Although early visual experience is essential for the proper development of visual cortex, Striem-Amit et al. show that the underlying connectivity structure of retinotopic mapping is retained even in congenitally blind individuals. This basic organisational principle emerges independently of visual input and persists despite lifelong experience-dependent plasticity.
Keywords: blindness, development, plasticity, vision
Although early visual experience is essential for the proper development of visual cortex, Striem-Amit et al. show that the underlying connectivity structure of retinotopic mapping is retained even in congenitally blind individuals. This basic organisational principle emerges independently of visual input and persists despite lifelong experience-dependent plasticity.
Abstract
Is visual input during critical periods of development crucial for the emergence of the fundamental topographical mapping of the visual cortex? And would this structure be retained throughout life-long blindness or would it fade as a result of plastic, use-based reorganization? We used functional connectivity magnetic resonance imaging based on intrinsic blood oxygen level-dependent fluctuations to investigate whether significant traces of topographical mapping of the visual scene in the form of retinotopic organization, could be found in congenitally blind adults. A group of 11 fully and congenitally blind subjects and 18 sighted controls were studied. The blind demonstrated an intact functional connectivity network structural organization of the three main retinotopic mapping axes: eccentricity (centre-periphery), laterality (left-right), and elevation (upper-lower) throughout the retinotopic cortex extending to high-level ventral and dorsal streams, including characteristic eccentricity biases in face- and house-selective areas. Functional connectivity-based topographic organization in the visual cortex was indistinguishable from the normally sighted retinotopic functional connectivity structure as indicated by clustering analysis, and was found even in participants who did not have a typical retinal development in utero (microphthalmics). While the internal structural organization of the visual cortex was strikingly similar, the blind exhibited profound differences in functional connectivity to other (non-visual) brain regions as compared to the sighted, which were specific to portions of V1. Central V1 was more connected to language areas but peripheral V1 to spatial attention and control networks. These findings suggest that current accounts of critical periods and experience-dependent development should be revisited even for primary sensory areas, in that the connectivity basis for visual cortex large-scale topographical organization can develop without any visual experience and be retained through life-long experience-dependent plasticity. Furthermore, retinotopic divisions of labour, such as that between the visual cortex regions normally representing the fovea and periphery, also form the basis for topographically-unique plastic changes in the blind.
Introduction
Ever since Hubel and Wiesel’s Nobel prize-winning work (Wiesel and Hubel, 1963, 1965) it has been clear that the maturation process and resulting characteristics of the core retinotopic areas of the human brain, in particular the primary and early visual areas, are extremely sensitive to visual input during certain (critical or sensitive) developmental periods. Abolition of visual input in animals during a critical period after birth can result in permanently impaired vision due to deficient development of visual processing in the brain. For instance, lack of input from one eye will render the ocular dominance columns of that eye nearly extinct and sight will never properly recover even if visual input is restored. Similar phenomena have also been reported in humans (Lewis and Maurer, 2005; Maurer et al., 2005; Birch et al., 2009; Chan et al., 2012; Dormal et al., 2012 although see also Kalia et al., 2014), and the common consensus is that lack of visual input will cause irreparable damage to the organization of the visual cortex. Furthermore, full visual deprivation causes visual areas to reorganize to process other inputs and sensory modalities (Merabet and Pascual-Leone, 2010; Voss and Zatorre, 2012). The reorganization of the primary visual cortex of the congenitally blind is thought to be so extreme as to result in its recruitment for language and memory tasks (Sadato et al., 1996; Amedi et al., 2003, 2004; Burton et al., 2003; Bedny et al., 2011). This has engendered the common belief that sight restoration should not be attempted on people fully blind from birth, because their brains cannot develop or retain the organization needed for processing vision.
However, one of the fundamental issues as regards brain organization, which may have important bearings on brain plasticity and potential practical implications for future sight restoration efforts, has yet to be thoroughly explored. It is currently not known to what extent the visual cortex can develop and retain its fundamental large-scale organizational principle—the topographic organization of the visual scene, also known as retinotopic mapping (Wandell et al., 2007a)—without any visual input. Further, even if such maps do develop without visual experience, can they be retained throughout life-long blindness, or do they dwindle as an outcome of the well-known usage-induced, experience-dependent, compensatory plasticity (Raz et al., 2007; Kupers and Ptito, 2013)?
Two recent studies have investigated the existence of traces of topographical organization in the blind’s striate cortex (the anatomical location of the primary visual cortex; in a mixed group of congenital and acquired blindness individuals; Butt et al., 2013) and its bilateral anatomical connections via the corpus callosum (Bock et al., 2013). The findings showed that in fact the earliest station of the visual cortical hierarchy retains aspects of its architecture patterns in blindness.
Here we explored whether this pattern is limited to the very first station of cortex, or whether this organization, which is the major organizing principle of the entire visual cortex (Wandell et al., 2007a), is dependent on visual experience for its creation and/or maintenance throughout life. This was studied using functional connectivity analysis based on intrinsic slow (<0.1 Hz) blood oxygen level-dependent fluctuations in 11 congenitally, fully blind subjects (Supplementary Table 1) and 18 sighted controls. Functional connectivity MRI (Biswal et al., 1995) in the absence of a task shows similar spatial patterns to those evident during a task (Fox and Raichle, 2007; Smith et al., 2009; Deco et al., 2011), and is highly correlated with underlying structural connectivity (Damoiseaux and Greicius, 2009), including not only direct but also indirect (Honey et al., 2009) and polysynaptic connectivity (Vincent et al., 2007). Importantly, resting-state functional connectivity also correlates with functional and structural anatomy changes due to brain plasticity (Guerra-Carrillo et al., 2014), and usage, reflecting ongoing developmental changes in network engagement (Dosenbach et al., 2007; Fair et al., 2007). Thus, functional connectivity is a sensitive measure to investigate both stable anatomical connectivity as well as plastic changes throughout life, in normal conditions, atypical development, and disease. These findings reinforce the validity of this approach to the study of the organization of the visual cortex without visual input, which is critical for studying neural networks that are not otherwise accessible, such as the retinotopic organization in the fully blind. We thus studied the segregation of visual functional networks in congenitally blind subjects based on the three retinotopic mapping principles of the intact visual cortex: eccentricity, laterality and elevation, which together constitute the complete mapping of the visual field.
Material and methods
Participants
Eleven congenitally blind individuals with no light perception (Supplementary Table 1) and 18 normally sighted healthy controls (with normal or corrected-to-normal vision; no age difference between the groups; P > 0.2) participated in the study. The age of the subjects ranged from 23 to 54 years. None had neurological or psychiatric conditions. The Tel-Aviv Sourasky Medical Centre Ethics Committee approved the experimental procedure, and written informed consent was obtained from each subject.
Functional imaging
Functional MRI data were obtained during a 9.1-min (182 volumes) resting-state scan (i.e. spontaneous blood oxygen level-dependent fluctuations) using a 3-T General Electric scanner with an InVivo 8-channel head coil. During the scan, subjects lay supine in the scanner with no external stimulation or explicit task. The sighted subjects were blindfolded and had their eyes shut for the duration of the scan. Two-dimensional functional images using blood oxygen level-dependent contrast were obtained with an Echo Planar Imaging sequence (repetition time = 3000 ms, echo time = 30 ms, 29–46 slices to obtain full-brain coverage, voxel size 3 × 3 × 4 mm, flip angle 90°). T1-weighted anatomical images were acquired using a 3D MPRAGE sequence (repetition time = 8.9 ms, echo time = 3.5 ms, inversion time = 450, 158 slices, voxel size 1 × 1 × 1 mm, flip angle 13°).
Functional MRI preprocessing
Data analysis was performed using the Brain Voyager QX 2.2 software package (Brain Innovation, Maastricht, The Netherlands) and complementary in-house preprocessing in MATLAB (MathWorks) using standard preprocessing procedures. The first two images of each scan were excluded from the analysis due to non-steady state magnetization. Preprocessing of functional scans included 3D motion correction, slice scan time correction, band-pass filtering (0.01–0.1 Hz), regression of spurious signals from the ventricles and white matter regions defined using a grow-region function embedded in Brain Voyager on the individual level and spatial smoothing with a 4 mm full-width at half-maximum Gaussian kernel. No head motions exceeded 2 mm in any given axis, or had spike-like motion of more than 1 mm in any direction. Data were then normalized to standard Talairach space (Talairach and Tournoux, 1988).
Defining a priori retinotopic regions of interest
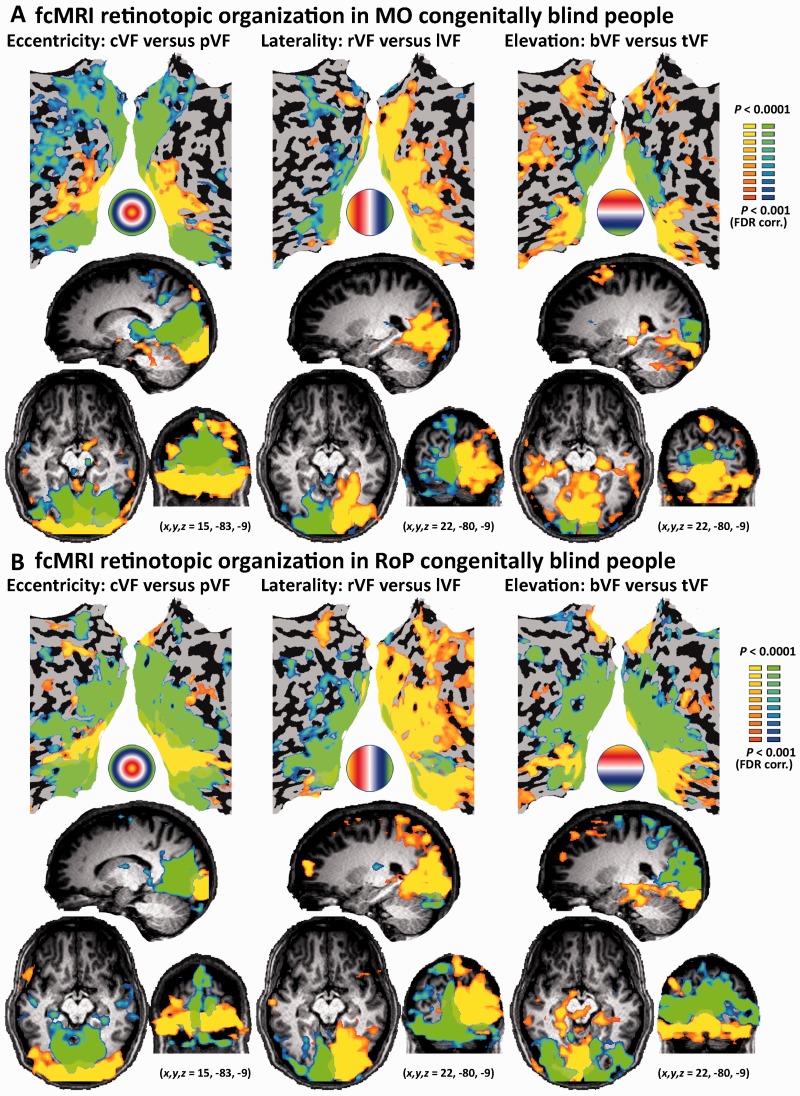
To define the primary visual cortex seeds for functional connectivity MRI analysis we scanned 14 normally sighted subjects using a standard phase-encoded retinotopic mapping protocol, with eccentricity and polar mapping of ring and wedge stimuli, respectively, to measure visual retinotopic mapping (Engel et al., 1994; Sereno et al., 1995; Wandell et al., 2007b; Wandell and Winawer, 2011), delivered during two separate experiments. The stimuli were projected by an LCD projector onto a tangent screen positioned over the subject’s forehead and viewed through a tilted mirror. In Experiment 1 an expanding annulus was presented, expanding from 0° to 34° of the subject’s visual field in 30 s, repeated 10 times. Experiment 2 presented a wedge with a polar angle of 22.5° that rotated around the fixation point, completing a full cycle in 30 s, repeated 20 times. Both the annulus in Experiment 1 and the wedge in Experiment 2 contained a flickering (6 Hz) radial checkerboard pattern according to standard retinotopic procedures (Engel et al., 1994) for field map mapping. In both cases there was a 30-s period of baseline (fixation) before and after the visual stimulus for baseline. Group phase analysis was conducted on the two experiments as done in other studies (Hertz and Amedi, 2010; Striem-Amit et al., 2011) resulting in group maps depicting the eccentricity and angle mapping. Angle mapping was then used to define the borders of V1 and V2, and the two maps were used to segregate them according to eccentricity (centre of visual field and periphery; Fig. 1A), laterality (right/left visual field responses; Fig. 1A) and elevation (lower and upper visual field responses; Fig. 1A), thus creating the seeds used for functional connectivity MRI analyses.
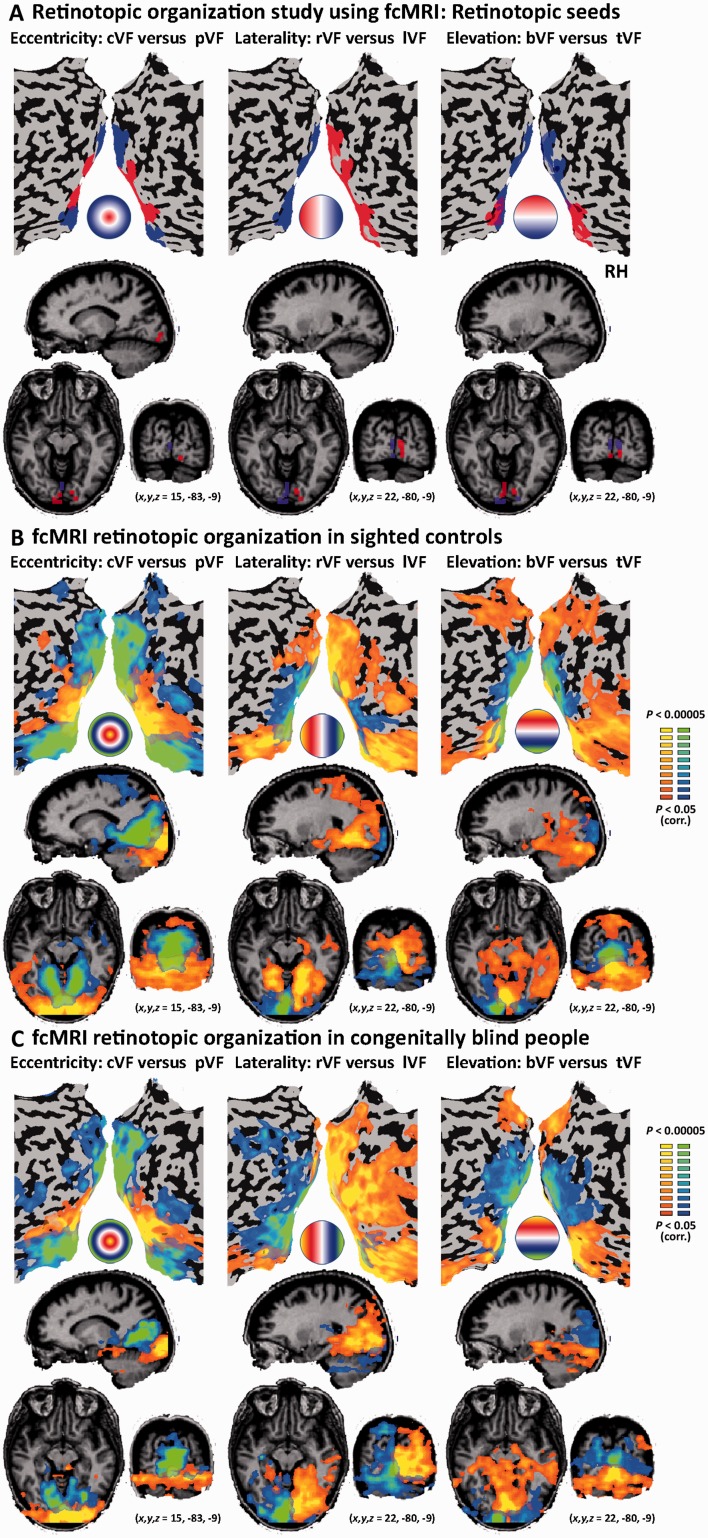
Figure 1.
Resting-state functional connectivity MRI retinotopic organization in congenitally fully blind participants. (A) Retinotopic functional connectivity MRI seeds separating the primary visual cortex according to the three fundamental topographical mapping principles of eccentricity (left: central and peripheral visual fields), laterality (middle: left and right visual fields) and elevation (right: top and bottom visual fields). The seeds were generated from an external retinotopic mapping experiment, and are displayed on brain slices and unfolded cortical sheets along with a circle depicting the corresponding parts in the visual fields. (B) Functional connectivity MRI retinotopic organization maps of a group of normally sighted controls (GLM random effect analysis, corrected for multiple comparisons) shows that visual field mapping principles can indeed be observed using resting-state functional connectivity MRI covering a large extent of the visual cortex from the seeds in the primary visual cortex to areas encompassing high-order ventral and dorsal visual streams. The circles depict the approximate corresponding areas in the visual field for both seeds in each colour map. (C) The group of participants fully blind from birth showed a similar arrangement of resting-state functional connectivity MRI topographic organization as seen in the sighted (GLM random effect analysis, corrected for multiple comparisons), replicating the known retinotopic organization pattern in the absence of visual experience. cVF = visual field; pVF = peripheral visual field; lVF = left visual field; rVF = right visual field; tVF = top visual field; bVF = bottom visual field.
Analysis of functional connectivity
Functional connectivity was computed for a priori defined seed regions of interest located in the primary visual cortex (V1), dividing it according to eccentricity, laterality, and elevation using a separate task-based retinotopic mapping external localizer from normally-sighted controls (see details above). Individual average time courses from each seed region of interest were sampled and z-normalized at the individual level. Partial correlation analysis (Zhang et al., 2008; Margulies et al., 2010) was performed in the following manner: for each seed, the time course was used as an individual predictor in a separate group analysis using a General Linear Model (GLM) in a hierarchical random effects (RFX) analysis (Friston et al., 1999), while regressing out the time course of the complementary visual field component to discard the shared variance and remain solely with the unique variance attributed to the seed (see Supplementary Fig. 1 for illustration). This analysis resulted in a group map for each of the six regions of interest. The two maps from each two complementary seeds in V1 (i.e. the group maps for central and peripheral visual fields in Fig. 1B) were overlaid to reveal the pattern across this axis. The minimum significance level of the results for the full group sizes (n = 11 for the blind, n = 18 for the sighted) was set to P < 0.05 corrected for multiple comparisons, using the spatial extent method based on the theory of Gaussian random fields (Friston et al., 1993), a set-level statistical inference correction. This was done based on the Monte Carlo stimulation approach extended to 3D data sets using the threshold size plug-in for BrainVoyager QX. Mapping the boundary of V1 and V2 (Fig. 6) was done in a comparable manner, using seeds within the left ventral (lower) V1 and V2, on both sides of the border between them, which refer to similar visual field locations in both areas. Group comparisons (Fig. 5 and Supplementary Fig. 6) were created using standard seed functional connectivity analysis, without using partial correlations (without regressing out the time course of another seed).
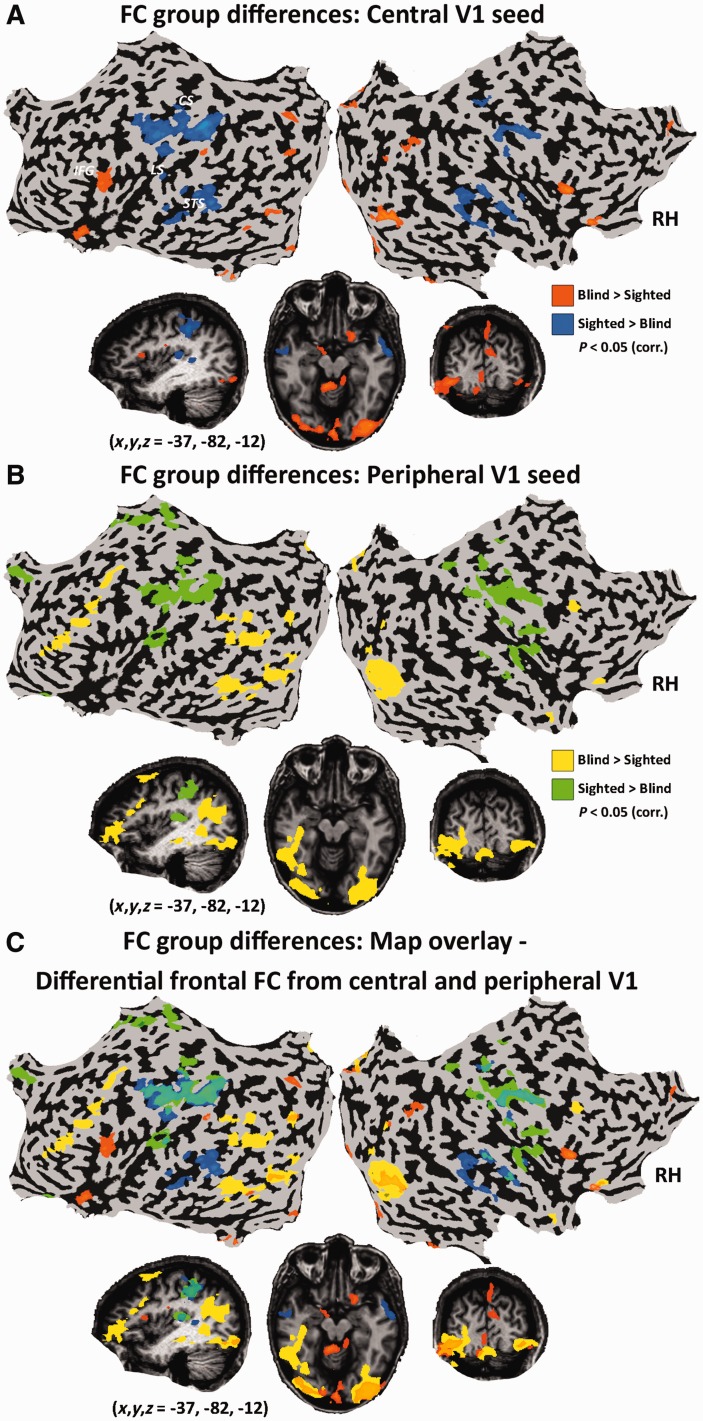
Figure 6.
Mapping the boundary between Brodmann areas 17 and 18 using functional connectivity MRI in the blind. (A) To reveal the boundary between Brodmann areas 17 and 18 we sampled two seeds, in comparable visual field locations, in ventral areas V1 and V2 defined retinotopically in the sighted group using visual stimuli (see ‘Materials and methods’ section). (B) Anatomical Brodmann areas 17 and 18 are displayed confirming our seed locations from an external atlas. (C) Group functional connectivity partial correlation analysis in the congenitally blind (random effect GLM) reflected the V1-V2 distinction along the entire ventral areal border. Overlaid black line shows the Brodmann area border. (D) Similar findings are shown in the intersubject consistency analysis (probability map showing the overlap of the functional connectivity MRI maps across the blind subjects).
Figure 5.
Functional connectivity MRI patterns of group differences across V1 retinotopic functional portions. To study the effect of blindness on the functional connectivity (FC) of different parts of V1, a contrast between the groups is presented for each seed (A, central V1; B, peripheral V1; without applying partial correlations) along the eccentricity axis (for a similar comparison along laterality and elevation see Supplementary Fig. 6) as well as the overlay of each complementary seed pair (C). While the within-visual-cortex pattern is generally similar in both seeds, different parts of V1 of the blind show altered functional connectivity to other non-visual cortices, especially in the frontal lobe, as compared to the sighted. CS = central sulcus; LS = lateral sulcus; STS = superior temporal sulcus; IFG = inferior frontal gyrus.
Analysis of blind subgroups
Since the blind varied in their aetiologies, this enabled us to investigate subgroups of the blind with differing prenatal visual development. Specifically, we investigated the retinotopic organization in subjects (n = 5) whose blindness resulted from retinopathy of prematurity (caused in these cases by oxygen treatment to premature infants), who prenatally had an intact visual system, and in subjects (n = 5) whose blindness resulted from microphthalmia, a developmental condition in which the eyes do not develop to their normal size (in this case, also causing full blindness), whose peripheral visual system was also deficient prenatally. For the subgroup analysis we applied a fixed effect GLM analysis at a highly significant threshold (P < 0.001 with false discovery rate correction; Genovese et al., 2002).
Analysis of spatial consistency
In addition to canonical group analyses (e.g. random effect GLM), we applied three different complementary analyses to examine the spatial consistency of retinotopic networks in the blind: (i) we plotted the overlap probability maps across all the blind subjects; (ii) we used an independent k-means clustering analysis to qualitatively assess the similarity between the maps of the blind and sighted subjects; and (iii) we quantified the similarity between the maps of the blind and sighted subjects using a concordance correlation coefficient.
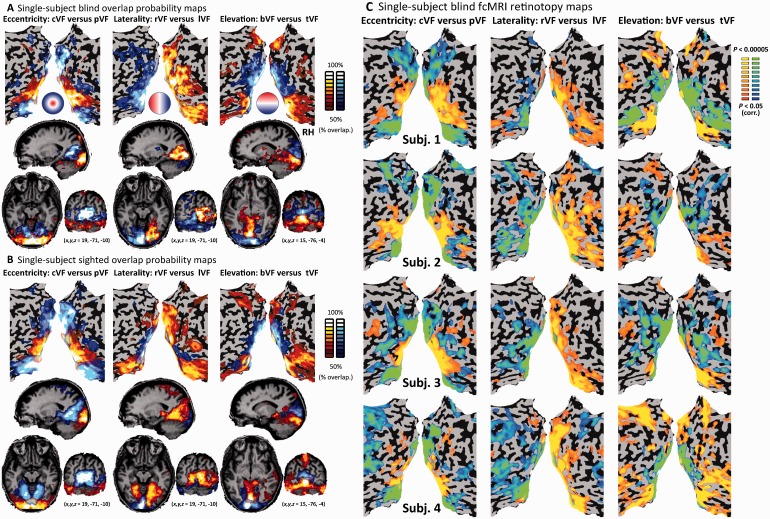
Overlap probability maps across subjects (Fig. 2A and Supplementary Fig. 3) were derived from single-subject activation contrast maps at a threshold of P < 0.05, corrected for multiple comparisons using the spatial extent method. The individual-level maps were overlaid and the overlap probability across the subjects was calculated on a voxel-by-voxel basis. Probability maps are shown at a minimal threshold of >50%, such that the smallest ratio of subjects showing activation in each presented voxel was 6 of 11 participants (although most voxels had a higher ratio, as indicated by the colour scale).
To capture the spatial similarity between the maps obtained for sighted and blind subjects, we performed a k-means clustering analysis. K-means is designed to partition n observations (in our case n = 29, data of all the blind and sighted individual subjects) into k clusters (in our case k = 2) in which each observation is assigned to the cluster with the nearest mean in an iterative converging analysis (1000 iterations), so as to minimize the within-cluster sum of squares (MacKay, 2003). The observations entered in the analysis were the concatenated retinotopic functional connectivity GLM statistical parametric maps of each subject from all 29 individual subjects, 18 sighted and 11 blind. Individual statistical parametric maps were masked by a visual cortex mask obtained with a retinotopic mapping rotating-wedge stimulus versus baseline contrast (see details on the localizer below). This mask was created at lenient threshold (P < 0.05 corrected for multiple comparisons, covering 149 404 mm3), so as to cover to the largest extent the visually-responsive cortex. Individual statistical parametric maps from each of the six V1 seeds (centre, periphery, left, right, top and bottom visual field) were concatenated and used as input for the k-means clustering analysis to test whether the clustering algorithm could tell the different groups apart. In a control analysis, the maps of each pair of complementing seeds for each subject (not concatenated; i.e. a statistical parametric map from the central visual field and a statistical parametric map from the peripheral visual field) were analysed, resulting in a clustering according to the seed rather than according to the group.
To quantify the spatial similarity between the sighted and blind group maps, we computed a concordance correlation coefficient (Lin, 1989) using in-house software written in MATLAB (MathWorks). Concordance correlation coefficient values range from 1 (perfect spatial similarity) to −1 (perfect spatial dissimilarity), and were computed for each seed between the group maps (e.g. between the central visual field map of the blind and that of the sighted). A mask of the visually-responsive cortex (also used for the k-means analysis) was used to compute the similarity between the group maps only for the relevant regions. The significance level was obtained using a permutation test while randomly shuffling voxels from one group map, and was corrected for multiple comparisons using the Bonferroni correction. We found a significant high spatial similarity between the group maps of the blind subjects and the sighted subjects for all seed regions of interest (Supplementary Table 2).
Figure 2.
Resting-state functional connectivity MRI retinotopic organization across single blind adult subjects. (A) The spatial reproducibility of the resting-state functional connectivity MRI retinotopic organization in the blind is emphasized by the probability map showing the overlap of the functional connectivity MRI maps across the blind subjects for each seed pair (for a stricter threshold replicating the same effects see Supplementary Fig. 3A). (B) The probability map shows the overlap of the functional connectivity MRI maps across the sighted subjects for each seed pair, and indicates comparable spatial reproducibility of the resting-state functional connectivity MRI retinotopic organization in both groups (see also Supplementary Fig. 3B for direct overlay of the two maps). (C) Resting-state functional connectivity MRI retinotopic organization maps are shown for four typical congenitally blind (with no light perception) subjects (one of whom, Subject 2, was blind due to micropthalmia), showing the topographic organization robustness even at the single subject level (see Fig. 4 for group analysis of microphthalmic subjects). cVF = visual field; pVF = peripheral visual field; lVF = left visual field; rVF = right visual field; tVF = top visual field; bVF = bottom visual field; RH = right hemisphere.
Spatial coregistration validation analyses
As the visual cortex of the blind shows some anatomical differences from that of the sighted in terms of grey matter and white matter integrity (Noppeney et al., 2005; Shimony et al., 2006; Pan et al., 2007), it may vary in size from that of sighted subjects (Butt et al., 2013) because of deficient underlying white matter. Thus, to verify that our functional connectivity retinotopic organization findings were not affected by the specific choice of coregistration method, we conducted two additional lines of control analyses: an analysis using stringent spatial smoothing (spatial smoothing of 12 mm full-width at half-maximum Gaussian kernel) to help align the functional data across the subjects (Supplementary Fig. 4), and a surface-based alignment across the subjects according to their cortical curvature (sulcus and gyrus) patterns (Supplementary Fig. 5), which is not affected by white matter volume differences. The curvature alignment across the subjects reached 100% subject overlap for most of the calcarine sulcus as well as other sulci, validating alignment success (Supplementary Fig. 5A). The topographic patterns of the visual cortex organization in the blind were evident in both these control analyses (Supplementary Fig. 5 and 6) to similar extents as in the main results.
Defining the location of category-selective visual areas and region of interest analysis
To localize the classical face (fusiform face area, FFA; Kanwisher et al., 1997) and scene-selective (parahippocampal place area, PPA; Epstein and Kanwisher, 1998) visual areas, we conducted an additional visual localizer experiment on a separate group of seven normally sighted participants, using a standard visual localizer experiment (detailed in Striem-Amit et al., 2012a). Twelve images from one visual category were presented in each epoch; each image was presented for 800 ms and was followed by a 200 ms blank screen (similar to standard visual localizer experiments; Hasson et al., 2003). The categories of stimuli were faces, houses, objects, letters, and textures. The FFA was defined as the peak group random-effect GLM activation cluster in the fusiform gyrus showing face selectivity when contrasting faces with all the other stimulus categories (P < 0.005, corrected). The PPA was similarly defined in the parahippocampal cortex according to the contrast houses versus all other categories (P < 0.05, corrected). GLM parameter estimators for the functional connectivity to the central and peripheral V1 were extracted for each of the groups (Fig. 3). A t-test was used to compare GLM parameter estimators for the two V1-subparts within each group (corrected for multiple comparisons), and an overall ANOVA was conducted computing the effects of group, eccentricity hemisphere and region (FFA, PPA) as well as their interactions.
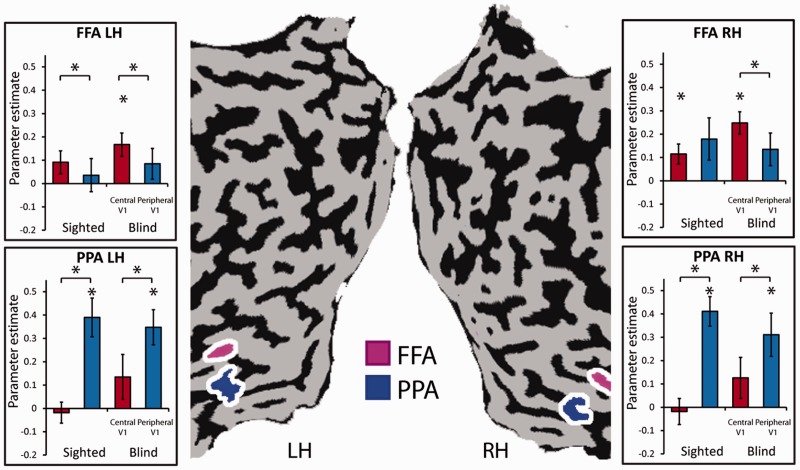
Figure 3.
Eccentricity bias as an organizing principle for human high-order object areas in the blind. The eccentricity preference of the FFA (marked in pink on the unfolded cortical sheets) and PPA (marked in blue; both defined using an external visual localizer in a normally sighted group) were computed using the functional connectivity MRI GLM parameter estimator, revealing the known bias for central visual field connectivity (in red bars) in the FFA and peripheral visual field connectivity (in blue bars) in the PPA in the blind. Error bars refer to SEM. LH = left hemisphere; RH = right hemisphere.
Results
Functional connectivity was computed for a priori defined seed regions of interest located in the primary visual cortex (V1) using a separate standard task-based retinotopic mapping localizer (Wandell et al., 2007a) in a separate group of normally sighted controls (n = 14). Regions of interest divided V1 (Fig. 1A) according to eccentricity (responses to central and peripheral visual fields), laterality (left and right visual fields) and elevation (top and bottom visual fields). Individual average time courses from each seed region of interest were extracted and z-normalized. In each complementing pair of visual field parts (e.g. central and peripheral visual field) each time course (e.g. central visual field) was used as an individual predictor in a separate group analysis using a GLM in a hierarchical random effects analysis (Friston et al., 1999), while regressing out the time course from the other seed (e.g. peripheral visual field), so as to minimize the common (correlated) components (Supplementary Fig. 1; i.e. partial correlation mapping; Zhang et al., 2008; Margulies et al., 2010). We then used this approach to investigate retinotopic organization functional connectivity in multiple levels and analyses. These included group random effect GLM analyses (Fig. 1), single subject analyses and across-subject consistency (Fig. 2), as well as a quantification of the similarity between the single subjects across the groups using an independent clustering analysis. Analyses were conducted at both the whole-brain level (Figs 1 and 2) and region of interest approaches (Fig. 3). A separate analysis was done in a subgroup of the blind who had experienced a malfunction in eye development, and who in addition to the absence of post-natal visual experience also did not have typical in utero visual development (Fig. 4). Furthermore, we compared between the groups on a seed-by-seed basis (Fig. 5).
Figure 4.
Resting-state functional connectivity MRI retinotopic organization with abnormal prenatal eye development. (A) Resting-state functional connectivity MRI retinotopic organization is shown in a subgroup of microphthalmic subjects, whose eyes did not develop properly (n = 4). This group still shows a similar pattern of visual cortex arrangement, replicating the known retinotopic organization in the absence of normal prenatal visual experience. (B) A subgroup of subjects blinded postnatally due to retinopathy of prematurity of similar group size (n = 5) showed effects qualitatively comparable to the microphthalmic subjects. cVF = visual field; pVF = peripheral visual field; lVF = left visual field; rVF = right visual field; tVF = top visual field; bVF = bottom visual field.
Group retinotopic organization functional connectivity MRI analysis
Resting-state funtional connectivity MRI random effect GLM analysis in the sighted control group replicated to a large extent the well-known retinotopic organization observed during task-based retinotopic phase mapping. We found robust connectivity to the central visual field in the occipital pole and the peripheral visual field in the anterior visual cortex extending well beyond V1 (Fig. 1B). We also found a gross division of the visual cortex into its left and right halves in their connectivity pattern to the right visual field and left visual field, respectively (Fig. 1B). Furthermore, we found a division to the upper and lower halves of visual cortex in connectivity to the bottom visual field and top visual field, respectively (Fig. 1B). These results support the validity of using functional connectivity analysis based on external group retinotopic seed regions of interest as a technique to map the organization within the visual cortex in the blind.
Importantly, the functional connectivity MRI random effect GLM analysis revealed an identical functional division of the visual cortex in the congenitally blind group, replicating the full extent of the visual cortex organization pattern according to the eccentricity, laterality and elevation divisions of labour (Fig. 1C). This was found despite the known general reduction of functional connectivity of the early visual cortex to other parts of the brain of the blind (see Supplementary Fig. 2 depicting a one region of interest seed functional connectivity analysis from the whole V1, as shown previously; Liu et al., 2007; Yu et al., 2008; Wang et al., 2013; Burton et al., 2014; Qin et al., 2014: reviewed in Bock and Fine, 2014). This connectivity-based organization according to retinotopic principles extended from our seed regions of interest in V1 throughout the classical retinotopic cortex and continued to the high-order visual cortex. It extended as far as the intraparietal sulcus in the dorsal stream and the fusiform and parahippocampal gyrus in the ventral stream.
Cross-subject consistency of retinotopic patterns in the blind
We further examined the consistency of this visual cortex functional connectivity topographical structure across the blind participants. We computed the functional connectivity statistical parametric map of visual field part activations in each of the subjects and plotted the cross-subject overlap probability map over all the individual subjects in the blind group (Fig. 2A and Supplementary Fig. 3A) and sighted group (Fig. 2B and Supplementary Fig. 3B). The retinotopic mapping principles of eccentricity, laterality and elevation in the visual field were also replicated across a large majority of the subjects (see Supplementary Fig. 3 for overlaid maps of the blind and sighted), as well as at the individual blind participant level (Fig. 2C). These findings were also replicated using alignment measures to correct for the potential size difference of the visual cortex of the blind. This included applying stringent spatial smoothing (with a 12 mm full-width at half-maximum Gaussian; Supplementary Fig. 4) to overcome intersubject variability, and aligning the data using cortex-based surface alignment rather than volume alignment (Supplementary Fig. 5).
Furthermore, we tested for fundamental differences between the blind and sighted subjects’ functional connectivity MRI retinotopic patterns by inspecting whether a k-means clustering analysis (MacKay, 2003) could clearly divide the functional connectivity maps of the subjects according to their group (with and without visual experience). First, we examined whether the algorithm would divide the maps according to the seed from which they were computed, or whether it would divide them according to the subjects’ sight status. The algorithm performed perfectly in clustering all the subjects’ eccentricity maps according to seed: it placed all the central visual field maps in one group and all the peripheral visual field in the other, regardless of whether the map belonged to a blind or sighted subject. It similarly flawlessly divided maps according to laterality and elevation principles (each tested separately). Critically, we tested what would happen when the algorithm was presented with the entire data set of maps grouped by subject (i.e. map data vectors from the six different seeds concatenated per subject), in which the only obvious source of grouping would be the visual experience. In this case it generated one cluster combining all the blind subjects with most (all but one) of the sighted subjects, and placed only one sighted subject in the second cluster, rather than generating a distinct cluster for each group. Furthermore, running the clustering analysis without this potential sighted outlier subject, again generated a cluster containing all the blind and most of the sighted in one cluster, and two other sighted subjects in another cluster. Thus, the intersubject variability in the sighted group appears to have been larger than the difference between the groups at the single-subject level, making the retinotopic organization functional connectivity patterns indistinguishable between the blind and sighted.
To quantify the spatial similarity between the blind and sighted retinotopic organizations we further computed the concordance correlation coefficient between the group maps (Lin, 1989 and see above). Highly significant concordance values were observed for all the retinotopic division maps (for all maps concordance correlation coefficient > 0.39, P < 0.001; see Supplementary Table 2 for details). Thus, the visual retinotopic functional connectivity networks were comparable to those of the sighted, even in the absence of any visual experience or light perception.
Eccentricity bias in high-order object areas in the blind
The retinotopic organization in the functional connectivity maps appeared to extend far beyond the early visual cortex. To investigate whether the blind visual cortex functional connectivity MRI retinotopy principles applied in the same way to high-order visual cortex areas, we inspected the eccentricity bias in two areas (localized in a separate visual localizer experiment) (Striem-Amit et al., 2012a; see marked portions in Fig. 3) which show opposite trends in the sighted according to their visual functional roles. The FFA (Kanwisher et al., 1997) normally shows a preference towards foveal representations that is related to the need for high-resolution vision during face processing (Hasson et al., 2002), and the PPA (Epstein and Kanwisher, 1998) shows peripheral visual field biases (Levy et al., 2001), presumably because scenes used in navigation are recognized at a coarser level and require large-scale integration of features. These two areas normally fall on either side of the retinotopic border (Hasson et al., 2002); thus their preference can also be used to estimate similarity in retinotopy border locations between groups. The functional connectivity preferences of these regions were assessed using an ANOVA with four main effects: group (blind/sighted), cortical region (FFA/PPA), eccentricity (centre/periphery) and hemisphere (right hemisphere/left hemisphere). Significant effects for cortical region (F = 9.033, P < 0.01), eccentricity (F = 5.171, P < 0.05) and hemisphere (F = 4.703, P < 0.05) were found, as well as significant interactions between cortical region × hemisphere (F = 7.74, P < 0.01) and importantly, also between the cortical region × eccentricity (F = 17.97, P < 0.001) indicating that indeed the FFA and PPA differ in their eccentricity functional connectivity preferences. Group effects were neither found in the main effect nor in any interaction (Supplementary Table 3), again suggesting that the blind pattern of functional connectivity was similar to that of the normally sighted. Furthermore, we directly investigated the effects within each region of interest in the blind using post hoc t-tests (corrected for multiple comparisons). The functional connectivity between the time courses of the FFA and central V1 was significant (t = 3.32 P < 0.01 and t = 5.18 P < 0.0005 for the left and right FFA, respectively), and significantly stronger than the functional connectivity between the peripheral V1 and FFA (t = 3.28 P < 0.005 and t = 4.41 P < 0.0005 for the left and right FFA, respectively). The reverse pattern was observed in the PPA, as the functional connectivity between the PPA and peripheral V1 was significant (t = 4.66 P < 0.001 and t = 3.33 P < 0.01 for the left and right PPA, respectively; Fig. 3) and significantly stronger than the PPA-central V1 functional connectivity (t = 5.80 P < 0.0001 and t = 4.79 P < 0.0001 for the left and right PPA, respectively). Therefore, the characteristic eccentricity biases pattern for the FFA and PPA were also evident in the absence of vision.
The role of typical prenatal development in retinotopic organization
Our results show that post-natal visual experience is not required for the creation and maintenance of macro-scale retinotopic organization. But to what extent is typical pre-natal visual experience necessary? To provide a preliminary investigation, we examined the visual cortex organization in the subgroup of subjects suffering from microphthalmia, a developmental deficiency in eye development that leads to the development of smaller (and in this case non-functional) eyes. Despite the reduced group size (n = 4), functional connectivity MRI retinotopy seed analysis indicated retention of the functional division of the cortical visual fields along the eccentricity, laterality and elevation axes throughout large portions of the visual cortex (Fig. 4A). This organization was not qualitatively different from that of a subgroup of participants suffering from retinopathy of prematurity whose visual system developed typically in utero but were injured due to postnatal oxygen treatment, being born prematurely (Fig. 4B; n = 5).
Plasticity in relation to retinotopic divisions
Our findings thus suggest that there are surprising similarities in the within-visual cortex organization of the blind and sighted. Are there also significant differences in visual cortex functional connectivity to other parts of the brain, due to life-long blindness, that are specific to retinotopically-defined segments of the visual cortex? Or is the original structure of visual field parts irrelevant to the plasticity pattern later emerging in the visual cortex in blindness? One distinct difference between visual field cortical organizations is found in the visual cortex areas related to the foveal and peripheral visual fields. For example, the peripheral V1 segment in the normally sighted shows anatomical connectivity to the auditory cortices and recruitment due to auditory attention (Eckert et al., 2008; Cate et al., 2009; Beer et al., 2011). Does this lead to different patterns of functional connectivity MRI in the blind? To assess this we compared the functional connectivity MRI of the blind and sighted groups separately in the central visual field seed and peripheral visual field seed (Fig. 5). We found that group differences within the visual cortex were generally similar across these two V1 segments (see Fig. 5C and Supplementary Fig. 2). However, different parts of V1 of the blind showed altered functional connectivity to other non-visual cortices, as compared to the sighted. Specifically, both portions of V1 of the blind showed reduced functional connectivity to the somatosensory and auditory cortices and increased connectivity to some portions of the lateral occipito-temporal cortex and inferior frontal lobe, as was previously reported (Liu et al., 2007; Yu et al., 2008; Wang et al., 2013; Bock and Fine, 2014; Burton et al., 2014). However, the different parts of V1 were functionally connected to different areas within the frontal lobe: the central V1 showed increased functional connectivity to the inferior frontal gyrus (mostly the pars triangularis, stronger on the left, near Broca’s area) whereas the peripheral V1 showed stronger functional connectivity to the dorsolateral prefrontal cortex, in the inferior frontal sulcus and middle frontal gyrus (also strongly left lateralized) and also to the posterior temporal lobe (posterior superior temporal sulcus and middle temporal gyrus). A similar comparison of both the laterality and elevation axes (Supplementary Fig. 6) showed a general pattern of increased segregation between functional networks of complementary parts of the primary visual cortex. Areas showing increased functional connectivity to the left hemisphere V1 as compared to the sighted (e.g. the left inferior frontal sulcus, the left retinotopic areas and the right auditory cortex) showed decreased functional connectivity to the right hemisphere V1 (whereas the reverse pattern was found in the right anterior frontal lobe). Areas showing increased functional connectivity to the upper part of V1 as compared to the sighted showed decreased functional connectivity to the lower part of V1 (both within the visual cortex and beyond it, in the right parietal lobe and left frontal lobe). Therefore, alongside the overall robust similarities between the visual cortex organizations between the two groups, the blind also displayed a pattern of plasticity which was uniquely altered for different portions of the visual cortex.
Functional connectivity MRI border analysis in the visual cortex of the blind
Our findings reveal the retention of the supra-areal retinotopic-principle organization (Buckner and Yeo, 2014), in which, for example, the eccentricity axis differences span V1 and its neighbouring extrastriate areas with no clear border between visual areas. But can functional connectivity also reveal areal borders in the blind, such as the V1-V2 border, as was recently shown in the sighted (Wig et al., 2014)? To test this directly, we compared the connectivity pattern from a seed in V1 with that of a seed in the same visual field in V2 (both in the ventral left central visual areas; Fig. 6A). Interestingly, while the entire ventral border between V1 and V2 could be constructed using such functional connectivity maps through both group random effect GLM analysis and inter-subject consistency measures, (Fig 6C and D; compare to Brodmann area definitions Fig. 6B), the dorsal border was not clearly visible (also when using dorsal V1 and V2 seeds; Supplementary Fig. 7). This may partly correspond to the decreased functional connectivity seen in the blind between the ventral aspect of V1 and the dorsal visual cortices (present in the group differences in the analysis of elevation; Supplementary Fig. 6). Alternatively, this may be ascribed to substantial areal organization differences. Thus, although the supra-areal organization appears to be preserved to a great extent in blindness, the areal borders themselves are not fully retained.
Discussion
Functional connectivity MRI measures have been used to reveal the effects of visual deprivation (Liu et al., 2007; Yu et al., 2008; Butt et al., 2013; Wang et al., 2013; Burton et al., 2014; Qin et al., 2014) throughout the sensory and multisensory cortices (Wallace et al., 2006; Carriere et al., 2007). Here, we largely replicated these results (Supplementary Fig. 2) but crucially, we used the new partial correlation approach with retinotopic-based seeds to investigate the retention of retinotopic organization in the visual cortex of the blind, which is not otherwise accessible non-invasively. Our findings suggest that postnatal visual experience is not necessary for at least certain levels of retinotopic large-scale functional connectivity to emerge, and to be retained throughout life, in humans.
Retention of functional organization in the visual cortex of the blind
These results strengthen other findings from our and several other groups in recent years that showed that some high-order areas of visual cortex of the early-onset blind retain the functional organization (for a specific object category or task) of the normally developing visual cortex, albeit via other modalities (Pietrini et al., 2004; Mahon et al., 2009; Ptito et al., 2009; Renier et al., 2010; Collignon et al., 2011, 2013; Bedny and Saxe, 2012; Reich et al., 2012; Striem-Amit et al., 2012a, b; Arnott et al., 2013; He et al., 2013; Kitada et al., 2013; Peelen et al., 2014; Striem-Amit and Amedi, 2014). There have only been a few attempts to investigate whether the general notion of functional organization retention extends to early visual processing stations. One such study reported contralateral activations in a single early-onset blind echo-locating expert (Thaler et al., 2011). More recently, two highly relevant studies investigated in depth the fine-scale organization of V1 in blind and sighted subjects; one using resting state connectivity (in a heterogeneous mix of congenital and late-onset, acquired blindness; Butt et al., 2013), and the other inspecting the anatomical connectivity in the corpus callosum splenium connecting the bilateral V1 (Bock et al., 2013). Both studies found a surprising level of preservation of connectivity patterns in blindness at the level of V1. Remarkably, even anopthalmic subjects (in which the input from the optic nerves to the thalamus and midbrain never existed or only existed temporarily; Stevenson and Hall, 2005), the corpus callosum splenium volume, diffusivity and overall topographic organization of fibres connecting bilateral V1 were retained (Bock et al., 2013). Our study is consistent in general with these studies, and expands upon them and for the first time reports the functional connectivity preservation of all three axes of retinotopic organization further beyond V1 even in the absence of visual input during early development (and to some extent even without normal prenatal development, in the microphthalmics). We show that these crucial functional properties can be found in the organization of the most critical early stations of the visual cortex hierarchy, and moreover, that these span the entire visual system from early retinotopic areas such as V1/V2 to object-related FFA and PPA. Furthermore, as functional connectivity attests not only to direct anatomical connectivity but is also affected by use-dependent plasticity, it is even more surprising that it retains some form of topographical organization in the full and life-long absence of visual experience and in the presence of cross-modal plasticity.
Mechanisms contributing to the development of retinotopic maps in the blind
The emergence of the retinotopic network may be due to a combination of mechanisms that are not experience-dependent, and take place prior to birth. These may be activity-independent topographically specific molecular factors (e.g. the combination of EphA-ephrin-A signalling in the cortex; Espinosa and Stryker, 2012) and activity-dependent effects of spontaneous retinal waves, coordinated waves of spontaneous activity, which sweep across the retina pre-natally, causing spatiotemporal patterns of neural activity to propagate forward in the visual system (Espinosa and Stryker, 2012). Our findings show that these are sufficient to drive and maintain the organization of macro-scale cortical maps (McLaughlin et al., 2003; Cang et al., 2005; Huberman et al., 2008; Cang and Feldheim, 2013; Ackman and Crair, 2014) even in the absence of later patterned vision or light perception.
To what extent is the large-scale retinotopic organization we observed here dependent upon prenatal retinal waves (Xu et al., 2011)? Can it develop fully based on other developmental topographically-specific molecular mechanisms that are not functionally or activity-dependent (Espinosa and Stryker, 2012; Reid, 2012)? It would be interesting to examine retinotopic functional connectivity in anopthalmic patients that do not develop retinas at all in order to address the necessity of this mechanism. In a preliminary attempt to investigate this question, we showed here that a small group of microphthalmic congenitally blind individuals (n = 4), who do not develop a full-sized functional retina (and in our case did not develop any functional vision) still possess a relatively retained retinotopic functional connectivity pattern (Fig. 4). Future studies with larger cohorts of fully anophthalmic blind participants may help assess whether this pattern emerges without any prenatal retinal activity. In any case, our findings of an intact large-scale topographic visual organization are not sufficient in themselves, to allow for functional sight restoration. Large-scale topographic patterns do not imply that finer-scale organization or experience-dependent mechanisms, which are necessary for the development of useful vision, such as those relevant for the creation, maintenance and refinement of binocular ocular dominance organization or orientation selectivity (Lewis and Maurer, 2005; Espinosa and Stryker, 2012; Levelt and Hübener, 2012; Maurer and Hensch, 2012; Reid, 2012) are present or inducible in the congenitally blind (Renier et al., 2014). Such fine-tuning, which is required for functional useful vision, still depends on intact sensory experience and pattern vision during a sequence of hyper-plastic critical developmental periods (Penn and Shatz, 1999; Hensch, 2005; Lewis and Maurer, 2005; Bengoetxea et al., 2012; Espinosa and Stryker, 2012; Voss, 2013; but see also Kalia et al., 2014). However, this hyperplasticity may be made possible even in adulthood via molecular manipulations which reopen critical-period development opportunities based on the principles naturally occurring in critical periods and following brain injury (e.g. excitatory-inhibitory balance) (Bavelier et al., 2010; Kuhlman et al., 2013; Nahmani and Turrigiano, 2014). Furthermore, extending the critical period and adult plasticity can also be aided through modifications of sensory input (such as delayed exposure to light) or sensory-motor interactions (Duffy and Mitchell, 2013; Hübener and Bonhoeffer, 2014), which may also have contributed to recently reported cases of developing pattern vision following early-onset blindness (Kalia et al., 2014).
The balance between plasticity and retained organization in the blind: division of labour within V1?
Our findings are not contradictory, but rather complementary, to previous findings of reduced overall functional connectivity of the visual cortex of the blind (also replicated in our data, see Supplementary Fig. 2; Liu et al., 2007; Yu et al., 2008; Wang et al., 2013; Burton et al., 2014; Qin et al., 2014). Partial correlation analysis regresses out the joint variance of visual areas, by systematically ignoring the connectivity changes which are common to all visual areas so as to focus on the internal structural features of the visual cortex. While we found that the internal structural organization of the visual cortex seems intact, its integration with other sensory cortices to form multisensory integration is likely to differ due to the lack of visual input (Hotting et al., 2004; Carriere et al., 2007). In fact, in a direct comparison of the blind and sighted subjects we found that not only were there differences due to life-long blindness as reported previously (Supplementary Fig. 2), but that blindness affects the central visual field part of V1 differently from the peripheral part (Fig. 5), where each segment connects more strongly to different areas in the frontal and temporal cortices. Central V1 showed increased functional connectivity to the left-lateralized inferior frontal gyrus pars triangularis near Broca’s area whereas peripheral V1 showed stronger functional connectivity to the dorsolateral prefrontal cortex, and also to the posterior temporal lobe. These differences might be related to the two apparently conflicting roles generally attributed to the early visual cortex of the congenitally blind: high-order cognitive functions such as language processing on one hand (Burton et al., 2003; Amedi et al., 2004; Bedny et al., 2011) and non-visual spatial (and also non-spatial) attention on the other (Gougoux et al., 2005; Garg et al., 2007; Collignon et al., 2011, 2013). This conflict also manifested in the seemingly counterintuitive functional connectivity pattern of enhanced functional connectivity to multiple unrelated areas of the frontal lobe with no apparent selectivity when larger seed areas of visual cortex are considered in the blind (Bock and Fine, 2014) despite the task-selective responses shown in multiple visual areas (Reich et al., 2012). These two different functional roles may simply be accounted for by two different segments of V1, which differ in the plasticity of their network properties as shown here. Such differences may also be carried over to higher order areas depending on their eccentricity biases, such as the lateral occipital cortex, which was also shown to be functionally connected to frontal language regions in the blind (Bedny et al., 2011; Watkins et al., 2012). While direct connectivity between language areas and V1 in normally sighted people is not evident normally in adulthood, functional connectivity may reflect indirect connections existing in the sighted as well, or connectivity patterns unique to the blind (Amedi et al., 2003; Bedny et al., 2011). In contrast, there is ample evidence that peripheral V1 is also connected to non-visual cortices and responds to auditory information and attention in sighted people (Eckert et al., 2008; Cate et al., 2009; Beer et al., 2011; Spierer et al., 2013; Vetter et al., 2014). Furthermore, previous studies have shown that the visual cortex is more strongly functionally connected to the fronto-parietal control network and ‘salience network’ in the blind (Wang et al., 2013; Burton et al., 2014). Here such changes were limited to the peripheral V1, possibly driving cross-modal attention plasticity. Thus, the division of V1 functional connectivity into segments according to eccentricity may help explain several non-converging lines of evidence regarding visual cortex organization and plasticity in the blind. The differences between the right and left hemispheres is consistent with previous findings of a functional connectivity disconnection between the two hemispheres (Butt et al., 2013; Burton et al., 2014), which may, as previously suggested, account for the greater spatial discrimination accuracy between non-visual inputs from the two sides of the sensory environment (Burton et al., 2014). The significance of the differences between the upper and lower sections of the V1 hemispheres is harder to interpret, but may relate to the cross-modal use by the blind of the dorsal part for non-visual tasks (Dormal et al., 2012), and the preference of the lower visual field and accordingly the upper visual cortex in such tasks (Danckert and Goodale, 2001). Relatedly, our finding of the retention of the V1-V2 areal border in the blind solely in the ventral aspect (Fig. 6) may result from the decreased functional connectivity found in the blind between the ventral aspect of V1 and the dorsal visual cortices (Supplementary Fig. 6). More speculatively, it may relate to a different areal organization in the blind, as has been shown in animal blindness models (Rakic et al., 1991; Kahn and Krubitzer, 2002). Hence, our findings suggest that there is a balance between plasticity and retained organization in the visual cortex of the blind. During development, spontaneous activity within the visual system may create the inner visual structure and sustain it sufficiently despite the absence of functionally significant retinotopically-organized input. However, the differential connectivity of parts of the retinotopically organized visual cortex also corresponds, and may relay unique patterns of plasticity and reorganization in the absence of sight, due to usage-dependent plasticity (Dosenbach et al., 2007; Fair et al., 2007). Therefore, even the robust plasticity in the blind builds on the bases of the visually-derived organization and connectivity pattern, in that it is specific to portions of V1 divided according to retinotopic principles.
Overall, these findings suggest that the balance of experience-independence and critical developmental periods should be further tested and revisited, for both the early and higher-order sensory areas. This is reported here for the case of vision, and may apply also to other sensory modalities and domains, including hearing and language capabilities, as well as multisensory processes, which also represent a balance between experience-dependent and independent developmental processes (Moore and Shannon, 2009; Butler and Lomber, 2013; Kral, 2013; Lewkowicz, 2014). These findings stress the relevance of anatomical connectivity patterns and innate developmental constraints (Mahon and Caramazza, 2011) for even the most robust plasticity: that of the central V1 of the blind for language processes.
Supplementary Material
Acknowledgements
We thank Sami Abboud, Uri Hertz, Fatma Imamoglu and Chris Filo Gorgolewski for helpful discussions.
Glossary
Abbreviations
- FFA
fusiform face area
- GLM
General Linear Model
- PPA
parahippocampal place area
Funding
This work was supported by a European Research Council grant (grant number 310809; A.A.), The Gatsby Charitable Foundation (A.A.), The James S. McDonnell Foundation scholar award (grant number 220020284; A.A.), The Israel Science Foundation (grant number ISF 1684/08; A.A.), The Edmond and Lily Safra Center for Brain Sciences (ELSC) Vision center grant (A.A.), the support of the Maratier family (A.A.), and the German Excellence Initiative Grant to the Berlin School of Mind and Brain (A.V.). None of the authors of this work has a financial interest related to this work.
Supplementary material
Supplementary material is available at Brain online.
References
- Ackman JB, Crair MC. Role of emergent neural activity in visual map development. Curr Opin Neurobiol. 2014;24:166–75. doi: 10.1016/j.conb.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedi A, Floel A, Knecht S, Zohary E, Cohen LG. Transcranial magnetic stimulation of the occipital pole interferes with verbal processing in blind subjects. Nat Neurosci. 2004;7:1266–70. doi: 10.1038/nn1328. [DOI] [PubMed] [Google Scholar]
- Amedi A, Raz N, Pianka P, Malach R, Zohary E. Early ‘visual' cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci. 2003;6:758–66. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- Arnott SR, Thaler L, Milne J, Kish D, Goodale MA. Shape-specific activation of occipital cortex in an early blind echolocation expert. Neuropsychologia. 2013;51:938–49. doi: 10.1016/j.neuropsychologia.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–71. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Pascual-Leone A, Dodell-Feder D, Fedorenko E, Saxe R. Language processing in the occipital cortex of congenitally blind adults. Proc Natl Acad Sci USA. 2011;108:4429–34. doi: 10.1073/pnas.1014818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Saxe R. Insights into the origins of knowledge from the cognitive neuroscience of blindness. Cogn Neuropsychol. 2012;29:56–84. doi: 10.1080/02643294.2012.713342. [DOI] [PubMed] [Google Scholar]
- Beer A, Plank T, Greenlee M. Diffusion tensor imaging shows white matter tracts between human auditory and visual cortex. Exp Brain Res. 2011;213:299–308. doi: 10.1007/s00221-011-2715-y. [DOI] [PubMed] [Google Scholar]
- Bengoetxea H, Ortuzar N, Bulnes S, Rico-Barrio I, Lafuente JV, Argando EG. Enriched and deprived sensory experience induces structural changes and rewires connectivity during the postnatal development of the brain. Neural Plasticity. 2012;2012:10. doi: 10.1155/2012/305693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Cheng C, Stager DR, Jr, Weakley DR, Jr, Stager DR., Sr The critical period for surgical treatment of dense congenital bilateral cataracts. J AAPOS. 2009;13:67–71. doi: 10.1016/j.jaapos.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bock AS, Fine I. Anatomical and functional plasticity in early blind individuals and the mixture of experts architecture. Front Hum Neurosci. 2014;8:971. doi: 10.3389/fnhum.2014.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock AS, Saenz M, Tungaraza R, Boynton GM, Bridge H, Fine I. Visual callosal topography in the absence of retinal input. Neuroimage. 2013;81:325–34. doi: 10.1016/j.neuroimage.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Yeo BTT. Borders, map clusters, and supra-areal organization in visual cortex. Neuroimage. 2014;93 (Pt 2):292–7. doi: 10.1016/j.neuroimage.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Diamond JB, McDermott KB. Dissociating cortical regions activated by semantic and phonological tasks: a FMRI study in blind and sighted people. J Neurophysiol. 2003;90:1965–82. doi: 10.1152/jn.00279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Raichle ME. Resting state functional connectivity in early blind humans. Front Syst Neurosci. 2014;8:51. doi: 10.3389/fnsys.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler BE, Lomber SG. Functional and structural changes throughout the auditory system following congenital and early-onset deafness: implications for hearing restoration. Front Syst Neurosci. 2013;7:92. doi: 10.3389/fnsys.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt OH, Benson NC, Datta R, Aguirre GK. The fine-scale functional correlation of striate cortex in sighted and blind people. J Neurosci. 2013;33:16209–19. doi: 10.1523/JNEUROSCI.0363-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Feldheim DA. Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci. 2013;36:51–77. doi: 10.1146/annurev-neuro-062012-170341. [DOI] [PubMed] [Google Scholar]
- Cang J, Rentería RC, Kaneko M, Liu X, Copenhagen DR, Stryker MP. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere BN, Royal DW, Perrault TJ, Morrison SP, Vaughan JW, Stein BE, Wallace MT. Visual deprivation alters the development of cortical multisensory integration. J Neurophysiol. 2007;98:2858–67. doi: 10.1152/jn.00587.2007. [DOI] [PubMed] [Google Scholar]
- Chan WH, Biswas S, Ashworth JL, Lloyd IC. Congenital and infantile cataract: aetiology and management. Eur J Pediatr. 2012;171:625–30. doi: 10.1007/s00431-012-1700-1. [DOI] [PubMed] [Google Scholar]
- Collignon O, Dormal G, Albouy G, Vandewalle G, Voss P, Phillips C, Lepore F. Impact of blindness onset on the functional organization and the connectivity of the occipital cortex. Brain. 2013;136:2769–83. doi: 10.1093/brain/awt176. [DOI] [PubMed] [Google Scholar]
- Collignon O, Vandewalle G, Voss P, Albouy G, Charbonneau G, Lassonde M, Lepore F. Functional specialization for auditory-spatial processing in the occipital cortex of congenitally blind humans. Proc Natl Acad Sci USA. 2013;108:4435–40. doi: 10.1073/pnas.1013928108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate AD, Herron TJ, Yund EW, Stecker GC, Rinne T, Kang X, Petkov CI, Disbrow EA, Woods DL. Auditory attention activates peripheral visual cortex. PLoS One. 2009;4:e4645. doi: 10.1371/journal.pone.0004645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–33. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Danckert J, Goodale MA. Superior performance for visually guided pointing in the lower visual field. Exp Brain Res. 2001;137:303–8. doi: 10.1007/s002210000653. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Dormal G, Lepore F, Collignon O. Plasticity of the dorsal “spatial” stream in visually deprived individuals. Neural Plast. 2012;2012:687–59. doi: 10.1155/2012/687659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–78. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy KR, Mitchell DE. Darkness alters maturation of visual cortex and promotes fast recovery from monocular deprivation. Curr Biol. 2013;23:382–6. doi: 10.1016/j.cub.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Kamdar NV, Chang CE, Beckmann CF, Greicius MD, Menon V. A cross-modal system linking primary auditory and visual cortices: evidence from intrinsic fMRI connectivity analysis. Hum Brain Mapp. 2008;29:848–57. doi: 10.1002/hbm.20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Rumelhart DE, Wandell BA, Lee AT, Glover GH, Chichilnisky EJ, Shadlen MN. fMRI of human visual cortex. Nature. 1994;369:525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–49. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 2007;104:13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1993;1:210–20. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–78. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Garg A, Schwartz D, Stevens AA. Orienting auditory spatial attention engages frontal eye fields and medial occipital cortex in congenitally blind humans. Neuropsychologia. 2007;5:2307–21. doi: 10.1016/j.neuropsychologia.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougoux F, Zatorre RJ, Lassonde M, Voss P, Lepore F. A functional neuroimaging study of sound localization: visual cortex activity predicts performance in early-blind individuals. PLoS Biol. 2005;3:e27. doi: 10.1371/journal.pbio.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Carrillo B, Mackey AP, Bunge SA. Resting-state fMRI: a window into human brain plasticity. Neuroscientist. 2014;20:522–33. doi: 10.1177/1073858414524442. [DOI] [PubMed] [Google Scholar]
- Hasson U, Harel M, Levy I, Malach R. Large-scale mirror-symmetry organization of human occipito-temporal object areas. Neuron. 2003;37:1027–41. doi: 10.1016/s0896-6273(03)00144-2. [DOI] [PubMed] [Google Scholar]
- Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–90. doi: 10.1016/s0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- He C, Peelen MV, Han Z, Lin N, Caramazza A, Bi Y. Selectivity for large nonmanipulable objects in scene-selective visual cortex does not require visual experience. Neuroimage. 2013;79:1–9. doi: 10.1016/j.neuroimage.2013.04.051. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hertz U, Amedi A. Disentangling unisensory and multisensory components in audiovisual integration using a novel multifrequency fMRI spectral analysis. Neuroimage. 2010;52:617–32. doi: 10.1016/j.neuroimage.2010.04.186. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035–40. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotting K, Rösler F, Röder B. Altered auditory-tactile interactions in congenitally blind humans: an event-related potential study. Exp Brain Res. 2004;159:370–81. doi: 10.1007/s00221-004-1965-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Effects of monocular deprivation in kittens. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964;248:492–97. doi: 10.1007/BF00348878. [DOI] [PubMed] [Google Scholar]
- Hübener M, Bonhoeffer T. Neuronal plasticity: beyond the critical period. Cell. 2014;159:727–37. doi: 10.1016/j.cell.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn DM, Krubitzer L. Massive cross-modal cortical plasticity and the emergence of a new cortical area in developmentally blind mammals. Proc Natl Acad Sci USA. 2002;99:11429–34. doi: 10.1073/pnas.162342799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia A, Lesmes LA, Dorr M, Gandhi T, Chatterjee G, Ganesh S, Bex PJ, Sinha P. Development of pattern vision following early and extended blindness. Proc Natl Acad Sci USA. 2014;111:2035–39. doi: 10.1073/pnas.1311041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada R, Okamoto Y, Sasaki AT, Kochiyama T, Miyahara M, Lederman SJ, Sadato N. Early visual experience and the recognition of basic facial expressions: involvement of the middle temporal and inferior frontal gyri during haptic identification by the early blind. Front Hum Neurosci. 2013;7:7. doi: 10.3389/fnhum.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A. Auditory critical periods: a review from system's perspective. Neuroscience. 2013;247:117–33. doi: 10.1016/j.neuroscience.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501:543–46. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R, Ptito M. Compensatory plasticity and cross-modal reorganization following early visual deprivation. Neurosci Biobehav Rev. 2013;41:36–52. doi: 10.1016/j.neubiorev.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Levelt CN, Hübener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–30. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat Neurosci. 2001;4:533–39. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev Psychobiol. 2005;46:163–83. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ. Early experience and multisensory perceptual narrowing. Dev Psychobiol. 2014;56:292–315. doi: 10.1002/dev.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68. [PubMed] [Google Scholar]
- Liu Y, Yu C, Liang M, Li J, Tian L, Zhou Y, Qin W, Li K, Jiang T. Whole brain functional connectivity in the early blind. Brain. 2007;130:2085–96. doi: 10.1093/brain/awm121. [DOI] [PubMed] [Google Scholar]
- MacKay DJ. New York, NY: Cambridge University Press; 2003. Information theory, inference and learning algorithms. [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O'Leary DDM. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–60. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Anzellotti S, Schwarzbach J, Zampini M, Caramazza A. Category-specific organization in the human brain does not require visual experience. Neuron. 2009;63:397–405. doi: 10.1016/j.neuron.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. What drives the organization of object knowledge in the brain? Trends Cogn Sci. 2011;15:97–103. doi: 10.1016/j.tics.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Bottger J, Long X, Lv Y, Kelly C, Schafer A, Goldhahn D, Abbushi A, Milham MP, Lohmann G, Villringer A. Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. MAGMA. 2010;23:289–307. doi: 10.1007/s10334-010-0228-5. [DOI] [PubMed] [Google Scholar]
- Maurer D, Hensch TK. Amblyopia: background to the special issue on stroke recovery. Dev Psychobiol. 2012;54:224–38. doi: 10.1002/dev.21022. [DOI] [PubMed] [Google Scholar]
- Maurer D, Lewis TL, Mondloch CJ. Missing sights: consequences for visual cognitive development. Trends Cogn Sci. 2005;9:144–51. doi: 10.1016/j.tics.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 2010;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Shannon RV. Beyond cochlear implants: awakening the deafened brain. Nat Neurosci. 2009;12:686–91. doi: 10.1038/nn.2326. [DOI] [PubMed] [Google Scholar]
- Nahmani M, Turrigiano GG. Adult cortical plasticity following injury: Recapitulation of critical period mechanisms? Neuroscience. 2014;283:4–16. doi: 10.1016/j.neuroscience.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Friston KJ, Ashburner J, Frackowiak R, Price CJ. Early visual deprivation induces structural plasticity in gray and white matter. Curr Biol. 2005;15:R488–90. doi: 10.1016/j.cub.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Pan WJ, Wu G, Li CX, Lin F, Sun J, Lei H. Progressive atrophy in the optic pathway and visual cortex of early blind Chinese adults: a voxel-based morphometry magnetic resonance imaging study. Neuroimage. 2007;37:212–20. doi: 10.1016/j.neuroimage.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Peelen MV, He C, Han Z, Caramazza A, Bi Y. Nonvisual and visual object shape representations in occipitotemporal cortex: evidence from congenitally blind and sighted adults. J Neurosci. 2014;34:163–70. doi: 10.1523/JNEUROSCI.1114-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AA, Shatz CJ. Brain waves and brain wiring: the role of endogenous and sensory-driven neural activity in development. Pediatr Res. 1999;45:447–58. doi: 10.1203/00006450-199904010-00001. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Furey ML, Ricciardi E, Gobbini MI, Wu WH, Cohen L, et al. Beyond sensory images: Object-based representation in the human ventral pathway. Proc Natl Acad Sci USA. 2004;101:5658–63. doi: 10.1073/pnas.0400707101. Epub 2004 Apr 5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptito M, Matteau I, Gjedde A, Kupers R. Recruitment of the middle temporal area by tactile motion in congenital blindness. Neuroreport. 2004;20:543–47. doi: 10.1097/WNR.0b013e3283279909. [DOI] [PubMed] [Google Scholar]
- Qin W, Xuan Y, Liu Y, Jiang T, Yu C. Functional connectivity density in congenitally and late blind subjects. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu051. Advance Access published on March 18, 2014. [DOI] [PubMed] [Google Scholar]
- Rakic P, Suner I, Williams RW. A novel cytoarchitectonic area induced experimentally within the primate visual cortex. Proc Natl Acad Sci USA. 1991;88:2083–87. doi: 10.1073/pnas.88.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Striem E, Pundak G, Orlov T, Zohary E. Superior serial memory in the blind: a case of cognitive compensatory adjustment. Curr Biol. 2007;17:1129–33. doi: 10.1016/j.cub.2007.05.060. [DOI] [PubMed] [Google Scholar]
- Reich L, Maidenbaum S, Amedi A. The brain as a flexible task-machine: implications for visual rehabilitation using non-invasive vs. invasive approaches. Curr Opin Neurol. 2012;25:86–95. doi: 10.1097/WCO.0b013e32834ed723. [DOI] [PubMed] [Google Scholar]
- Reid RC. From functional architecture to functional connectomics. Neuron. 2012;75:209–17. doi: 10.1016/j.neuron.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier LA, Anurova I, De Volder AG, Carlson S, VanMeter J, Rauschecker JP. Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron. 2010;68:138–48. doi: 10.1016/j.neuron.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier L, De Volder AG, Rauschecker JP. Cortical plasticity and preserved function in early blindness. Neurosci Biobehav Rev. 2014;41:53–63. doi: 10.1016/j.neubiorev.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibanez V, Deiber MP, Dold G, Hallett M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–28. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–93. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Shimony JS, Burton H, Epstein AA, McLaren DG, Sun SW, Snyder AZ. Diffusion tensor imaging reveals white matter reorganization in early blind humans. Cereb Cortex. 2006;16:1653–61. doi: 10.1093/cercor/bhj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci. 2009;106:13040–45. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierer L, Manuel AL, Bueti D, Murray MM. Contributions of pitch and bandwidth to sound-induced enhancement of visual cortex excitability in humans. Cortex. 2013;49:2728–34. doi: 10.1016/j.cortex.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Stevenson RE, Hall JG. Human malformations and related anomalies. Oxford University Press. 2005. [Google Scholar]
- Striem-Amit E, Amedi A. Visual cortex extrastriate body-selective area activation in congenitally blind people seeing by using sounds. Curr Biol. 2014;24:687–92. doi: 10.1016/j.cub.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Striem-Amit E, Cohen L, Dehaene S, Amedi A. Reading with sounds: sensory substitution selectively activates the visual word form area in the blind. Neuron. 2012a;76:640–52. doi: 10.1016/j.neuron.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Striem-Amit E, Dakwar O, Reich L, Amedi A. The large-scale organization of “visual” streams emerges without visual experience. Cereb Cortex. 2012b;22:1698–709. doi: 10.1093/cercor/bhr253. [DOI] [PubMed] [Google Scholar]
- Striem-Amit E, Hertz U, Amedi A. Extensive cochleotopic mapping of human auditory cortical fields obtained with phase-encoding fMRI. PLoS One. 2011;6:e17832. doi: 10.1371/journal.pone.0017832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme: 1988. [Google Scholar]
- Thaler L, Arnott SR, Goodale MA. Neural correlates of natural human echolocation in early and late blind echolocation experts. PLoS One. 2011;6:e20162. doi: 10.1371/journal.pone.0020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter P, Smith FW, Muckli L. Decoding Sound and Imagery Content in Early Visual Cortex. Curr Biol. 2014;24:1256–1262. doi: 10.1016/j.cub.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Voss P. Sensitive and critical periods in visual sensory deprivation. Front Psychol. 2013;4 doi: 10.3389/fpsyg.2013.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss P, Zatorre RJ. Organization and reorganization of sensory-deprived cortex. Curr Biol. 2012;22:R168–73. doi: 10.1016/j.cub.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Carriere BN, Perrault TJ, Vaughan JW, Stein BE. The development of cortical multisensory integration. J Neuroscience. 2006;26:11844–49. doi: 10.1523/JNEUROSCI.3295-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007a;56:366–83. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007b;56:366–83. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Winawer J. Imaging retinotopic maps in the human brain. Vision Res. 2011;51:718–37. doi: 10.1016/j.visres.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Qin W, Liu Y, Zhang Y, Jiang T, Yu C. Altered resting-state network connectivity in congenital blind. Hum Brain Mapp. 2013;35:2573–81. doi: 10.1002/hbm.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Cowey A, Alexander I, Filippini N, Kennedy JM, Smith SM, et al. Language networks in anophthalmia: maintained hierarchy of processing in ‘visual' cortex. Brain. 2012;135:1566–77. doi: 10.1093/brain/aws067. [DOI] [PubMed] [Google Scholar]
- Wig GS, Laumann TO, Petersen SE. An approach for parcellating human cortical areas using resting-state correlations. Neuroimage. 2014;93 (Pt 2):276–91. doi: 10.1016/j.neuroimage.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–17. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28:1029–40. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Xu HP, Furman M, Mineur YS, Chen H, King SL, Zenisek D, et al. An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron. 2011;70:1115–27. doi: 10.1016/j.neuron.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Liu Y, Li J, Zhou Y, Wang K, Tian L, et al. Altered functional connectivity of primary visual cortex in early blindness. Hum Brain Mapp. 2008;29:533–43. doi: 10.1002/hbm.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100:1740–48. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.