Abstract
Objectives:
This study aimed to assess the perforation rate of intraoral barriers for a direct digital sensor according to the barrier application.
Methods:
Four types of plastic barriers with different thicknesses and one type of latex finger cot were applied using six modified techniques. The perforations in barrier samples of six groups were examined by a water pressure test. The differences in the perforation rates among the six barrier applications were calculated.
Results:
The least perforation occurred in Group 4 (0.08-mm-thick single barrier, 22%) and the most in Group 1 (0.04-mm-thick single barrier, 58%). An ANOVA test revealed statistical differences in the perforation rate among the groups (p = 0.00; 95% confidence interval, 0.326–0.403).
Conclusions:
The use of double barriers can be helpful in reducing the perforation rate of intraoral barriers.
Keywords: infection control, dental, radiography
Introduction
Digital radiography has now become widely accepted in the field of dentistry and has led to many changes in oral and maxillofacial radiology. Digital radiography has several advantages over film radiography. To begin with, digital radiography has a wider dynamic range; therefore, the radiation exposure to patients can be reduced. Furthermore, the elimination of chemical processing and processing errors saves time and reduces radiation exposure. In addition, images can be stored permanently without any degradation of quality. Because of these benefits, digital radiography has replaced film radiography in dental practice. Unlike film-based imaging, intraoral digital sensors are used repeatedly and infection control has become an increasingly important issue.
According to the guidelines of the Center for Disease Control and Prevention (CDC), these digital radiographic sensors are classified as semicritical devices, because they have contact with mucous membranes.1 Ideally, these semicritical devices should be heat-sterilized or high-level disinfected between patients to prevent cross infection. However, currently, there is no intraoral digital sensor that can withstand heat sterilization or be completely immersed in a high-level disinfectant. Therefore, it is important to protect digital sensors by using barriers.
Limited literature is available on the effectiveness of an intraoral direct digital sensor barrier.2,3 Hokett et al2 reported a perforation rate of 44–51% when using a single plastic barrier under clinical conditions and recommended the use of a latex finger cot to significantly reduce the perforation rate. This high perforation rate of the plastic barrier brings the safety of the single-barrier technique into question. Furthermore, the perforation rate may vary by material type or barrier thickness, but previous reports have not addressed these issues. The aim of this study was to investigate the perforation rate of the intraoral plastic barriers for a direct digital sensor according to the modified barrier applications.
Methods and materials
Sample collection
Four types of low-density polyethylene (LDPE) barriers (intraoral cover; Joong-Ang Vinyl Package, Pusan, Republic of Korea) with different thicknesses and one type of latex finger cot (3908L; Grafco®, Atlanta, GA) were used on patients undergoing direct digital periapical radiography in the Department of Oral and Maxillofacial Radiology at Dankook University Dental Hospital, Cheonan, Chungnam, Republic of Korea. All periapical radiographs were taken by skilled technicians using a parallelling technique with the complementary metal-oxide semi-conductor sensors (EZ Sensor; Vatech, Hwaseong, Republic of Korea) and a positioning device (Rinn XCP; Dentsply, Philadelphia, PA). Children and disabled patients with weak occlusal forces were excluded from this study. Furthermore, cases in which barriers were used without a positioning device were excluded from this study. Based on the combinations of barriers, six modified techniques were applied to cover the sensor and positioning device (Table 1). All barriers were applied after combining the sensor with the positioning device to avoid perforation before placement in the patients' mouth (Figure 1). After the radiographic images were taken, all barrier samples were carefully removed and collected manually; forceps were not used to avoid perforation. This research (registration number H-1409/010/004) was authorized by the institutional review board at Dankook University Dental Hospital.
Table 1.
Application of barriers in each group
| Groups | Inner barrier material | Size (mm), width × length × thickness | Outer barrier material | Size (mm), width × length × thickness |
|---|---|---|---|---|
| Group 1 | LDPE | 90 × 170 × 0.04 | Not used | |
| Group 2 | LDPE | 90 × 170 × 0.05 | Not used | |
| Group 3 | LDPE | 90 × 170 × 0.06 | Not used | |
| Group 4 | LDPE | 90 × 170 × 0.08 | Not used | |
| Group 5 | LDPE | 90 × 170 × 0.04 | LDPE | 90 × 170 × 0.04 |
| Group 6 | LDPE | 90 × 170 × 0.04 | Latex finger cot | 30 × 70 × 0.08 |
LDPE, low-density polyethylene.
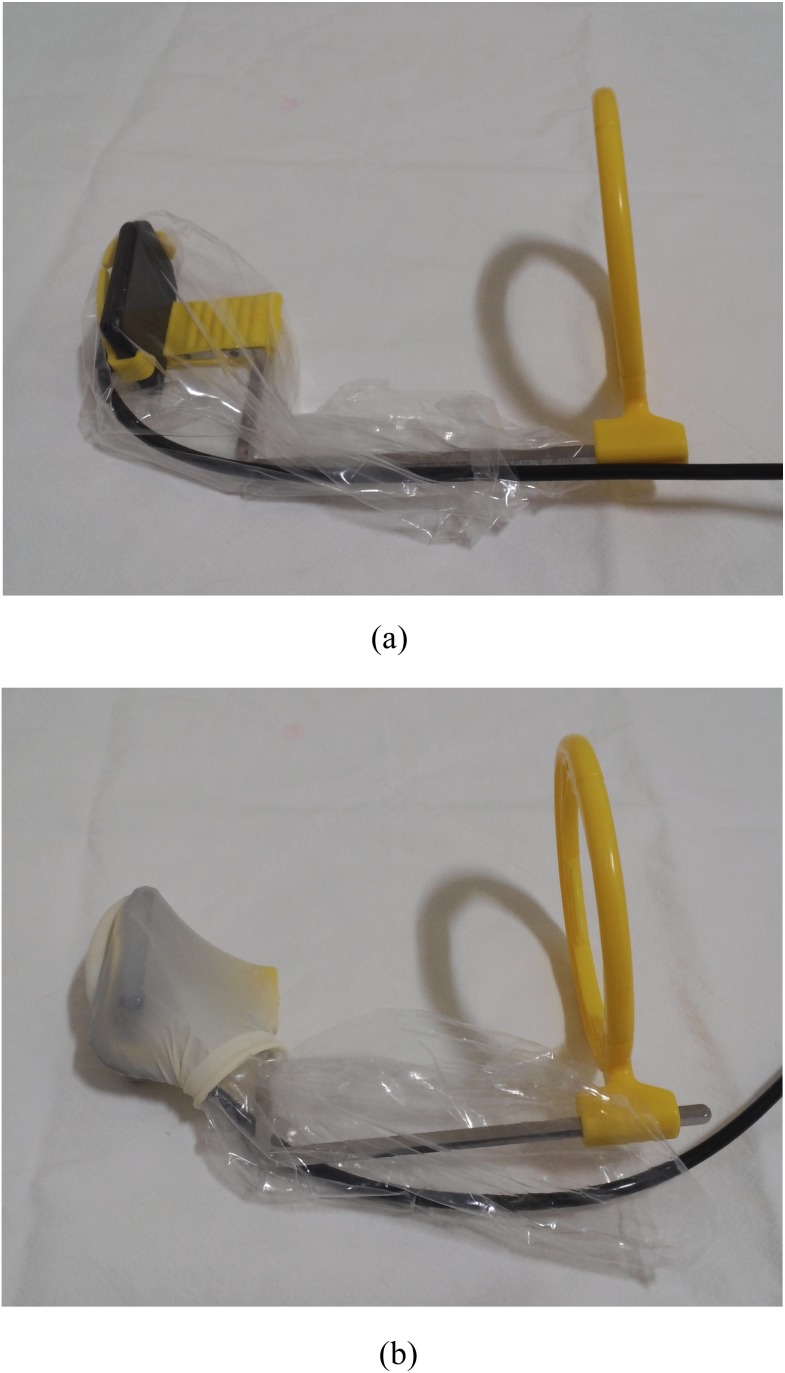
Figure 1.
(a) A plastic barrier with 0.04-mm thickness was applied after combining the sensor with a positioning device. (b) A plastic barrier was applied to a positioning device with a latex finger cot.
Water pressure test
The perforations of all barrier samples were examined by a water pressure test. The water pressure test has been approved by the Food and Drug Administration for testing for leakage in latex and vinyl gloves.4 All water pressure tests were performed by the same observer. First, each barrier was filled with about 200 ml of water and sealed by three twist turns of the opening. The surface of the barrier was dried using paper towels, and moderate hand pressure was applied for 20 s. Water leakage was examined by visual inspection, and the number of perforations was recorded. In cases where the barriers had a leakage on the side sealing line without any tooth indentation, the leakage was considered to be owing to product defects; these barriers were excluded from the study. When double barriers were used, the perforation rate of the inner and the outer barriers were recorded separately (Groups 5 and 6). A total of 800 barrier samples were collected and divided into six groups according to the applied barrier techniques. Then, the water pressure test was performed. Ten unused barriers in each group were tested as control.
Statistical analysis
The perforation rate of each group was calculated. For the double barrier groups, the perforation rate was calculated on the basis of inner barrier because the perforation of the outer barrier did not contaminate the sensor and the positioning device. Furthermore, the latex barriers were smaller than the plastic barrier envelope, and it was impossible to cover all areas of the inner barriers. Thereafter, the average number of perforations was calculated for each group by dividing the total number of perforations by the number of perforated samples. Inner barriers with more than two perforations were considered to be barriers with multiple perforations, and the rate of multiple perforations was calculated for each group. The differences in the perforation rates and numbers were evaluated by a one-way ANOVA test and post hoc Scheffe's post comparison, by using IBM SPSS® Statistics v. 21 (IBM Corporation, Armonk, NY). A statistical significance level of p < 0.05 was used.
Results
The perforation rates were 22–58%, and the perforation occurred the least in Group 4 (0.08-mm-thick single barrier) and the most in Group 1 (0.04-mm-thick single barrier) (Table 2). ANOVA revealed statistical differences in the perforation rate between the groups (p = 0.00; 95% confidence interval, 0.326–0.403). The perforation rate decreased significantly in Groups 3 (0.06 mm), 4 (0.08 mm), 5 (0.04 + 0.04 mm) and 6 (0.04 mm + finger cot) as compared with Group 1 (0.04 mm). Compared with Group 2 (0.05 mm), only Group 4 (0.08 mm) showed a significantly lower perforation rate.
Table 2.
Number of barrier perforations in each group
| Groups | Technique | Inner barrier perforation | Outer barrier perforation | Perforations of both barriers | Perforation rate (%) | 95% confidence interval | p-valuea |
|---|---|---|---|---|---|---|---|
| Group 1 | 0.04 | 58/100 | 58b | 0.48–0.68 | |||
| Group 2 | 0.05 | 47/100 | 47b,c | 0.37–0.57 | |||
| Group 3 | 0.06 | 35/100 | 35c,d | 0.25–0.45 | |||
| Group 4 | 0.08 | 22/100 | 22d | 0.14–0.30 | 0.00 | ||
| Group 5 | 0.04 + 0.04 | 25/100 | 35/100 | 24/100 | 25c,d | 0.16–0.34 | |
| Group 6 | 0.04 + finger cot | 32/100 | 26/100 | 12/100 | 32c,d | 0.23–0.41 |
Obtained using one-way ANOVA.
The different characters show the statistically significant differences among the groups obtained using Scheffe's post comparison.
ANOVA also revealed statistical differences in the average number of perforations and the rate of multiple perforations among the groups (p < 0.05) (Table 3). Groups 4–6 had lower perforation rates than did Group 1. Moreover, the average number of perforations and the rate of multiple perforations in Groups 4–6 were significantly lower than in Group 1. So even in the case of perforation, the degree of contamination in Groups 4–6 was presumed to be lower than that in Group 1. There were no leakages in the barriers of the control samples.
Table 3.
Average number of perforations and the rate of multiple perforations in each group
| Groups | Technique | Average number of perforations at inner barrier | 95% confidence interval | p-valuea | Rate of multiple perforations (%) | 95% confidence interval | p-valuea |
|---|---|---|---|---|---|---|---|
| Group 1 | 0.04 | 1.17b | 0.88–1.46 | 34b | 0.25–0.43 | ||
| Group 2 | 0.05 | 1.02b,c | 0.46–1.12 | 24b,c | 0.15–0.33 | ||
| Group 3 | 0.06 | 0.69b,c | 0.43–0.95 | 15c | 0.08–0.22 | ||
| Group 4 | 0.08 | 0.49c | 0.15–0.83 | 0.00 | 7c | 0.02–0.12 | 0.002 |
| Group 5 | 0.04 + 0.04 | 0.45c | 0.27–0.63 | 13c | 0.06–0.20 | ||
| Group 6 | 0.04 + Finger cot | 0.52c | 0.32–0.71 | 11c | 0.05–0.17 |
Obtained using one-way ANOVA.
The different characters show the statistically significant differences among groups obtained using Scheffe's post comparison.
Discussion
This is the first study that calculated the perforation rate of intraoral barriers on the basis of barrier thickness. There has been no research or regulation that addresses the requirements of the material and thickness of intraoral barriers for direct digital radiography. Therefore, the results of this study will be useful for infection control in dental clinics.
This study investigated the perforation rates of six modified applications of intraoral barriers. First, Group 1 (single use of a 0.04-mm-thick LDPE barrier) exhibited a perforation rate of 58%, which is a relatively high perforation rate compared with the 44% reported in a previous study,2 but a relatively low perforation rate compared with the 83% reported in another study.3 These high perforation rates indicate that the single use of a 0.04-mm-thick plastic barrier is not safe enough to prevent the contamination of digital sensors and positioning devices.
This study also reveals that the use of a latex finger cot significantly reduces the leakage rate; this corresponds to the findings of an earlier study.2,3 However, the perforation rate of Group 6 (with the latex finger cot) was 32%, and it differed considerably from the earlier reports of 0%,2 6%3 and 55%.3
In addition, the result of Group 6, which used the latex finger cot, was insignificant; however, this group showed a higher rate of perforation than did Groups 4 (0.08-mm-thick single barrier) and 5 (0.04-mm-thick double barrier). The reason for this difference is unclear, because information on the material and the thickness of the barriers was not provided in previous studies. The reason for the higher perforation rate of Group 6 was thought to be the small size of the latex finger cot that made it impossible to cover the positioning device completely. This was revealed when the perforation aspect of the double barrier groups was inspected.
20 of the 32 inner barrier perforations in Group 6 occurred without an outer latex perforation (Table 2). Thus, it could be speculated that these perforations occurred in the area that was not covered by the latex finger cot. By contrast, in Group 5, the sizes of both LDPE barriers were the same, and there was only one case of inner barrier perforation without an outer barrier perforation (Table 2). Therefore, it is believed that just covering the sensor area with a latex finger cot also posed the risk of contaminating the positioning device. In addition to the small size, there was another practical disadvantage of the use of a latex finger cot. When covering the sensor, opening the latex finger cot required the use of two hands, and one more hand was required for holding the sensor and the positioning device in place.
When a 0.05-mm-thick plastic barrier was used alone (Group 2), the perforation rate decreased, but the difference was not statistically significant. The perforation rates of Groups 3 and 4 were significantly lower than Group 1, and they showed comparable effectiveness with the use of a latex finger cot. By contrast, the rigidity of the plastic barrier increased with an increase in barrier thickness; this caused a poking sensation in the mucous membrane of the patients. In the case of the single use of a 0.06-mm-thick plastic barrier (Group 3), a few sensitive patients complained of discomfort. The use of the 0.08-mm-thick plastic barrier caused a sharp pain in many patients; therefore, this barrier is considered difficult to use in clinical practice. The double barriers of 0.04-mm-thick LDPE (Group 5) also showed significantly better results than did a single 0.04-mm-thick barrier. This result corresponds with that of an earlier study, which reported that the use of double gloves in orthopaedic surgery is safer than that of single gloves.5 Moreover, the perforation rate of the 0.04-mm-thick double barriers is comparable to the perforation rate of a 0.08-mm-thick single barrier. In addition, a double-barrier application did not cause any pain or practical problems in a periapical radiography procedure.
A limitation of this study is that a water pressure test was used to compare the obtained results with the results reported in a previous paper, but the water leakage of the barriers does not necessarily imply a contamination of the radiographic equipment. Limited literature is available on bacterial contamination of a direct digital sensor in intraoral radiography. Wenzel et al6 reported that direct digital sensor and cord were not contaminated by oral bacteria at all in bitewing radiography. However, the sample size was only 14, and further research on bacterial contamination of direct digital sensors is required.
Unlike research on the bacterial contamination of a direct intraoral sensor, several studies on the bacterial contamination of an image plate have been reported. However, the contamination rates of the image plate were high; they were reported to be 100%,7 100%,8 56%,9 57%10 and 17%11 in earlier papers. There was also leakage or extraction failure in the case of an image plate.7,11 In digital intraoral radiography, infection control is important irrespective of the system used. There have been some studies that have shown the potential use of chlorhexidine gargle and gas sterilization for infection control.10,12
In summary, this study proved that there are statistical differences in perforation rates according to the applications of the intraoral barriers. The use of a double barrier can reduce the perforation rate by more than half as compared with the use of a single barrier. However, the risk of contamination cannot be completely eliminated with a barrier application. Therefore, the development of new barriers, new techniques or waterproof sensors is required. Until then, the use of a double barrier is suggested; furthermore, the radiographic equipment should be cleaned and disinfected between patients as recommended by the CDC guideline.1
References
- 1.Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, Malvitz DM; Centers for Disease Control and Prevention (CDC). Guidelines for infection control in dental health-care settings–2003. MMWR Recomm Rep 2003; 52: 1–61. [PubMed] [Google Scholar]
- 2.Hokett SD, Honey JR, Ruiz F, Baisden MK, Hoen MM. Assessing the effectiveness of direct digital radiography barrier sheaths and finger cots. J Am Dent Assoc 2000; 131: 463–7. [DOI] [PubMed] [Google Scholar]
- 3.Hubar JS, Gardiner DM. Infection control procedures used in conjunction with computed dental radiography. [In German.] Int J Comput Dent 2000; 3: 259–67. [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Patient examination gloves and surgeon's gloves: sample plans and test method for leakage defects; adulteration. Code of Federal Regulations Title 21 Volume 8 Sec. 800.20. [Updated 1 April 2014; cited 18 August 2014.] Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=800.20
- 5.Al-Habdan I, Corea JR, Sadat-Ali M. Double or single gloves: which is safer in pediatric orthopedic surgery. J Pediatr Orthop 2006; 26: 409–11. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel A, Frandsen E, Hintze H. Patient discomfort and cross-infection control in bitewing examination with a storage phosphor plate and a CCD-based sensor. J Dent 1999; 27: 243–6. [DOI] [PubMed] [Google Scholar]
- 7.Negron W, Mauriello SM, Peterson CA, Arnold R. Cross-contamination of the PSP sensor in a preclinical setting. J Dent Hyg 2005; 79: 8. [PubMed] [Google Scholar]
- 8.Peterson CA, Mauriello SM, Arnold RR, Webster-Cyriaque J. Infection control for a digital imaging sensor. J Dent Res 2002; 81: A–111. [Google Scholar]
- 9.Kalathingal SM, Moore S, Kwon S, Schuster GS, Shrout MK, Plummer K. An evaluation of microbiologic contamination on phosphor plates in a dental school. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107: 279–82. doi: 10.1016/j.tripleo.2008.05.025 [DOI] [PubMed] [Google Scholar]
- 10.Kalathingal S, Youngpeter A, Minton J, Shrout M, Dickinson D, Plummer K, et al. An evaluation of microbiologic contamination on a phosphor plate system: is weekly gas sterilization enough? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109: 457–62. doi: 10.1016/j.tripleo.2009.09.035 [DOI] [PubMed] [Google Scholar]
- 11.MacDonald DS, Waterfield JD. Infection control in digital intraoral radiography: evaluation of microbiological contamination of photostimulable phosphor plates in barrier envelopes. J Can Dent Assoc 2011; 77: b93. [PubMed] [Google Scholar]
- 12.Hunter A, Kalathingal S, Shrout M, Plummer K, Looney S. The effectiveness of a pre-procedural mouthrinse in reducing bacteria on radiographic phosphor plates. Imaging Sci Dent 2014; 44: 149–54. doi: 10.5624/isd.2014.44.2.149 [DOI] [PMC free article] [PubMed] [Google Scholar]