive tissue surrounding the arteries at the base of the brain’.
Taken together, the close similarity with histamine headache strongly favours vascular distortion in explaining post-lumbar puncture rather than movement of larger intracranial structures.
Pickering was a famous physician and clinician scientist. Born in Northumberland, and brought up by his widowed mother, George was an academically precocious schoolboy in Newcastle and London (Dulwich College); and at Cambridge where he turned from physiology and natural sciences to medicine. As a medical student at St Thomas’s Hospital, Sir Joseph Barcroft (1872–1947) steered him in the direction of Sir Thomas Lewis (1881–1945), who coined the phrase ‘clinical science’, when a vacancy arose in Lewis’s team through ‘temperamental incompatibility’ of another member of the department. At University College Hospital, Pickering was close to the acerbic and outspoken neurosurgeon, Wilfrid Trotter (1872–1939). There he worked on blood flow and (re)discovered renin. That brought him to the attention of Sir Charles Wilson (Lord Moran: 1882–1977) who, in 1939, encouraged the move to St Mary’s Hospital Medical School, leading the Medical Unit, where after World War II, Pickering created an atmosphere that made St Mary’s ‘the place where people wanted to get to, both from abroad and at home’. His work on hypertension ranged from the physiology of angiotensin to genetic and epidemiological studies showing that blood pressure is distributed as a continuous trait in the population—a view with which Sir Robert Platt (1900–78) took exception regarding hypertension as having a biphasic distribution. On this, Pickering was proved correct. In 1956, he moved to Oxford as regius professor of medicine, working on baroreceptor mechanisms of the carotid sinus and the pathogenesis of atherosclerosis. It was in Oxford that Ralph Ross Russell, working with Pickering, chancing to see a platelet embolus passing through a retinal vessel in a patient experiencing amaurosis fugax, summoned an excited Pickering who arrived on the ward in his pyjamas. This laid to rest the concept of migrating arterial spasm as the cause of transient cerebral ischaemia. But, for us, the work on lumbar puncture headache cannot be regarded as Pickering’s greatest moment in clinical science. The series is small; the observations incomplete; the controls missing; the documentation minimal; and the conclusions hardly novel. Nonetheless, interest in lumbar puncture headache persists, as the paper by Yen-Feng Wang and colleagues from Taiwan in the present issue, showing that post-dural puncture headache is associated with more rostral and extensive periradicular and epidural leakages extending beyond the site of puncture, illustrates (page 1492).