Sir,
There has been a growing interest in the role of the cerebellum in primary dystonia. Although this role is not yet fully understood (Sadnicka et al., 2012), an elegant paper by Hubsch et al. (2013) in Brain showed that in writer’s cramp, a form of task-specific primary hand dystonia, the cerebellum has lost its ability to modulate sensorimotor plasticity of the motor cortex. As part of their study, they used the paired-associative stimulation protocol, a transcranial magnetic stimulation-based intervention to induce motor cortex plasticity, combined with either intermittent or continuous theta burst stimulation (TBS) over the cerebellum. Neither form of cerebellar TBS induced any change of the paired-associative stimulation effect in patients with writer’s cramp as opposed to control subjects. The authors hypothesized that this suggests that the cerebellum has lost its effect on some relevant sensorimotor integrative functions and/or that the continuously hyperactive state of the cerebellum renders it refractory to TBS.
We wish to build on this by sharing our results of a cerebellar, single session TBS intervention study in patients with writer’s cramp, specifically designed to address the question whether such an intervention has any effect on dystonia severity and writing performance. The reason for this was that, based on the growing body of evidence of this role of the cerebellum in dystonia, many have already speculated on the cerebellum being a putative target for neuromodulatory interventions in dystonia. More specifically, the quite robust finding of cerebellar hyperactivity in dystonia led to the idea that applying transcranial cerebellar inhibition might have therapeutic effects.
Ten patients (nine right-handed and one left-handed) with writer’s cramp were included. All but one received both continuous TBS and sham TBS with an interval of 3 months, to exclude any carryover effect. The subjects were randomized between the two groups: first continuous TBS or first sham TBS. One patient only received the real stimulation. Two healthy individuals were asked to perform the same tasks as the writer’s cramp group, mainly to see whether our kinematic writing analyses indeed detected the expected baseline abnormalities in the patients with writer’s cramp.
Transcranial magnetic stimulation was delivered through a C-B60 figure-8 coil (MagVenture) connected to a Magpro-X-100 stimulator. First, the active motor threshold was determined over the hotspot, the optimal site of the magnetic coil for eliciting motor evoked potentials, in the right first dorsal interosseus muscle (or left in the one patient with left-sided writer’s cramp). Active motor threshold was defined as the minimum stimulator intensity required to obtain motor evoked potentials with an amplitude of at least 200 μV in at least 5 of 10 trails while the subject maintained a low-level tonic contraction (10% of maximal voluntary contraction) in right first dorsal interosseus. We used the continuous TBS protocol, which has been proven to effectively suppress the cerebellum activity. Continuous TBS was applied at 80% of the active motor threshold over the hemicerebellum ipsilateral to the dominant hand (1 cm inferior and 3 cm lateral from the inion) during real stimulation. For the sham stimulation, the coil was placed ∼5 cm lower over the neck muscles. This left the cerebellum unaffected in previous studies (Hoffland et al., 2012).
Subjects were asked to do a series of simple writing tasks before and directly after TBS: (i) drawing circles with a 2-cm diameter (three times); (ii) drawing circles with a 0.5-cm diameter (three times); (iii) drawing circles with a 2-cm diameter followed by a continuous series of loops over a distance of 20-cm length (twice); and (iv) drawing circles with a 2 cm diameter followed by three continuous loops, a short lifting from the tablet and again three loops over a 20-cm length (twice). Previously, Zeuner et al. (2007) concluded that drawing circles was the best method to assess motor impairment in patients with writer’s cramp. Subjects were encouraged to perform the tasks as fast as possible. The circles and loops were drawn with a standard-shaped, wireless, electronic, inking pen on normal sheets of paper that were placed on a Wacom Pen Tablet (Intuos 3). A custom-made program (developed at Donders Institute for Brain, Cognition and Behaviour, for Windows XP in Borland® Delphi 7) running on a Pentium-IV XP laptop was used for recording pen position and axial pen pressure. The sampling rate was set at 200 Hz.
All data analyses were performed off-line in Matlab [Version 7.8.0.347 (R2009a)]. Pen-tip displacements were filtered with a cut-off frequency of 10 Hz by a low-pass third-order Butterworth filter, which was applied in two directions. The filtered pen position in x- and y-coordinates and its filtered first and second time derivatives (i.e. velocity and acceleration) were displayed for visual inspection of all trials.
Analyses focused on the vertical movement as this is the main movement component in handwriting (Zeuner et al., 2007). Therefore, pen movements were segmented into successive up and down strokes based on the maximal and minimal vertical position. Only strokes that had the required vertical size (i.e. 20 mm or 5 mm ± 25%) were selected for further analysis. The first stroke of a series of circles or loops of a trial was omitted for analysis.
For each stroke the following variables were calculated and used as dependent variables in the analysis: stroke duration (in s), trajectory length (in cm), mean velocity (in mm/s), mean axial pen pressure (in N), and the number of vertical velocity peaks. Zeuner et al. (2007) used stroke frequency, but mean stroke duration provides the same information and can be more easily calculated for short series of strokes (such as in our fourth task). The number of velocity peaks per stroke was calculated by counting the number of zero crossings of the vertical acceleration for each stroke and dividing by two. A fluent stroke has a single velocity peak which is characteristic of fluent open-loop performance. Therefore, the number of velocity peaks per stroke can be used as a measure of disfluency.
Analyses of variance (following a general linear model, with repeated measures) were conducted on the means over up and down strokes per trial separately for each of the dependent variables and apart for each of the four drawing tasks. Within-subject factors were Trial (there were two trials in each task, or we discarded the first trial in case of three), measurement Period (before or after stimulation) and nature of Stimulation (continuous TBS versus sham TBS). Statistical significance was set at P < 0.05, but because of the number of variables we analysed, we applied a Bonferroni correction, after which significance was set at P < 0.004.
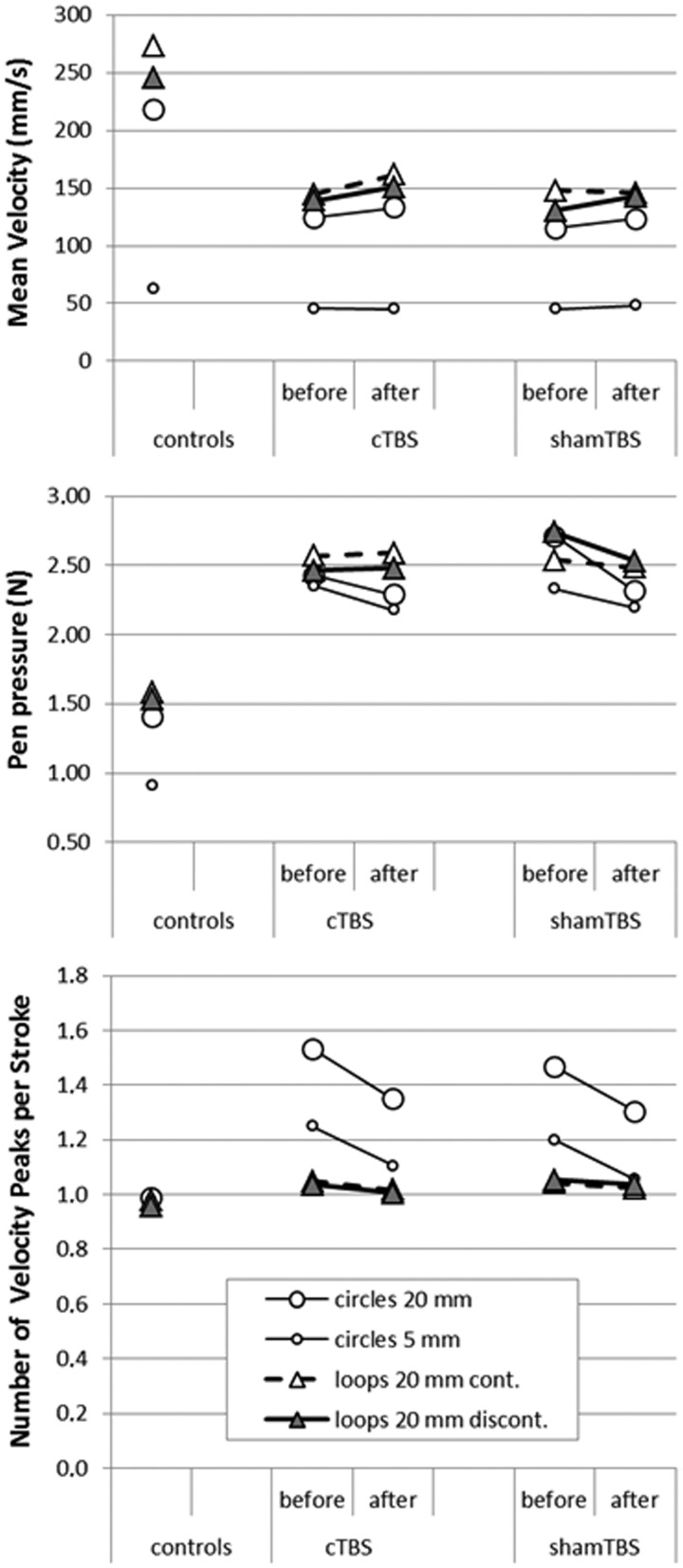
Results are displayed in Tables 1–3, and Fig. 1. The control group had, as expected, a lower pen pressure, a higher mean velocity and their writing was more fluent compared to the patients with writer’s cramp, indicating that our writing analysis was able to detect relevant writing abnormalities (data not shown).
Table 1.
Patient means and standard deviations, averaged over trials and series, for three variables that characterize performance in the four writing tasks
| Circles 20 mm |
Circles 5 mm |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | 95% CI of mean | Mean | SD | 95% CI of mean | |
| n strokes (up+down; sum over two trials) | 27.3 | 7.4 | 21.6–32.9 | 31.9 | 8.4 | 25.5–38.4 |
| Mean stroke length (mm) | 30.4 | 2.0 | 28.0–32.0 | 8.3 | 1.0 | 7.6–9.1 |
| Mean stroke duration (ms) | 325.0 | 134.0 | 222.0–428.0 | 211.0 | 61.0 | 164.0–257.0 |
| Loops 20 mm continuous |
Loops 20 mm discontinuous |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | 95% CI of mean | Mean | SD | 95% CI of mean | |
| n strokes (up+down; sum over two trials) | 51.7 | 17.6 | 38.1–65.2 | 58.1 | 22.3 | 41.0–75.3 |
| Mean stroke length (mm) | 30.4 | 5.9 | 25.8–34.9 | 29.3 | 4.8 | 25.6–33.0 |
| Mean stroke duration (ms) | 215.0 | 57.0 | 171.0–259.0 | 221.0 | 56.0 | 178.0–264.0 |
CI = confidence interval; SD = standard deviation.
Table 2.
Results of statistical tests (t/F and P-values) on (i) effects of stimulation (differences between trials before and after stimulation); and (ii) the interaction between the effect of stimulation and the type of stimulation (continuous TBS and shamTBS)
| Circles 20 mm |
Circles 5 mm |
Loops 20 mm continuous |
Loops 20 mm discontinuous |
|||||
|---|---|---|---|---|---|---|---|---|
| t(10) | P | t(10) | P | t(10) | P | t(10) | P | |
| Before-after | ||||||||
| Velocity | 7.55 | 0.029 | 0.51 | 0.500 | 1.93 | 0.207 | 8.37 | 0.023 |
| Pen pressure | 5.74 | 0.048 | 3.47 | 0.105 | 0.01 | 0.921 | 0.56 | 0.478 |
| Disfluency | 1.50 | 0.260 | 5.42 | 0.053 | 1.98 | 0.202 | 6.62 | 0.037 |
| ContinuousTBS_ShamTBS before-after | ||||||||
| Velocity | 0.01 | 0.929 | 0.64 | 0.450 | 3.37 | 0.109 | 0.02 | 0.797 |
| Stroke duration | 0.04 | 0.850 | 0.52 | 0.494 | 0.77 | 0.409 | 0.07 | 0.801 |
| Pen pressure | 6.51 | 0.038 | 0.04 | 0.849 | 0.25 | 0.633 | 1.33 | 0.287 |
| Disfluency | 0.01 | 0.947 | 0.00 | 0.995 | 0.29 | 0.608 | 0.66 | 0.443 |
Table 3.
Mean velocity, pen pressure and disfluency for controls in a single series of writing tasks and for patients with writer’s cramp in four series of the four writing tasks (before and after continuous TBS and before and after sham TBS)
| Velocity | Mean (in mm/s) | SD | 95% CI lower bound | 95% CI upper bound | |
|---|---|---|---|---|---|
| Controls | Circles 20 mm | 217.6 | 23.0 | 10.6 | 424.6 |
| Circles 5 mm | 63.2 | 5.2 | 16.6 | 109.8 | |
| Loops 20 mm cont. | 272.9 | 4.2 | 235.3 | 310.6 | |
| Loops 20 mm discont. | 245.2 | 5.3 | 197.6 | 292.9 | |
| cTBS (before) | Circles 20 mm | 127.7 | 63.7 | 78.7 | 176.6 |
| Circles 5 mm | 46.9 | 19.7 | 31.8 | 62.1 | |
| Loops 20 mm cont. | 147.1 | 56.9 | 103.4 | 190.8 | |
| Loops 20 mm discont. | 141.3 | 49.6 | 103.2 | 179.4 | |
| cTBS (after) | Circles 20 mm | 137.5 | 66.3 | 86.6 | 188.5 |
| Circles 5 mm | 46.6 | 14.9 | 35.2 | 58.0 | |
| Loops 20 mm cont. | 165.3 | 53.3 | 104.5 | 183.8 | |
| Loops 20 mm discont. | 153.7 | 55.7 | 92.4 | 164.5 | |
| shamTBS (before) | Circles 20 mm | 111.6 | 50.3 | 72.9 | 150.3 |
| Circles 5 mm | 44.4 | 20.0 | 29.0 | 59.8 | |
| Loops 20 mm cont. | 144.1 | 51.6 | 104.5 | 183.8 | |
| Loops 20 mm discont. | 128.5 | 46.9 | 92.4 | 164.5 | |
| shamTBS (after) | Circles 20 mm | 120.0 | 60.3 | 73.7 | 166.3 |
| Circles 5 mm | 47.3 | 19.3 | 32.5 | 62.2 | |
| Loops 20 mm cont. | 143.5 | 45.2 | 108.8 | 178.2 | |
| Loops 20 mm discont. | 141.2 | 50.6 | 102.4 | 180.1 | |
| Pen pressure | Mean (N) | SD | 95% CI Lower bound | 95% CI Upper bound | |
|---|---|---|---|---|---|
| Controls | Circles 20 mm | 1.405 | 0.343 | −1.683 | 4.493 |
| Circles 5 mm | 0.915 | 0.037 | 0.584 | 1.247 | |
| Loops 20 mm cont. | 1.583 | 0.073 | 0.930 | 2.237 | |
| Loops 20 mm discont. | 1.532 | 0.045 | 1.130 | 1.935 | |
| cTBS (before) | Circles 20 mm | 2.477 | 1.219 | 1.540 | 3.414 |
| Circles 5 mm | 2.419 | 1.173 | 1.518 | 3.322 | |
| Loops 20 mm cont. | 2.619 | 1.113 | 1.764 | 3.475 | |
| Loops 20 mm discont. | 2.535 | 1.181 | 1.626 | 3.442 | |
| cTBS (after) | Circles 20 mm | 2.327 | 1.068 | 1.506 | 3.147 |
| Circles 5 mm | 2.228 | 1.070 | 1.405 | 3.051 | |
| Loops 20 mm cont. | 2.638 | 1.179 | 1.731 | 3.545 | |
| Loops 20 mm discont. | 2.519 | 1.147 | 1.638 | 3.401 | |
| shamTBS (before) | Circles 20 mm | 2.757 | 1.094 | 1.916 | 3.597 |
| Circles 5 mm | 2.398 | 1.095 | 1.555 | 3.239 | |
| Loops 20 mm cont. | 2.582 | 0.836 | 1.939 | 3.225 | |
| Loops 20 mm discont. | 2.765 | 0.867 | 2.099 | 3.432 | |
| shamTBS (after) | Circles 20 mm | 2.326 | 0.851 | 1.673 | 2.980 |
| Circles 5 mm | 2.211 | 9.961 | 1.472 | 2.950 | |
| Loops 20 mm cont. | 2.501 | 0.924 | 1.790 | 3.210 | |
| Loops 20 mm discont. | 2.570 | 0.902 | 1.877 | 3.263 | |
| Disfluency | Mean number of peaks per stroke | SD | 95% CI Lower bound | 95% CI Upper bound | |
|---|---|---|---|---|---|
| Controls | Circles 20 mm | 0.987 | 0.006 | 0.939 | 1.034 |
| Circles 5 mm | 0.979 | 0.030 | 0.709 | 1.249 | |
| Loops 20 mm cont. | 0.985 | 0.004 | 0.948 | 1.022 | |
| Loops 20 mm discont. | 0.959 | 0.006 | 0.910 | 1.008 | |
| cTBS (before) | Circles 20 mm | 1.471 | 1.167 | 0.574 | 2.368 |
| Circles 5 mm | 1.215 | 0.487 | 0.841 | 1.589 | |
| Loops 20 mm cont. | 1.044 | 0.078 | 0.984 | 1.103 | |
| Loops 20 mm discont. | 1.032 | 0.056 | 0.989 | 1.074 | |
| cTBS (after) | Circles 20 mm | 1.309 | 0.565 | 0.875 | 1.743 |
| Circles 5 mm | 1.090 | 0.190 | 0.945 | 1.236 | |
| Loops 20 mm cont. | 1.015 | 0.055 | 0.973 | 1.057 | |
| Loops 20 mm discont. | 1.003 | 0.049 | 0.966 | 1.041 | |
| shamTBS (before) | Circles 20 mm | 1.514 | 0.789 | 0.908 | 2.120 |
| Circles 5 mm | 1.211 | 0.282 | 0.994 | 1.428 | |
| Loops 20 mm cont. | 1.045 | 0.087 | 0.979 | 1.112 | |
| Loops 20 mm discont. | 1.055 | 0.076 | 0.997 | 1.113 | |
| shamTBS (after) | Circles 20 mm | 1.329 | 0.594 | 0.872 | 1.785 |
| Circles 5 mm | 1.053 | 0.160 | 0.931 | 1.176 | |
| Loops 20 mm cont. | 1.027 | 0.077 | 0.968 | 1.086 | |
| Loops 20 mm discont. | 1.036 | 2.500 | 0.996 | 1.076 | |
cTBS = continuous TBS; SD = standard deviation; CI = confidence interval; cont. = continuous; discont. = discontinuous.
Figure 1.
Results on writing tasks before and after cTBS and sham TBS. Mean velocity (top), pen pressure (middle) and disfluency (bottom) for control subjects in a single series of writing tasks and for patients with writer’s cramp in four series of the four writing tasks [before and after continuous TBS (cTBS) and before and after sham TBS].
Our goal was to examine whether continuous TBS over the cerebellum had any effect on writing impairment in writer’s cramp. Basically, there were no significant differences in writing performance after cerebellar continuous TBS versus sham in our group of patients with writer’s cramp. Although four of the parameters changed significantly after TBS (velocity in 20 mm circles and 20 mm loops discontinuous, pen pressure in 20 mm circles and disfluency in 20 mm loops discontinuous), this applied to both continuous TBS and sham TBS. These results are most likely the result of a learning effect that occurred, despite the fact that participants already performed the tasks required quite often before the actual experiment, or of a placebo effect.
One interaction effect (Table 2, pen pressure in the 20 mm circles) seemed to be significant, but did not survive Bonferroni correction. Moreover, for this measure, the decrease (which would be an improvement) after sham TBS was actually larger than the decrease after continuous TBS.
Our results therefore indicate that a single session of cerebellar inhibition through continuous TBS has no effect on writing performance in writer’s cramp. This is in keeping with the inferences made by Hubsch et al. (2013) based on their experiments, and our study could be seen as the more clinical counterpart of their study. In our study, we aimed to achieve cerebellar inhibition. A recent study by Sadnicka et al. (2014) explored cerebellar stimulation by using a single session of transcranial direct current stimulation (anodal versus sham) over the cerebellum in patients with writer’s cramp. This also failed to modulate motor cortex plasticity. Although the focus in that study was on plasticity responses, they performed some clinimetrics of writing and this also remained unchanged. The authors, similar to Hubsch et al. (2013) observed a marked variability in plasticity responses in the patients, which complicates attempts to try and reduce exaggerated plasticity as a universally applicable treatment opportunity in this type of dystonia.
In conclusion, although the cerebellum is clearly implicated in the pathophysiology of dystonia, non-invasively modulating cerebellar activity does not hold promise as a real therapeutic intervention, at least not in task-specific, focal hand dystonias such as writer’s cramp. It might be that a single session of cerebellar inhibition is not sufficient to induce any measurable and sustainable change. A recent study with a sham-controlled, 2-week cerebellar continuous TBS intervention in cervical dystonia was able to detect a clinical improvement that was paralleled by changes in measurements of cerebellar-brain inhibition and motor cortex plasticity (Koch et al., 2014). Patient selection might also be an issue, which should perhaps be based on individual plasticity responses. This chapter in dystonia research is therefore not closed yet.
Funding
The work presented here was made possible by a grant from the ‘Wetenschapsfonds Dystonie’ of the Dutch Dystonia Society.
Conflict of interest
Dr Van de Warrenburg receives research support from the Gossweiler Foundation, the Radboud University Medical Centre, BBMRI-NL, the ‘Wetenschapsfonds Dystonie’ of the Dutch Dystonia Society and the Dutch Royal Society for Physical Therapy. Dr J. van Gaalen receives research support from the Gossweiler Foundation.
References
- Hoffland BS, Bologna M, Kassavetis P, Teo JTH, Rothwell JC, Yeo CH, et al. Cerebellar theta burst stimulation impairs eyeblink classical conditioning. J. Physiol. 2012;590:887–97. doi: 10.1113/jphysiol.2011.218537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubsch C, Roze E, Popa T, Russo M, Balachandran A, Pradeep S, et al. Defective cerebellar control of cortical plasticity in writer's cramp. Brain. 2013;136:2050–62. doi: 10.1093/brain/awt147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Porcacchia P, Ponzo V, Carrillo F, Cáceres-Redondo MT, Brusa L, et al. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stim. 2014;7:564–72. doi: 10.1016/j.brs.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Sadnicka A, Hoffland BS, Bhatia KP, van de Warrenburg BP, Edwards MJ. The cerebellum in dystonia – Help or hindrance? Clin. Neurophysiol. 2012;123:65–70. doi: 10.1016/j.clinph.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Sadnicka A, Hamada M, Bhatia KP, Rothwell JC, Edwards MJ. Cerebellar stimulation fails to modulate motor cortex plasticity in writing dystonia. Mov. Disord. 2014 doi: 10.1002/mds.25881. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zeuner KE, Peller M, Knutzen A, Holler I, Münchau A, Hallett M, et al. How to assess motor impairment in writer’s cramp. Mov. Disord. 2007;22:1102–9. doi: 10.1002/mds.21294. [DOI] [PubMed] [Google Scholar]