Astrocyte-derived Sonic Hedgehog helps to maintain the integrity of the blood-brain barrier, but many of its downstream effectors are unknown. Podjaski et al. show that in multiple sclerosis, Sonic Hedgehog upregulates netrin-1 expression on the CNS vasculature, inducing an autocrine response that reduces blood-brain barrier permeability and dampens neuroinflammation.
Keywords: multiple sclerosis, experimental autoimmune encephalomyelitis, blood–brain barrier, netrin 1, neuroinflammation
Astrocyte-derived Sonic Hedgehog helps to maintain the integrity of the blood-brain barrier, but many of its downstream effectors are unknown. Podjaski et al. show that in multiple sclerosis, Sonic Hedgehog upregulates netrin-1 expression on the CNS vasculature, inducing an autocrine response that reduces blood-brain barrier permeability and dampens neuroinflammation.
Abstract
Blood–brain barrier function is driven by the influence of astrocyte-secreted factors. During neuroinflammatory responses the blood–brain barrier is compromised resulting in central nervous system damage and exacerbated pathology. Here, we identified endothelial netrin 1 induction as a vascular response to astrocyte-derived sonic hedgehog that promotes autocrine barrier properties during homeostasis and increases with inflammation. Netrin 1 supports blood–brain barrier integrity by upregulating endothelial junctional protein expression, while netrin 1 knockout mice display disorganized tight junction protein expression and barrier breakdown. Upon inflammatory conditions, blood–brain barrier endothelial cells significantly upregulated netrin 1 levels in vitro and in situ, which prevented junctional breach and endothelial cell activation. Finally, netrin 1 treatment during experimental autoimmune encephalomyelitis significantly reduced blood–brain barrier disruption and decreased clinical and pathological indices of disease severity. Our results demonstrate that netrin 1 is an important regulator of blood–brain barrier maintenance that protects the central nervous system against inflammatory conditions such as multiple sclerosis and experimental autoimmune encephalomyelitis.
Introduction
The blood–brain barrier, also referred to as the neurovascular unit, is a critical gateway that regulates the passage of molecules and cells between the blood and the CNS. Barrier properties of this specialized microvascular network derive from inter-endothelial adhesion processes, mediated by tight junction and adherens junction proteins. Endothelial cells have the capacity to modulate barrier permeability by regulating the expression levels of tight and adherens junction proteins and recruiting them into junctional complexes within lipid raft membrane microdomains (Wosik et al., 2007). Within these complexes, transmembrane proteins including occludin, claudin and junctional adhesion molecule, as well as intracellular adaptor proteins such as zonula occludens 1 (now known as TJP1), α-catenin (now known as CTNNA1) and p120 (now known as CTNND1) promote para-cellular barrier function (Sixt et al., 2001; Abbott, 2005; Man et al., 2007). Astrocyte endfeet, which cover almost the entire basolateral surface of the brain endothelium, provide factors such as sonic hedgehog (SHH) and angiotensin that dynamically regulate junctional protein expression promoting a competent blood–brain barrier (Wosik et al., 2007; Alvarez et al., 2011a). In several inflammatory brain pathologies, including multiple sclerosis, trauma and stroke, pro-inflammatory mediators contribute to junctional complex breakdown, causing blood–brain barrier dysfunction (Unterberg et al., 2004; Kebir et al., 2007; Cayrol et al., 2008; Altman and Rutledge, 2010).
Netrins are laminin-related proteins that regulate cell migration and influence cell–cell and cell–substrate adhesion (Srinivasan et al., 2003; Kang et al., 2004; Park et al., 2004; Shekarabi et al., 2005; Jarjour et al., 2008; Lejmi et al., 2008; Moore et al., 2008, 2009). Although netrins are best known to regulate axon guidance and glial cell migration, they also exert angiogenic (Wilson et al., 2006) and anti-inflammatory (Rosenberger et al., 2009; Grenz et al., 2011) functions. Both netrin 1 (NTN1) and netrin 4 (NTN4) increase endothelial proliferation, vascular branching, and vessel density (Wilson et al., 2006; Navankasattusas et al., 2008; Hoang et al., 2009; Larrieu-Lahargue et al., 2010), which ameliorates pathological symptoms in conditions of ischaemia (Wilson et al., 2006; Hoang et al., 2009; Durrani et al., 2012). Although SHH was previously reported to regulate blood–brain barrier homeostasis through activation of the co-receptor SMO (Alvarez et al., 2011b), additional downstream mediators of SHH-dependant signalling remain to be identified.
In this study we evaluated if netrin 1 is a downstream effector of SHH signalling on blood–brain barrier endothelium and the effect of netrin 1 on barrier and immunological responses during neuroinflammatory responses. We found that SHH stimulated netrin 1 expression on human blood–brain barrier endothelial cells, which resulted in increased tight junction protein expression and reduced blood–brain barrier permeability. In contrast, the absence of netrin 1 altered tight junction protein expression and promoted barrier leakage in vivo. To investigate the role of netrin 1 during inflammatory conditions, we applied netrin 1 to experimental autoimmune encephalomyelitis (EAE) mice therapeutically and prophylactically. Netrin 1 stabilized the blood–brain barrier, reduced disease severity and it was most protective when given early in the disease process.
Materials and methods
Human primary brain-derived endothelial and astrocyte cell cultures
Human brain-derived endothelial cells
With informed consent and ethical approval (ethical approval number HD04.046), human temporal lobe material was obtained from patients who underwent surgical treatment for intractable temporal lobe epilepsy. Primary human brain-derived endothelial cells were isolated as described elsewhere (Alvarez et al., 2011b).
Astrocyte conditioned media
To harvest astrocyte conditioned media, human foetal astrocytes [obtained from the human foetal repository in the Albert Einstein College of Medicine, New York] were isolated and grown in complete Dulbecco's modified Eagle medium (DMEM, Invitrogen), supplemented with 10% foetal bovine serum (FBS) (Alvarez et al., 2011b). Astrocyte conditioned media was harvested every 7 days from confluent astrocyte monolayers, and filtered when added to human brain-derived endothelial cells.
Mouse brain-derived endothelial cell primary cultures
Primary cultures of mouse brain endothelial cells were prepared from 8-week-old C57BL/6 mice. The brains were isolated without perfusion and meninges/choroid plexuses were removed. After mincing and homogenization the parenchymal tissue was digested in DMEM containing 0.7 mg/ml collagenase type II (Worthington Biochemical) and 39 U/ml DNase I (Worthington Biochemical) for 75 min at 37°C. Myelin was removed by centrifugation at 1000g for 20 min in 20% bovine serum albumin (BSA)-DMEM (Sigma). The remaining pellet was then shaken for 1 h at 37°C with a mixture of 1 mg/ml collagenase-dispase (Roche) and 39 U/ml DNase I in DMEM. The microvessels were obtained using a 33% continuous Percoll® gradient (1000g for 10 min) and were plated on 6-well culture dishes coated with 5 μg/ml collagen type IV (Sigma). Mouse brain endothelial cells were cultured in DMEM supplemented with 20% (v/v) FBS (Sigma), 1 ng/ml basic fibroblast growth factor (Roche), 100 μg/ml heparin (Sigma), 1.4 μM hydrocortisone (Sigma), and antibiotic-antimycotic solution (Invitrogen). Puromycin (10 μg/ml; Sigma) was added to the media for the first 48 h of culture. After 72 h, 4 μg/ml of puromycin was maintained in the culture media. The media was replaced every 24 h during the first 3 days. A confluent monolayer was formed following 4–6 days culture.
RNA isolation and reverse transcription quantitative PCR
Human brain-derived endothelial cells were cultured to confluency, then treated for 24 h in fresh endothelial cell medium, supplemented without any treatment or with either recombinant SHH (100 ng/ml R&D systems), 40% astrocyte conditioned media or TNF and interferon gamma (IFNγ) (both at 100 U/ml). Cells were trypsinized, washed and then lysed with TRIzol® (Invitrogen). RNA was isolated with the MinElute® columns (Qiagen) according to manufacturer's instructions and treated with DNase (Qiagen). To obtain cDNA, a total of 2 μg per RNA sample, random hexaprimers (Roche) and Moloney murine leukemia virus reverse transcriptase enzyme (Invitrogen) were used for the reverse transcription reaction at 42°C. cDNA samples were prepared in duplicate with the respective TaqMan® FAM-labelled MGB probes (netrin 1: Hs00924151_m1; netrin 3 Hs00352403_g1; netrin 4: Hs00221915_m1; DCC: Hs00180437_m1; neogenin: Hs00933949_m1; unc5h-A: Hs01113563_m1; unc5hB: Hs00402127_m1; unc5h-C: Hs00186620_m1; unc5h-D: Hs00369888_m1; all by Applied Biosystems), together with human ACTB as an endogenous reference control and amplified and measured with the ABI PRISM 770 Sequence Detection System (Applied Biosystems). Each probe was tested for its efficiency by including a serial dilution of cDNA from human foetal brain homogenates (positive control). The ΔCT method (CT = cycle threshold = cycle at which gene of interest is detected in a linear range; ΔCT = difference between CT of the gene of interest and the CT of the internal gene control actin) was used to compare levels of mRNA.
RNA sequencing
The RNA from three distinct primary cultures of human brain-derived endothelial cell preparations was sent to Genome Quebec for RNA sequencing analysis. In brief, RNA was quantified, quality was measured and mRNA was fragmented to build a cDNA library. RNA sequencing was performed by generating between 89 and 209 million paired reads per library using the Illumina HiSeq 2000/2500 sequencer (Illumina). Quantification was done by assembling aligned RNA-Seq reads into transcripts and their abundance was estimated in fragments per kilobase of exon per million fragments mapped (FPKM) using the Cufflinks program (http://cufflinks.cbcb.umd.edu).
Protein isolation and western blot analysis
Confluent human brain-derived endothelial cells were treated for 24 h with either 40% astrocyte conditioned media or a mix of TNF and IFNγ (each at 100 U/ml GIBCO), respectively, compared to untreated human brain-derived endothelial cells. For protein isolation the cells were scraped off, lysed with SDS lysis buffer (final 2% SDS, Baculo Gold protease inhibitors) and sonicated. Protein was quantified using the BCA detection kit (Pierce). In addition, the brains from netrin 1 knockout, heterozygous and wild-type mice (CD-1 background obtained from Marc Tessier-Lavigne, Rockefeller University) (Serafini et al., 1996) were dissected at postnatal Day 0. Brains were homogenized, lysed in 2% SDS buffer, sonicated and protein was quantified. All procedures with animals were performed in accordance with the Canadian Council on Animal Care guidelines for the use of animals in research. SDS-PAGE (Bio-Rad) was performed by loading 20 µg of denatured protein per lane, and blotted onto a PVDF-membrane (Bio-Rad). After blocking with 5% skimmed milk in Tris-buffered saline–0.1% Tween (TBST), the primary antibody was applied, followed by washes with TBST and by incubation with secondary horseradish peroxidase (HRP)-coupled antibodies (rabbit anti-goat-HRP, rabbit anti-rat-HRP, and rabbit anti-mouse-HRP (all at 1:2000 Dako), goat anti-rabbit (1:5000 Jackson Laboratories). Specific bands were developed with the ECL substrate (Amersham, ECL Plus detection kit). Protein bands were then measured by densitometry using Bio-Rad Gel Doc System and Quantity One® software. Primary antibodies: Goat anti-netrin 4 (1:500 R&D Systems), rabbit anti-netrin 1 (1:5000 serum BLAZE), goat anti-neogenin (1:50 Santa Cruz C-20), goat anti-unc5B (R&D Systems), rabbit anti-unc5C (Tompson Lab), rabbit anti-occludin (1:500 BD Biosciences), mouse anti-JAM-A (1:300 BD Biosciences), rabbit anti-claudin-5 (1:250). A mouse anti-actin (1:20 000, Sigma-Aldrich) antibody was used to detect ACTB as a reference control.
Human and mouse CNS tissue preparation for histological analysis
Brain tissue was obtained from 10 patients with clinical and neuropathological multiple sclerosis diagnosis. Autopsy samples were immediately frozen in liquid nitrogen. White matter multiple sclerosis tissue samples were selected on the basis of MRI and lesions were classified according to standard histopathological criteria, as previously published by our group (Kebir et al., 2007; Cayrol et al., 2008). All patients and controls, or their next of kin, had given informed consent for autopsy and use of their brain tissue for research purposes. Ethical approval was given prior to autopsy. We identified lesion sites and normal-appearing white matter by the presence or absence of immune cell infiltrates and demyelination, respectively.
For EAE, adult female mice (C57BL/6), with or without EAE, were anaesthetized and PBS-perfused. Brains and spinal cords were removed, embedded in O.C.T. compound and cryo-sectioned into 7-μm thin sections for further staining procedures. To evaluate the degree of leucocyte infiltration and demyelination, we stained CNS sections with Luxol Fast blue and haematoxylin/eosin as described (Alvarez et al., 2011b) and microscopically analysed sections with Open Lab software.
To assess the effect of netrin 1 loss during blood–brain barrier development, we removed the brains of netrin 1 knockout, heterozygous and wild-type mice immediately after birth. Samples were embedded in O.C.T. compound and cryosectioned into 7-μm thin sections for further staining procedures.
Immunofluorescent staining
In vitro
Human and murine blood–brain barrier endothelial cells were grown to confluency in 8-well plastic chamber slides and then treated for 24 h with either 40% astrocyte conditioned media, a mix of TNF and IFNγ (at 100 U/ml each; GIBCO), or recombinant netrin 1 (100 ng/ml; R&D Systems). Cells were washed and then fixed at room temperature with 70% ethanol. Immunocytofluorescent staining was performed as previously reported (Alvarez et al., 2011b) using the following primary antibodies: rat anti-netrin 1 (1:100, R&D Systems), mouse anti-p120 (1:100, Invitrogen), rabbit anti-ZO-1 (1:100, Invitrogen), rabbit anti-alpha catenin (1:50, Invitrogen), rabbit anti-occludin (1:100, Invitrogen) and mouse anti-claudin-5. Qualitative microscopic evaluation was performed using a LEICA SP5 confocal microscope as described previously (Alvarez et al., 2011b).
In situ
Tissue cryosections were fixed in acetone for 10 mins at −20°C and blocked with 10% FBS in PBS, permeabilized with 0.05% Tween in PBS and incubated with primary antibodies (diluted in 3% FBS), followed by washes, secondary antibody incubation and final washes. The sections were mounted in Mowiol® containing TOPRO-3 (1:300, Invitrogen). Primary antibodies/reagents: mouse anti-GFAP-Cy3 (1:2000, Sigma), Esculentum lectin-FITC (1:2000, Vector Laboratories), and rat anti-netrin 1 (1:100, R&D Systems). Primary antibodies for tight junction protein detection in netrin 1 knockout and wild-type mice: rabbit anti-ZO-1 (1:100, Invitrogen), rabbit anti-occludin (1:50, Invitrogen), rabbit anti-claudin-5 (1:30, Invitrogen). Alexa Fluor®-conjugated secondary antibodies (Invitrogen) were diluted 1:500 in PBS. Qualitative microscopic evaluation was performed as described above.
In vivo blood–brain barrier permeability to serum proteins
Serum protein leakage was determined by simultaneous staining of plasma proteins and blood vessel markers in brains and spinal cords from wild-type and netrin 1 knockout mice as well as netrin 1-treated versus PBS-treated EAE mice. Immunostainings were performed as described above with the following primary antibodies/reagents: rabbit anti-laminin (1:1500, Dako), Esculentum-lectin-FITC (1:2000, Vector Laboratories), rat anti-CD31 (1:1500, BD Biosciences), goat anti-apoB (1:300, Abcam), rabbit anti-fibrinogen (1:1500, Innovative Research) and goat anti-mouse Alexa Fluor® 488-conjugated (Molecular Probes). We used a LEICA confocal microscope for microscopic evaluation. Plasma protein leakage was determined by multiplying fluorescent (mean pixel) intensity and the area of leakage (number of pixels) of the respective fluorescent serum protein marker, using the ImageJ software.
In vitro blood–brain barrier permeability assay
Experimental conditions were prepared in triplicates. Human brain-derived endothelial cells were plated on gelatin-coated 3-μm pore size Boyden chambers at a density of 1.3 × 104 cells per well. When cells reached confluency, they were treated with ECM media, supplemented with either 40% (vol/vol) astrocyte conditioned media or netrin 1 (100 ng/ml R&D Systems) as previously described (Tepavcevic et al., 2014). After 24 h, 50 μg/ml of bovine serum albumin-FITC (Invitrogen) or 50 μg/ml of dextran 10 kDa-Alexa Fluor® 647 (Invitrogen) were added to the upper chambers. Aliquots of 50 μl were harvested from each upper and lower chamber 2 h after tracer molecule addition and quantified with a fluorescence multimode plate reader (Biotek, Synergy 4). Baseline diffusion at 0 h was subtracted from the diffusion rates at 2 h. Permeability coefficient was calculated as previously described (Siflinger-Birnboim et al., 1987; Alvarez et al., 2011b).
Transendothelial electrical resistance measurement
The electrical properties of confluent monolayers of primary mouse brain-derived endothelial cells were measured with the Electric Cell-substrate Impedance Sensing (ECIS) methodology using the ECIS Zθ instrument and 8W10E + electrode arrays (Applied Biophysics). The transendothelial electrical properties of cells (3 × 104 per well) grown as a monolayer were measured for 65 h, after which cells were inflamed with IFNγ/TNF (100 U/ml each, R&D systems) for 30 h and finally stimulated with netrin 1 (100 ng/ml). The electrical properties (impedance) of the cells were measured at 1000 Hz over a period of 115 h.
Enzyme-linked immunosorbent assay
Netrin detection
To quantify levels of soluble netrin 1 in conditioned supernatants from astrocytes, and human brain-derived endothelial cells, in serum from healthy young adults and multiple sclerosis patients or CSF, we analysed samples by ELISA. For this purpose, human netrin 1 ELISA detection kit from Cusabio (CSB-E11899h) was used. For astrocyte brain-derived endothelial cell co-cultures, primary cultures of human brain-derived-endothelial cells were seeded on the top of the transwell (gelatin-coated 3-μm pore size Boyden chambers), and primary cultures of human foetal astrocytes were seeded in the bottom chambers, and allowed to grow to confluence in complete media for 3 days. Culture media was changed for fresh media without serum, and after 2 days samples were collected from top and bottom chambers and netrin 1 levels were assessed by ELISA.
Cytokine/chemokine detection
To assess whether netrin 1 has anti-inflammatory effects on human brain-derived endothelial cells, the levels of IL-6, CSF2 (previously known as GM-CSF), IL-8, CCL2 (previously known as MCP-1), and CLCX10 (previously known as IP-10) were measured in conditioned supernatants from human brain-derived endothelial cells with BD Biosciences BD OptEIA™ ELISA kits, following the manufacturer's protocols. To obtain supernatants, we treated confluent human brain-derived endothelial cells for 24 h with fresh media, supplemented with either recombinant netrin 1 (100 ng/ml R&D Systems), with TNF and IFNγ (each at 100 U/ml, GIBCO), or with a mix of TNF/IFNγ plus netrin 1.
Experimental autoimmune encephalomyelitis
EAE was induced by subcutaneous injections of 200 μg MOG35-55 (Sheldon Biotechnology Centre) in a total of 100 μl emulsion of complete Freund's adjuvant (supplemented with 2 mg/ml of Mycobacterium tuberculosis; Fisher Scientific) per mouse (C57BL/6) on Day 0. Pertussis toxin (300 ng/mouse, diluted in sterile PBS; Sigma Aldrich) was injected intraperitoneally on Days 0 and 2. For netrin 1 treatment regimens we injected mice intraperitoneally with either 1 μg/ 200 μl of recombinant mouse netrin 1 (R&D Systems, diluted in sterile PBS), or 200 μl of PBS alone every other day starting 2 days before EAE induction and up to Day 12 (prophylactic) or from Day 10 (every other day) to Day 22 (therapeutic) (Alvarez et al., 2011b; Larochelle et al., 2012). For histological analysis of the CNS, we collected the right brain hemisphere and halves of cervical, thoracic and lumbar spinal cords. CNS tissues were embedded in O.C.T. compound, frozen, and cryosectioned into 7-μm thin sections for further staining procedures. To evaluate the degree of leucocyte infiltration and demyelination, we stained CNS sections with Luxol Fast blue and haematoxylin and eosin as described (Alvarez et al., 2011b) and microscopically analysed sections with the Open Lab software.
Flow cytometry analyses
Flow cytometric analyses of in vitro cell culture
To evaluate netrin surface expression by human brain-derived-endothelial cells, cells were scraped off with cold 2 mM EDTA in PBS. Cells were incubated with primary antibodies (1:100 rat anti-netrin 1 R&D Systems or rat IgG2a isotype control, diluted in 0.1% FBS in PBS), washed and incubated with secondary antibodies, and washed again before analysis. To test cell adhesion molecule expression, human brain-derived endothelial cells were grown to full confluency, washed, and treated with netrin 1 (100 ng/ml) for 24 h, concomitantly with or without 0.1 U/ml of TNF and IFNγ. Cells were scraped off and incubated with the following antibodies: anti-human ICAM1 (CD54)-FITC, anti-human ALCAM (CD166)-PE, anti-human MCAM (CD146)-APC, anti-human HLA class I-PE (BD Biosciences) or isotype controls.
Flow cytometry analyses of ex vivo murine leukocytes
Mouse immune cells were isolated from the CNS compartment (left brain hemisphere and lumbar spinal cords), draining lymph nodes, and spleen. We then recorded total cell counts and treated cells with 20 ng/ml PMA, 1 μg/ml ionomycin and 2 μg/ml brefeldin A overnight. Cells were then stained with fluorochrome-conjugated antibodies against CD45, CD44, CD3, CD4, CD8 and CD11b (BD Biosciences). For intracellular stainings, we fixed and permeabilized with 1% formaldehyde, supplemented with 0.1% saponin. Cells were then stained with antibodies against IL-17, IFNγ, IL-10, IL-6 and CSF2 (GM-CSF; BD Biosciences) washed and analysed with the BD LSRII flow cytometer.
Gating
In the CNS, infiltrated leucocytes were distinguished from brain resident microglia by gating on CD45-highly fluorescent cells. Microglia are CD11b positive and CD45-low positive gate. For both spleen and lymph nodes, we used the same CD45-high gating to identify leucocytes. IL-17, IFNγ, IL-10, IL-6 and CSF2 (GM-CSF) populations were gated on CD45-high, CD3+ cells. Data acquisition was performed using a BD LSR II and analysis was performed with FACS DIVA and FlowJo Software. For quantitative analysis, the geometric mean of fluorescence intensity was compared to the isotype control.
Statistical analysis
We repeated each experimental condition with at least three different sample preparations and analysed data with GraphPad Prism software. Values are represented as mean and standard error of the mean (SEM). Values were considered statistically significant when P-values were *P ≤ 0.05, **P ≤ 0.01, or ***P ≤ 0.001. To compare endothelial cell responses with and without netrin 1 treatment by western blot, quantitative PCR, and ELISA analysis we used a paired Student t-test. For determination of netrins in vitro effect on blood–brain barrier permeability, we used a one-way ANOVA. To compare netrin 1 knockout mice with wild-types, or netrin 1 with PBS-treated EAE mice for protein extravasation or immune cell accumulation, we used an unpaired t-test and ANOVA statistical analysis.
Results
Netrin 1 expression is controlled by SHH and regulated by inflammation
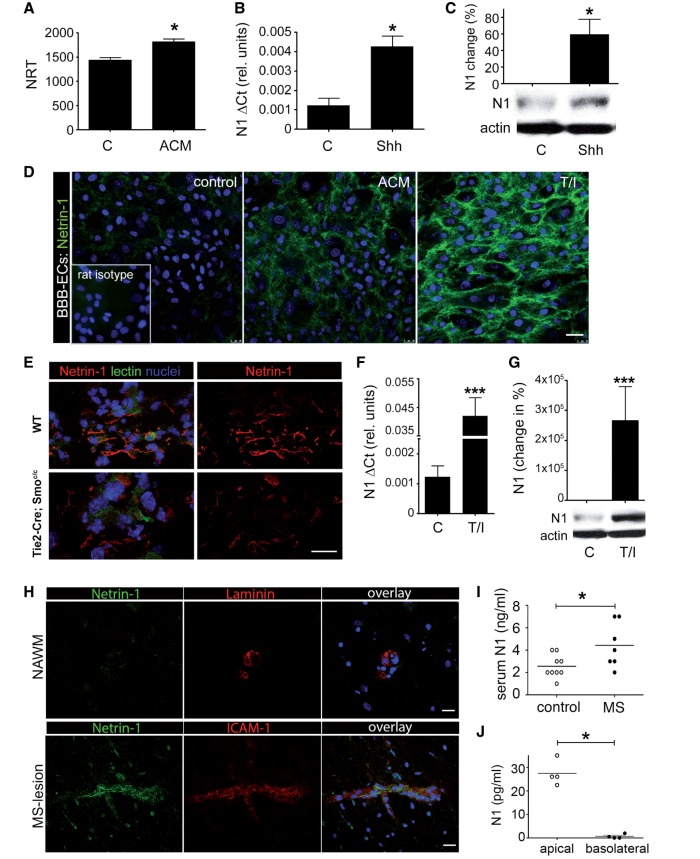
Using primary cultures of human blood–brain barrier endothelial cells combined with RNA sequencing technologies (Fig. 1A), we identified netrin 1 as a downstream effector of the hedgehog influence on the blood–brain barrier, suggesting an autocrine and glial cell autonomous influence of netrin 1 on blood–brain barrier functions. To confirm our quantitative RNA sequencing data, we first evaluated which netrin and netrin receptors were expressed in cultures of human brain-derived endothelial cells. We found moderate to low levels of netrin 1 expression in resting (non-treated) human brain-derived-endothelial cells (Fig. 1A–D), moderate levels of netrin 4 (Supplementary Fig. 1A and B) and high levels of the netrin receptor neogenin (Supplementary Fig. 1C and D). The netrin receptors UNC5B and UNC5C were detected at low levels (Supplementary Fig. 1E–H), whereas netrin 3, DCC, UNC5A and UNC5D were undetectable [Supplementary Fig. 1(i)]. Human brain-derived endothelial cells significantly upregulated netrin 1 expression upon treatment with SHH or astrocyte factors (Fig. 1A–D). In vivo, murine microvascular CNS blood vessels express netrin 1 (Fig. 1E) and genetic deletion of the SHH receptor SMO on endothelial cells (Tie2-Cre: Smoc/c) decreased netrin 1 expression on both meningeal and parenchymal microvessels. Collectively, our data demonstrate that netrin 1 expression on human brain-derived endothelial cells is regulated by SHH, at least in part through its interaction with SMO.
Figure 1.
Netrin 1 expression at the blood–brain barrier. Quantitative RNA sequencing analysis (A), quantitative PCR (B) and western blot (C) analyses of netrin 1 (N1) expression by primary cultures of human brain-derived endothelial cells. Endothelial cells were either untreated (control, C), or grown with either 20% (v/v) astrocyte conditioned media (ACM) or with human recombinant SHH (hrShh) (0.1 μg/ml). Netrin 1 mRNA levels are presented as the number of reads per transcript (NRT) or as the difference in cycle threshold (ΔCt) relative to actin. Netrin 1 protein levels are represented as percentile change of netrin expression compared to control brain-derived endothelial cells, normalized to 0. Representative western blots for netrin 1 are shown. All values are normalized to actin. In vitro immunocytofluorescent stainings for netrin 1 (D) (in green), on untreated (control), astrocyte conditioned media, or TNF/IFNγ (T/I) treated primary cultures of human brain-derived endothelial cells (nuclei in blue). Isotope control antibodies are shown in inserts. Netrin 1 (red) and tomato lectin (green) immunostainings (E) of CNS sections from E14 WT and smo endothelial deficient (Tie2-Cre;Smoc/c) mouse embryos. Quantitative PCR (F) and western blot (G) analyses of netrin 1 expression by primary cultures of human blood–brain barrier-endothelial cells either resting (C) or treated with T/I. In situ immunostainings for netrin 1 (H) in normal-appearing white matter (NAWM top row) and active lesions of patients with multiple sclerosis (bottom row). Co-staining for vessel marker laminin and ICAM1 (red) and nuclei (TOPRO, blue) are shown. Netrin 1 was measured by ELISA (I) in serum collected from patients with multiple sclerosis (n = 7), and healthy donors (n = 9) or in the top and bottom chamber (J) of transwells in which human astrocytes and human brain-derived endothelial cells were co-cultured. For all panels, *P ≤ 0.05; ***P ≤ 0.001. Data shown are the mean ± SEM of n = 3–5 experiments. Immunostaining panels shown are representative of 9–15 CNS sections from three patients or animals. Scale bar = 10 μm.
We next assessed the influence of inflammation on netrin 1 expression by blood–brain barrier endothelial cells. Primary cultures of human blood–brain barrier endothelial cells activated with the pro-inflammatory cytokines TNF and IFNγ significantly upregulated netrin 1 mRNA (Fig. 1F) and protein (Fig. 1D and G). Using post-mortem brain sections of individuals affected with multiple sclerosis, a demyelinating disease characterized by focal inflammation and disruption of the blood–brain barrier (Alvarez et al., 2011a; Gaitan et al., 2011) we found that blood vessels in non-inflamed normal-appearing white matter express low levels of netrin 1, whereas those associated with perivascular lesions displayed increased netrin 1 immuno-reactivity (Fig. 1H and Supplementary Fig. 2A). A similar pattern of expression was observed in CNS sections of mice affected by EAE (Supplementary Fig. 2B and C). Although netrin 1 expression was seen at the blood–brain barrier in human and mouse CNS sections, we also detected netrin 1 expression by astrocytes (Supplementary Fig. 2A) and within inflammatory infiltrates. To determine the in vivo relevance of our findings, we measured netrin 1 levels in serum from patients with multiple sclerosis compared with healthy donors. Netrin 1 was significantly increased by ∼2-fold in serum of patients with multiple sclerosis, as compared to controls (Fig. 1I). Furthermore, we found that primary co-cultures of human brain-derived endothelial cells with astrocytes allowed for the directional release of netrin 1 from the apical (luminal) side of human brain-derived endothelial cells (Fig. 1J). We therefore conclude that the vascular expression of netrin 1 in the CNS is minimal during homeostasis and increases significantly with neuroinflammation.
Netrins reduce diffusion across the blood–brain barrier in vitro and in vivo
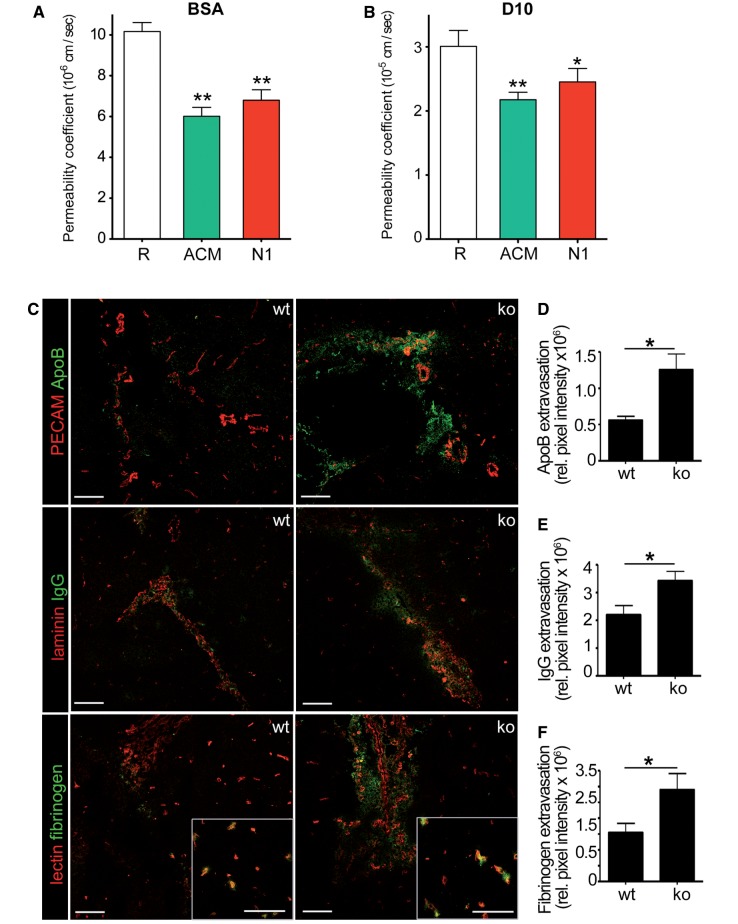
The supporting role of netrin 1 on barrier function was determined by measuring the permeability of human brain-derived endothelial cells to two fluorescently labelled tracer molecules of different size (Fig. 2A and B). We found that netrin 1 significantly reduced the diffusion of bovine serum albumin (BSA) and dextran 10 kDa across human brain-derived endothelial cells, and that netrin 1 did not induced human brain-derived endothelial cell proliferation (Supplementary Fig. 3A). To determine whether netrin 1 affects blood–brain barrier function in vivo, we used netrin 1 knockout newborn mice (Supplementary Fig. 3B–F) (Serafini et al., 1996), which die within 24 h after birth. In these animals, we did not detect differences in the number of CNS vessels (Supplementary Fig. 3E and F). However, endogenous serum proteins fibrinogen, IgG, and APOB significantly accumulated in the brains of netrin 1 knockout mice (Fig. 2C–F). Therefore, we conclude that absence of endogenous netrin 1 increased the extravasation of plasma proteins into the brain parenchyma, confirming the human in vitro data demonstrating that netrin 1 contributes to blood–brain barrier integrity.
Figure 2.
Netrin 1 enhances endothelial barrier properties in vitro and in vivo. Permeability coefficient of bovine serum albumin (BSA) (A) and dextran (D10) (B) across primary cultures of human brain-derived endothelial cells, either untreated/resting (R), or treated with astrocyte conditioned media, or recombinant netrin 1 (N1). In situ immunostainings (C) for blood-derived plasma proteins apolipoprotein B (ApoB), Immunoglobulin G (IgG), and fibrinogen, in netrin 1 knockout mice (ko) compared to wild-type mice (wt). Inserts represent magnifications of fibrinogen (green) extravasation in microvessels from wild-type and knockout animals, respectively. Immunostainings for PECAM, tomato lectin and laminin serve to identify vessels. Data shown are the mean ± SEM from n ≥ 5 independent experiments and of n = 12 sections from four animals per group. Plasma protein extravasation was quantified (D–F) by multiplying the mean pixel fluorescent intensity of serum protein and the area of leakage. Scale bars = 20 μm. (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
Netrin 1 regulates the expression of junctional proteins at the blood–brain barrier
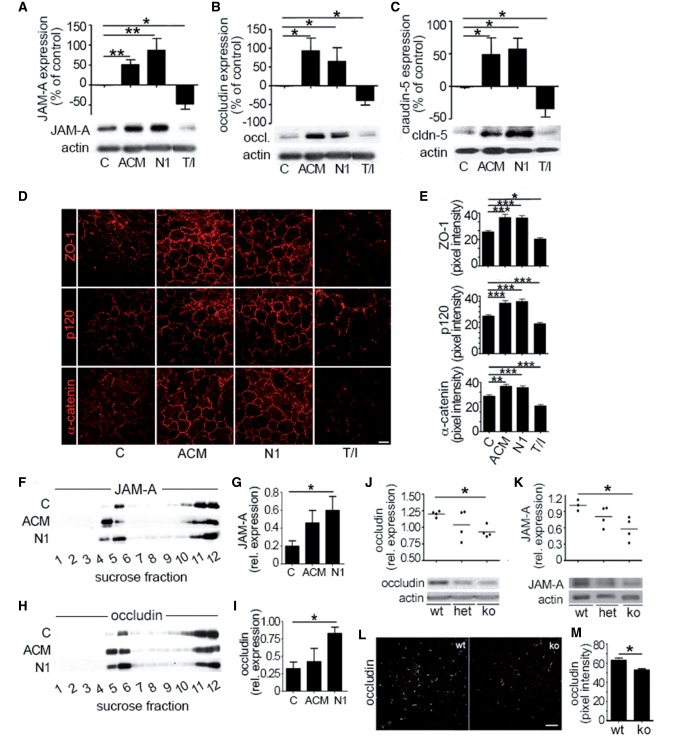
We then investigated whether the reduced barrier permeability in the presence of netrin 1 resulted from a change in junctional protein expression. Human brain-derived endothelial cells treated with netrin 1 upregulated tight junction proteins JAM-A (now known as F11R), occludin and claudin 5 (Fig. 3A–C) and increased ZO-1 (now known as TJP1), p120 (CTNND1), and alpha catenin expression at interendothelial junctions (Fig. 3D and E) as compared to untreated cells. This effect was similar to treatment with astrocyte conditioned media (positive control). We also found that netrin 1 upregulated the expression of claudin 5 in primary cultures of mouse brain endothelial cells (Supplementary Fig. 4A). In contrast, human brain-derived-endothelial cells stimulated with TNF and IFNγ, exhibited decreased and fragmented tight junction protein expression (Fig 3A–E). Barrier promoting factors are known to recruit tight junction proteins into cholesterol-enriched lipid raft membrane microdomains of brain endothelial cells (Wosik et al., 2007; Dodelet-Devillers et al., 2009) characterized by high expression of cholesterol, GM1 ganglioside and CD59 (Supplementary Fig. 4A–C). We found that netrin 1 promoted the recruitment of JAM-A (Fig. 3F and G) and occludin to human brain-derived endothelial cell lipid raft membrane microdomains (Fig. 3H and I) confirming the ability of netrin 1 to induce both the expression and the proper localization of tight junction proteins into functional junctional complexes. Furthermore, in netrin 1 knockout animals occludin and JAM-A were less abundant in brain homogenates, when compared to those of wild-type littermates (Fig. 3J–M). Taken together, our data demonstrate that netrin 1 is required for proper expression of junctional proteins and to promote optimal blood–brain barrier function.
Figure 3.
Netrin 1 regulates junctional protein levels in vitro and in vivo. Expression of junctional proteins JAM-A (A), occludin (B), and claudin-5 (C) in primary cultures of human brain-derived endothelial cells untreated (control, C) or treated with astrocyte conditioned media, netrin I (N1) or T/I, by western blots. In vitro immunocytofluorescent stainings (D) of junctional proteins ZO-1, p120, and α-catenin in untreated (C), or in astrocyte conditioned media (ACM), netrin 1 or T/I treated brain-derived endothelial cells. Quantification of fluorescent pixel intensity of D is shown in E. Effect of netrin 1 on tight junction proteins JAM-A (F and G) and occludin (H and I) and accumulation into lipid raft membrane microdomains. Human brain-derived endothelial cells were treated as in A–C and cell membrane fractions were separated by sucrose density centrifugation. Lipid raft fractions (4 and 5) were isolated and western blot analysis of JAM-A (F and G) and occludin (H and I) was performed. Expression and quantification of tight junction proteins occludin (J), and JAM-A (K) in brain homogenates from netrin 1 knockout (ko) mice compared to heterozygote (het) and wild-type (wt) mice, by western blot. In situ immunostainings of occludin (L) in brains of netrin 1 knockout (ko) mice compared to wild-type mice. Quantification of fluorescence intensity of occludin expression is shown in (M). All data shown are the mean ± SEM from n ≥ 3–5 independent experiments, from three to five human brain-derived endothelial cell donors and/or ≥ 5 animals. Scale bars = 10 μm (D), 20 μm (L). (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
Netrin 1 has anti-inflammatory effects at the blood–brain barrier
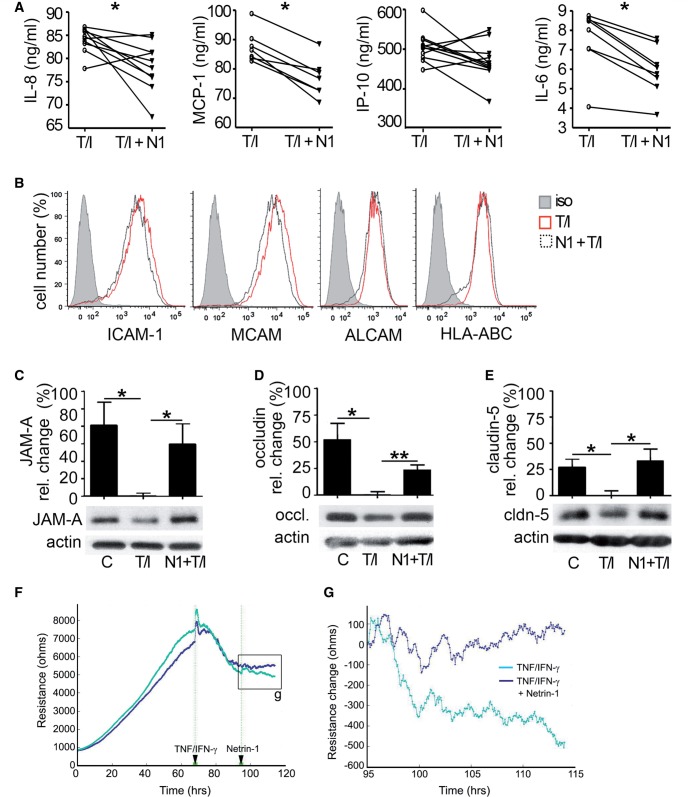
Recent reports provided evidence that netrin 1 has anti-inflammatory properties in the lungs (Ly et al., 2005; Rosenberger et al., 2009; van Gils et al., 2012) and therefore we elected to evaluate whether netrin 1 could counteract pro-inflammatory cytokine activation of human brain-derived endothelial cells. First, we found that addition of netrin 1 to resting brain-derived endothelial cells did not affect the secretion of MCP-1/CCL2 or IL-8/CXCL8 (data not shown). However, on TNF/IFNγ activated human brain-derived endothelial cells netrin 1 significantly reduced the secretion of IL8/CXCL8, MCP-1/CCL2 and IL-6 (Fig. 4A), but not the expression of IP-10 (CXCL10) nor the adhesion molecules ICAM1, MCAM and ALCAM (Fig. 4B). To then assess the potential of netrin 1 to counteract pro-inflammatory cytokine-induced loss of tight junction proteins at the blood–brain barrier, we applied netrin 1 concomitantly with TNF and IFNγ to human brain-derived endothelial cells. Netrin 1 prevented the downregulation of the tight junction molecules JAM-A, occludin and claudin 5, which were otherwise significantly downregulated when pro-inflammatory cytokines were added alone (Fig. 4C–E). We also measured the effect of inflammation and netrin 1 treatment using primary cultures of mouse brain endothelial cells and found that netrin 1 promoted barrier function by counteracting the reduction in impedance induced by TNF and IFNγ on these cells (Fig 4F and G). Collectively, our data demonstrate that netrin 1 provides an anti-inflammatory influence on the blood–brain barrier by decreasing chemokine secretion by CNS endothelial cells and by protecting against cytokine-induced barrier disruption.
Figure 4.
Netrin 1 counteracts the detrimental outcome induced by inflammation on the human and murine blood–brain barrier. Production of IL8, MCP-1, IP-10, and IL6 by primary cultures of human brain-derived endothelial cells activated with 100 U/ml of TNF/IFNγ (T/I) alone or in combination with netrin 1 (N1) was measured by ELISA (A). Expression of cell adhesion molecules (ICAM1, MCAM and ALCAM) by human brain-derived endothelial cells was also determined under the same conditions using flow cytometry (B). The expression of JAM-A (C), occludin (D) and claudin-5 (E) by human brain-derived endothelial cells was measured by western blot under the same conditions. (F) Impedance readout at 1000 Hz from primary cultures of mouse brain endothelial cells exposed to IFNγ and TNF when confluent. Netrin 1 treatment was applied 30 h post-inflammation. (G) High power view of the impedance change induced by netrin 1 treatment on inflamed mouse brain endothelial cells. Data (C–E) represent the mean percentile change ± SEM of expression normalized to actin from n = 5 independent experiments. Individual points (A) represent absolute concentrations of cytokines for n = 3–10 independent experiments. (*P ≤ 0.05; **P ≤ 0.01).
Early netrin 1 therapy reduces EAE severity and limits early immune cell infiltration
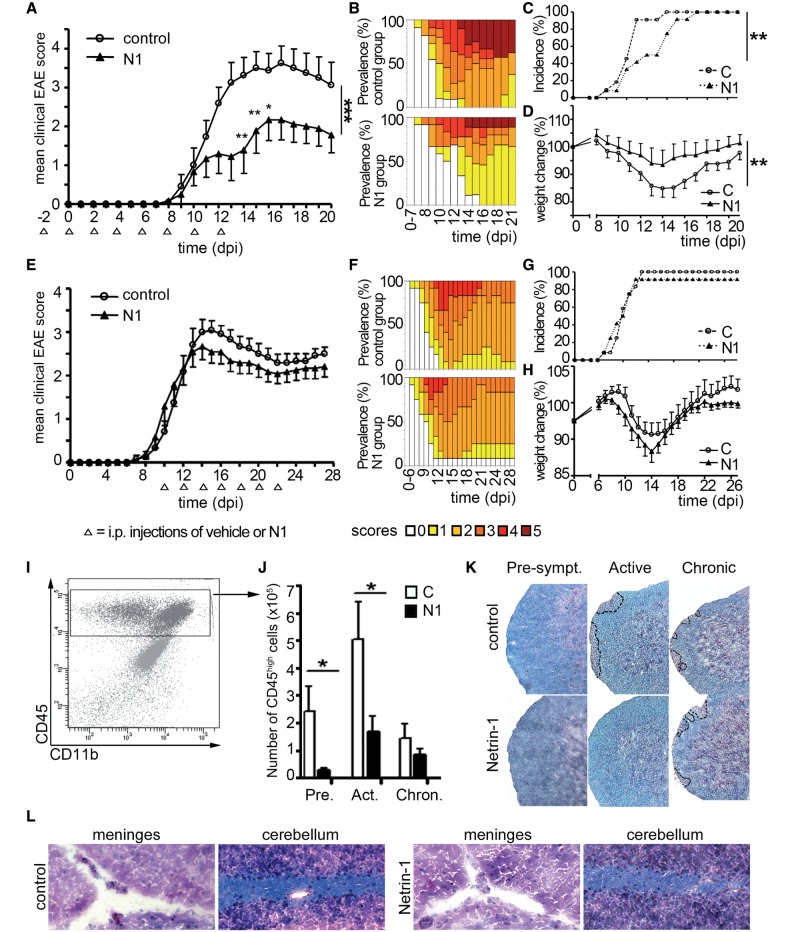
Blood–brain barrier breakdown and immune cell accumulation in the CNS are hallmarks of multiple sclerosis and EAE. To evaluate whether netrin 1 therapy regulates blood–brain barrier dysfunction, immune cell invasion and disease severity, we injected MOG35-55-induced-EAE mice with recombinant mouse netrin 1 using a prophylactic treatment strategy. Animals treated with netrin 1 consistently exhibited less severe disease scores (Fig. 5A), lower prevalence of severe scores (Fig. 5B), delayed disease onset (Fig. 5C), and retained more body weight throughout the disease course (Fig. 5D). However, netrin 1 injections at the onset of symptoms provided no significant clinical improvement (Fig. 5E–H). At the pathological level, the prophylactic regimen significantly decreased the number of CD45high leucocytes infiltrating the CNS (Fig. 5I, J and Supplementary Fig. 5A). Netrin 1-treated mice also had fewer and smaller inflammatory lesions compared to vehicle-treated mice during the presymptomatic and active stages in the spinal cord (Fig. 5K), and to a lesser extent in the brain (Fig. 5L). Netrin 1 did not reduce the number of microglia, when compared to vehicle treatment (Supplementary Fig. 5A), but induced a significant reduction in the number of infiltrating CD11b+ myeloid cells (Supplementary Fig. 5B) and CD4+ T lymphocytes (Supplementary Fig. 5C) 20 days post-induction. In the CNS from mice treated with netrin 1, we also detected a smaller proportion of T cells secreting IFNγ (Supplementary Fig. 6A), and IL-17 (Supplementary Fig. 6B), but not IL-10 (Supplementary Fig. 6C) when compared to vehicle-treated animals. Interestingly, netrin 1 treatment seemed to increase the proportion of IL-10-secreting T lymphocytes in secondary lymphoid tissues (Supplementary Fig. 6F and I). Taken together, our results demonstrate that prophylactic administration of netrin 1 decreases clinical and pathological severity of auto-antigen-induced CNS autoimmune inflammation.
Figure 5.
Netrin 1 reduces severity of EAE. Mean cumulative clinical scores (A and E), score prevalence per treatment group (B and F), disease incidence (C and G) and weight change (D and H) were recorded from MOG35-55-immunized C57BL/6 mice injected intraperitoneally with vehicle control (C) or recombinant netrin 1 (N1). Treatment (open arrowheads) was initiated either before disease induction (A–D) or at the onset of symptoms (E–H). Data shown are representative of n = 3 independent experiments for the prophylactic regimen (A–D; n = 44 animals per group) and of n = 2 independent experiments for the therapeutic regimen (E–H, n = 24 animals per group). CNS infiltrating leucocytes (CD45high population (I) were quantified by flow cytometry (J) in the preclinical (Pre; Day 8), active (Act; Day 13) and chronic (Chron; Day 20 post-induction) phase of EAE, in controls animals and in animals treated prophylactically with netrin 1. Data shown are the mean ± SEM of n = 12 animals per group (selected to be representative of the mean score of the group, at sacrifice), from three independent experiments. Luxol Fast blue/haematoxylin and eosin (LHE) stainings (K) of spinal cord sections of control and netrin 1-treated animals in the preclinical (Pre), active (Act) and chronic (Chron) phase of EAE. Areas of immune cell infiltration are shown with dotted lines. LHE staining of brain sections (L) from control (left) and netrin 1-treated (right) animals in the active phase of EAE. Data shown are representative of n = 12 sections, from four animals per group, and from n = 3 independent experiments. (*P ≤ 0.05; **P ≤ 0.01).
Netrin 1 reduces blood–brain barrier breakdown under inflammatory conditions
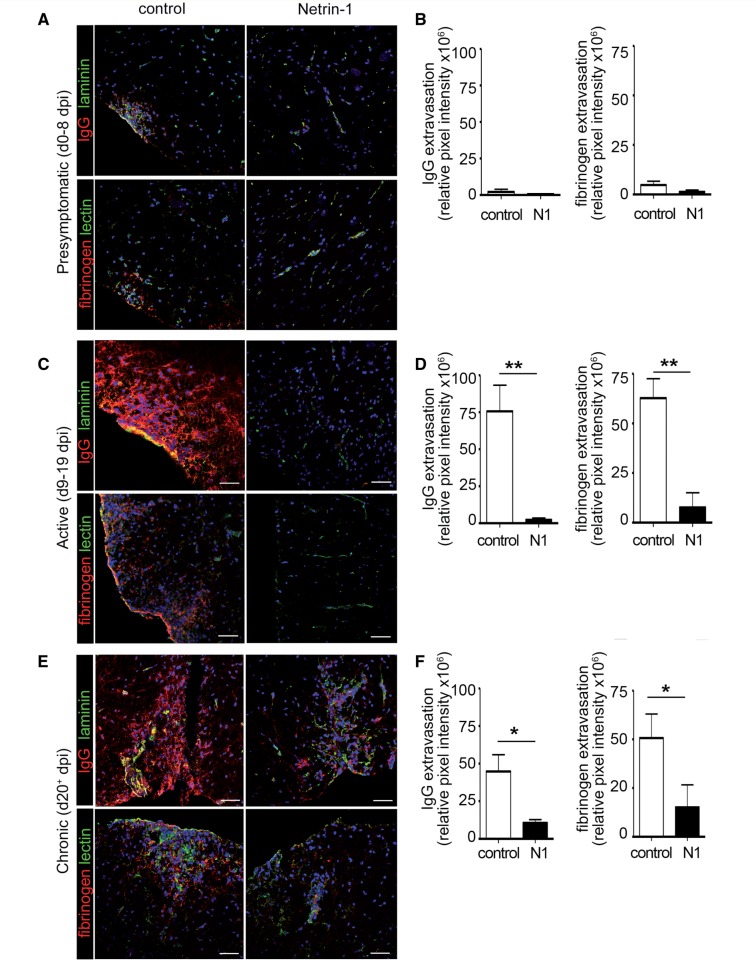
To evaluate the effect of prophylactic administration of netrin 1 on the blood–brain barrier, we determined serum protein extravasation into the CNS at distinct EAE stages. During the presymptomatic phase netrin 1-treated mice showed minimal fibrinogen and IgG extravasation into the CNS, as compared to controls (Fig. 6A and B). In contrast, the extent of plasma protein extravasation increased significantly in control mice during the active stage of the disease and was barely detectable in netrin 1-treated mice (Fig. 6C and D). This pattern was maintained during the chronic stage (Fig. 6E and F). Collectively, our data demonstrate that prophylactic administration of netrin 1 promotes blood–brain barrier integrity and reduces neuroinflammation.
Figure 6.
Netrin 1 reduces plasma protein leakage across the blood–brain barrier during EAE. In situ immunostainings of immunoglobulin G (red, IgG) and fibrinogen (red) in spinal cord sections of control or netrin 1 treated EAE mice, during presymptomatic (Day 8 post-induction) (A), active (Day 13 post-induction) (C), and chronic (Day 20 post-induction) (E) disease stages. Laminin and tomato lectin were used as counter stainings for vessels. Quantitative analysis of IgG and fibrinogen extravasation in control or netrin 1-treated EAE animals during presymptomatic (B), active (D) and chronic (F) stages of disease. For B, D and F, data shown are the mean of n = 6 animals. Scale bars = 20 μm. (*P ≤ 0.05; **P ≤ 0.01).
Discussion
Astrocyte-derived factors influence the phenotype of the CNS vasculature. We recently showed that the barrier phenotype and immunoquiescence of the blood–brain barrier is influenced by astrocyte-secreted SHH (Alvarez et al., 2011b). Herein, we report that under homeostatic conditions SHH induces netrin 1 expression by the CNS endothelium to support blood–brain barrier function. However, inflammatory conditions highly upregulate netrin 1 expression on CNS endothelium, a phenomenon that stabilizes the blood–brain barrier, limits immune cell infiltration and improves symptoms during the clinical course of EAE. The barrier tightening induced by netrin 1 was not caused by increased endothelial cell proliferation, even though netrins support angiogenesis (Wilson et al., 2006; Nacht et al., 2009; Larrieu-Lahargue et al., 2010). In our study, barrier strengthening occurred, at least in part, as a consequence of netrin-induced tight junction and adherens junction molecule upregulation. Levels of both transmembrane and intracellular components of the junctional complex increased in response to netrin 1. In addition, treatment of human brain-derived endothelial cells with netrin 1 enriched junctional proteins in lipid raft membrane microdomains, where proteins effectively interact to form functional clusters that support barrier integrity (Dodelet-Devillers et al., 2009). In vivo, genetically-induced loss of function of netrin 1 altered tight junction protein levels and caused blood–brain barrier disruption, allowing serum proteins to extravasate into the brain parenchyma.
Rosenberger et al. (2009) also showed barrier tightening in an endothelial cell line in response to recombinant netrin 1 protein and hypoxic preconditioning. Yet, the effect of netrin 1 on blood–brain barrier stability remained unknown. In our study, netrin 1 prevented the loss of tight junction proteins when human brain-derived endothelial cells were simultaneously challenged with pro-inflammatory cytokines and it decreased the secretion of pro-inflammatory cytokines and chemokines by human brain-derived endothelial cells. Netrin 1 is known to reduce the levels of pro-inflammatory mediators in the blood (Mutz et al., 2010), the peritoneal cavity (Mirakaj et al., 2011), bronchio-alveolar fluids (Rosenberger et al., 2009) and in the kidney (Tadagavadi et al., 2010). When we injected netrin 1 during the presymptomatic phase of EAE, we also observed milder disease severity and less CNS tissue destruction. Netrin 1 stabilized the blood–brain barrier and limited immune cells and serum proteins from entering the CNS. Although the effect of netrin 1 on the development of EAE could be in part due to blood–brain barrier stabilization, increasing evidence indicates that the initial infiltration of leucocytes across the blood–brain barrier is unlikely to depend on alteration of the tight junction (Pfeiffer et al., 2011). Furthermore, several reports indicate that netrin 1 directly acts on leucocytes to prevent their migration in various tissues (Ly et al., 2005; Ranganathan et al., 2013). It is therefore likely that netrin 1 acts both at the blood–brain barrier and on immune cells to prevent their infiltration in brain during EAE. This would be in agreement with the prophylactic efficacy reported herein and with the lack of therapeutic effect of netrin 1 treatment. Collectively, these observations are very supportive of the strong anti-inflammatory effect of netrin 1 on autoimmune CNS diseases, whether locally at the blood–brain barrier, or more systemically on the peripheral immune system.
Our study demonstrates that netrin 1 is a novel intrinsic regulator of blood–brain barrier function that is upregulated upon inflammation in multiple sclerosis and EAE. Netrin 1 protects and supports blood–brain barrier function during EAE, particularly when given as an early therapeutic treatment. Thus, netrin 1 represents a potential therapeutic strategy to neurological conditions in which blood–brain barrier disruption and inflammation are pathological hallmarks.
Supplementary Material
Glossary
Abbreviations
- EAE
experimental autoimmune encephalitis
- IFNγ
interferon gamma
Funding
This study was supported by operating grants from the Canadian Institutes of Health Research (CIHR; MOP81880) and the Multiple Sclerosis Society of Canada (MSSC) to A.P. and T.E.K., C.P. holds a Banting and Best scholarship from the CIHR. M.A.L, C.L. and O.S.L. hold scholarships and fellowships from the MSSC. J.I.A. holds The David L Torrey career developmental award from the MSSC. N.A. holds a New Investigator Award from the CIHR. A.P. is a Senior Scholar of the Fonds de Recherche du Québec-Santé. T.E.K. holds a Chercheur National award from the Fonds de Recherche du Québec-santé and a Killam Foundation Scholar award.
Supplementary material
Supplementary material is available at Brain online.
References
- Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25:5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman R, Rutledge JC. The vascular contribution to Alzheimer's disease. Clin Sci. 2010;119:407–21. doi: 10.1042/CS20100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Bio Biophys Acta. 2011a;1812:252–64. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011b;334:1727–31. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–45. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- Dodelet-Devillers A, Cayrol R, van Horssen J, Haqqani AS, de Vries HE, Engelhardt B, et al. Functions of lipid raft membrane microdomains at the blood-brain barrier. J Mol Med. 2009;87:765–74. doi: 10.1007/s00109-009-0488-6. [DOI] [PubMed] [Google Scholar]
- Durrani S, Haider K, Ahmed RP, Jinag S, Ashraf M. Cytoprotective and proangiogenic activity of ex-vivo netrin-1 transgene overexpression protects the heart against ischemia/reperfusion injury. Stem Cells Dev. 2012;21:1769–78. doi: 10.1089/scd.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitan MI, Shea CD, Evangelou IE, Stone RD, Fenton KM, Bielekova B, et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann Neurol. 2011;70:22–9. doi: 10.1002/ana.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenz A, Dalton JH, Bauerle JD, Badulak A, Ridyard D, Gandjeva A, et al. Partial netrin-1 deficiency aggravates acute kidney injury. PLoS One. 2011;6:e14812. doi: 10.1371/journal.pone.0014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang S, Liauw J, Choi M, Guzman RG, Steinberg GK. Netrin-4 enhances angiogenesis and neurologic outcome after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:385–97. doi: 10.1038/jcbfm.2008.128. [DOI] [PubMed] [Google Scholar]
- Jarjour AA, Bull SJ, Almasieh M, Rajasekharan S, Baker KA, Mui J, et al. Maintenance of axo-oligodendroglial paranodal junctions requires DCC and netrin-1. J Neurosci. 2008;28:11003–14. doi: 10.1523/JNEUROSCI.3285-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Yi MJ, Zhang W, Feinleib JL, Cole F, Krauss RS. Netrins and neogenin promote myotube formation. J Cell Biol. 2004;167:493–504. doi: 10.1083/jcb.200405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–5. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle C, Cayrol R, Kebir H, Alvarez JI, Lecuyer MA, Ifergan I, et al. Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain. 2012;135(Pt 10):2906–24. doi: 10.1093/brain/aws212. [DOI] [PubMed] [Google Scholar]
- Larrieu-Lahargue F, Welm AL, Thomas KR, Li DY. Netrin-4 induces lymphangiogenesis in vivo. Blood. 2010;115:5418–26. doi: 10.1182/blood-2009-11-252338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejmi E, Leconte L, Pedron-Mazoyer S, Ropert S, Raoul W, Lavalette S, et al. Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc Natl Acad Sci USA. 2008;105:12491–6. doi: 10.1073/pnas.0804008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly NP, Komatsuzaki K, Fraser IP, Tseng AA, Prodhan P, Moore KJ, et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102:14729–34. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S, Ubogu EE, Ransohoff RM. Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol. 2007;17:243–50. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirakaj V, Gatidou D, Potzsch C, Konig K, Rosenberger P. Netrin-1 signaling dampens inflammatory peritonitis. J Immunol. 2011;186:549–55. doi: 10.4049/jimmunol.1002671. [DOI] [PubMed] [Google Scholar]
- Moore SW, Biais N, Sheetz MP. Traction on immobilized netrin-1 is sufficient to reorient axons. Science. 2009;325:166. doi: 10.1126/science.1173851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SW, Correia JP, Lai Wing Sun K, Pool M, Fournier AE, Kennedy TE. Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1. Development. 2008;135:2855–64. doi: 10.1242/dev.024133. [DOI] [PubMed] [Google Scholar]
- Mutz C, Mirakaj V, Vagts DA, Westermann P, Waibler K, Konig K, et al. The neuronal guidance protein netrin-1 reduces alveolar inflammation in a porcine model of acute lung injury. Crit Care. 2010;14:R189. doi: 10.1186/cc9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacht M, St Martin TB, Byrne A, Klinger KW, Teicher BA, Madden SL, et al. Netrin-4 regulates angiogenic responses and tumor cell growth. Exp Cell Res. 2009;315:784–94. doi: 10.1016/j.yexcr.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Navankasattusas S, Whitehead KJ, Suli A, Sorensen LK, Lim AH, Zhao J, et al. The netrin receptor UNC5B promotes angiogenesis in specific vascular beds. Development. 2008;135:659–67. doi: 10.1242/dev.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, et al. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci USA. 2004;101:16210–5. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer F, Schafer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, et al. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122:601–14. doi: 10.1007/s00401-011-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan PV, Jayakumar C, Mohamed R, Dong Z, Ramesh G. Netrin-1 regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2-mediated PGE2 production. Kidney Int. 2013;83:1087–98. doi: 10.1038/ki.2012.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–14. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Shekarabi M, Moore SW, Tritsch NX, Morris SJ, Bouchard JF, Kennedy TE. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J Neurosci. 2005;25:3132–41. doi: 10.1523/JNEUROSCI.1920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siflinger-Birnboim A, Del Vecchio PJ, Cooper JA, Blumenstock FA, Shepard JM, Malik AB. Molecular sieving characteristics of the cultured endothelial monolayer. J Cell Physiol. 1987;132:111–7. doi: 10.1002/jcp.1041320115. [DOI] [PubMed] [Google Scholar]
- Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol. 2001;153:933–46. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell. 2003;4:371–82. doi: 10.1016/s1534-5807(03)00054-6. [DOI] [PubMed] [Google Scholar]
- Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol. 2010;185:3750–8. doi: 10.4049/jimmunol.1000435. [DOI] [PubMed] [Google Scholar]
- Tepavcevic V, Kerninon C, Aigrot MS, Meppiel E, Mozafari S, Arnould-Laurent R, et al. Early netrin-1 expression impairs central nervous system remyelination. Ann Neurol. 2014;76:252–68. doi: 10.1002/ana.24201. [DOI] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–9. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–43. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, et al. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–4. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosik K, Cayrol R, Dodelet-Devillers A, Berthelet F, Bernard M, Moumdjian R, et al. Angiotensin II controls occludin function and is required for blood-brain barrier maintenance: relevance to multiple sclerosis. J Neurosci. 2007;27:9032–42. doi: 10.1523/JNEUROSCI.2088-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.