Abstract
Objectives:
To evaluate the diagnostic value of diffusion-weighted MRI for differentiating metastatic from non-metastatic retropharyngeal lymph nodes (RLNs) in patients with nasopharyngeal carcinoma (NPC).
Methods:
Untreated patients with NPC (n = 145) were scanned with both morphological MRI and diffusion-weighted imaging (DWI). RLNs (n = 335) were classified as metastatic on the basis of response to therapy as assessed on follow-up MRI. Morphological (short- and long-axial diameters) and functional [mean apparent diffusion coefficient (ADC) and minimum ADC values] parameters of the RLNs were derived from DWI and compared between metastatic and non-metastatic groups. A receiver operating characteristic curve and the area under the curve were used to evaluate the effectiveness of individual criteria and to generate threshold values to diagnose RLN metastases.
Results:
Statistically significant differences between metastatic and non-metastatic RLNs were found for all four parameters derived from DWI (p < 0.001). At threshold values, accuracies of the ADC-based criteria (0.938 and 0.965 for mean and minimum ADC values, respectively) were greater than that of size-based criteria (0.838 and 0.809 for short- and long-axial diameters). The minimum ADC value at the threshold of 0.89 × 10−3 mm2 s−1 was the most effective of all parameters in differentiating metastatic from non-metastatic RLNs with the sensitivity of 95.7%, specificity of 95.1% and accuracy of 96.5%.
Conclusions:
DWI is feasible for differentiating metastatic RLNs from non-metastatic nodes in patients with NPC with high accuracy, and the minimum ADC derived from DWI could serve as a standard clinical marker for disease status.
Keywords: nasopharyngeal carcinoma, retropharyngeal lymph node (RLN), magnetic resonance imaging (MRI), diffusion-weighted imaging (DWI), apparent diffusion coefficient (ADC) value
Introduction
Nasopharyngeal carcinoma (NPC) is highly prevalent in the southeastern part of China. For decades, a combination of radiotherapy and chemotherapy has been used as the standard treatment for newly diagnosed patients with NPC. The disease poses a unique problem for pathological staging, as the nasopharynx has a well-developed network of lymphatic and regional lymph nodes, which are commonly involved in NPC.1–3
Retropharyngeal lymph nodes (RLNs) are the first echelon lymph nodes typically involved in NPC. Spread of the disease to RLNs has been associated with a poor prognostic outcome in patients with NPC, and therefore, a diagnosis of RLN metastasis has been classified as N1 stage disease.4,5 However, the deep anatomical location of RLNs makes it difficult to diagnose metastatic disease histopathologically. Diagnostic criteria for distinguishing metastatic from benign lymph nodes have been limited to those obtained by standard MRI and are based on measurements of the size and shape of nodes and signal intensities that correspond to the presence of capsular spread and central necrosis. A minimal axial diameter of ≥6 mm is the main diagnostic parameter currently used to differentiate metastatic from non-metastatic lesions, but the accuracy of the diagnosis is 87.5%.6
Adding a functional component to imaging, such as diffusion-weighted imaging (DWI), has become increasingly crucial in the assessment of malignant tumours. DWI is a non-invasive characterization of diverse tissues based on water diffusion properties. Any architectural alteration in the proportion of extracellular to intracellular water protons will change the diffusion coefficient of the tissue. To date, several reports have indicated that DWI is a useful method in the differentiation of metastatic from non-metastatic cervical lymph nodes in patients with head and neck tumours, on the basis that it provides a more detailed characterization of tissue at both a microscopic level and in terms of an actual pathological process.7–9 However, studies exploiting DWI to distinguish metastatic from benign RLNs in patients with NPC are limited. Here, DWI was evaluated as a possible clinical strategy to differentiate metastatic from non-metastatic RLNs in patients with NPC and compared with the current standard of size-based criteria.
Methods and materials
Ethics statement
Informed written consent was obtained from all patients prior to the initiation of the study. The collection of patient data for the purposes of this study was performed following the approval from the institutional review board of Sun Yat-sen University (Guangdong, China).
Patients
Clinical data were retrospectively collected from 145 patients with newly diagnosed and non-disseminated NPC from April 2011 to December 2012. The cohort included 98 males and 47 females with a median age of 48 years (age range, 26–67 years). All patients underwent a pre-treatment evaluation that included 3.0-T MRI and DWI of the neck and nasopharynx, chest radiography, abdominal ultrasonography and a whole-body bone scan. The medical records and findings of imaging studies were reviewed, and in all patients, staging was performed according to the 2009 staging system of the seventh edition of the American Joint Committee on Cancer. T category classification of patients was as follows: 36 (T1); 9 (T2a); 44 (T2b); 35 (T3); 21 (T4). N category classification was as follows: 8 (N0); 79 (N1); 36 (N2); 8 (T3a); 8 (T3b).
Imaging protocol
MRI was performed with the Siemens 3.0-T Trio Tim scanner with a head and neck combined coil (Siemens, Malvern, PA). Patients were scanned from the suprasellar cistern to the inferior margin at the sternal end of the clavicle. The following sequences were obtained from all patients: T1 weighted fast spin-echo (SE) images in the axial, coronal and sagittal planes (repetition time, 500–600 ms; echo time, 10–20 ms) and T2 weighted fast SE images in the axial plane (repetition time, 4000–6000 ms; echo time, 95–110 ms). Axial DWI was obtained prior to intravenous injection of gadolinium using a SE echo planar imaging sequence (repetition time, 5000–6000 ms; echo time, 85–100 ms) with the chemical-shift selective fat-suppression technique. The sequence was repeated for two values or motion-probing gradients (b = 0 and 1000 s mm−2). Contrast-enhanced T1 weighted imaging (T1WI) scans in the axial and sagittal planes and fat-suppressed in the coronal plane (section thickness, 5 mm; field of view, 22 cm; frequency matrix size, 320 × 224 pixels; repetition time, 320–350 ms; echo time, 10–20 ms) were acquired. A 0.1 mmol kg−1 body weight bolus injection of gadopentate dimeglumine was administered for contrast-enhanced sequences.
Image assessment
Two experienced radiologists independently evaluated the MR images. Any disagreements were resolved by consensus. All RLNs that could be identified on MR and diffusion-weighted images were included in this study. To ensure the accuracy of the MRI assessments of RLN, nodes that were not clearly defined on normal MRI and could only be identified on DWI were not acceptable for further analyses. The MRI assessment included central necrosis, extra capsular neoplastic spread (ENS), size criteria (short-axial and long-axial diameters) and contrast enhancement patterns (homogeneous and heterogeneous; strong, moderate and absent) of each node. Central necrosis was defined as a focal area of high signal intensity on T2 weighted imaging (T2WI) and low signal intensity on T1WI that showed no enhancement after contrast material administration regardless of the presence or absence of a surrounding rim of enhancement. ENS was diagnosed on the basis of nodal capsular irregularity with enhancement and infiltration of adjacent fat. The axial diameter measurements were made on T2 weighted axial images. Short-axial diameters corresponded to the widest diameter of the lymph node in the axial plane that was perpendicular to the maximal long-axial diameters. For each node, a region of interest (ROI) was drawn on diffusion-weighted images and the apparent diffusion coefficient (ADC) map. Both mean and minimum ADC values were measured while attempting to avoid inclusion of the lymph node margins. The mean ADC value was obtained by a single measurement covering the entire area of the node, whereas the minimum ADC value was extracted from the manual placement of at least five circular ROIs that encompassed five voxels (approximately 6 mm2). The degree of enhancement was considered to be strong if it was similar to that of normal mucosa, moderate if less than that of normal mucosa and absent if the lesion showed no relative increase in signal with the contrast agent.
Treatment
Details regarding the radiation therapy (RT) technique performed at the cancer centre of Sun Yat-sen University have been reported previously.10–12 Briefly, all patients were treated with definitive-intent RT. Patients received one cycle of irradiation, where the total dosage was between 66 and 83 Gy. All RLNs that were visible on MRI were incorporated into the gross tumour volume when the target volume was drawn. A total of 46 patients with local or regionally advanced disease (classified as T3 or T4, or N2 or N3 lesions) received neoadjuvant, concurrent or adjuvant chemotherapy.
Follow-up and assessment of retropharyngeal lymph nodes
Tumour response was assessed by MRI and flexible nasopharyngoscopy performed while the patient was undergoing RT (45–50 Gy) and 14–30 days after the completion of RT. Residual primary tumours, cervical lymph nodes or RLNs were noted, and close follow up was performed through further clinical study and MRI. All patients were regularly monitored for at least 15 months with a maximum follow up of 23 months and a mean follow up of 19 months.
The nature of the RLNs was assessed based on MRI performed after completion of RT. A RLN was considered to be positive for malignant involvement if, on the follow-up MRI, it had significantly decreased in size and was undetectable or it showed stability in size but progressed.6 If the RLN was still detectable after RT but had decreased in size by >30%, it was considered to be a partial resolution,13 and analysis continued until diagnosis of the node could be made. If a node showed stability in size, but T2WI and T1WI contrast enhancements were significantly reduced, a positive RLN was still diagnosed. A RLN was considered to be negative for malignant involvement, if it showed stability in size and the patient remained disease free.6 Nodes were excluded from the study if a diagnosis could not be made by the completion of the follow-up.
Statistical analysis
The data were analysed with SPSS® software v. 19.0 (IBM Corporation, 2010, Armonk, NY). One-way ANOVA and a two-sided Student's t-test were used to evaluate data obtained from metastatic vs non-metastatic nodes. A p-value <0.05 was considered to be statistically significant. The receiver operating characteristic (ROC) curve and the area under the curve (AUC) were used to evaluate the effectiveness of different criteria. The curve represents the relationship between the sensitivity and specificity. The overall accuracy is represented by the AUC, and the larger the AUC, the better the test. The sensitivity, specificity and accuracy were calculated according to standard definitions.
Results
Assessment of RLNs
Prior to treatment, MRI revealed 335 RLNs in 139/145 (95.9%) patients with NPC. At 14–30 days after completion of RT, the radiographic responses of these 335 RLNs were distributed as follows: complete resolution of the lymph node, 89 (26.6%); partial resolution, 124 (37.0%); and stability, 122 (36.4%). The clinical behaviour of the 124 RLNs with partial resolution could be differentiated on the basis of later follow-up MRI images; 118 lymph nodes gradually decreased in size, and the remaining 6 lymph nodes remained stable in size until the completion of the follow-up study. However, the T2WI and contrast-enhanced T1WI signals of these six nodes were both significantly reduced. Of the 122 RLNs displaying stability at the first MRI following treatment, 20 (16.4%) lymph nodes demonstrated progressive disease at later time points, while 102 (83.6%) lymph nodes remained stable or had no evidence of progressive disease at further follow up. On the basis of the clinical response of RLNs as assessed on follow-up MRI, 233 out of the 335 identifiable RLNs (69.6%) were found to be metastatic (positive; Figures 1 and 2). The remaining 102 (30.4%) RLNs were classified as benign (negative; Figure 2).
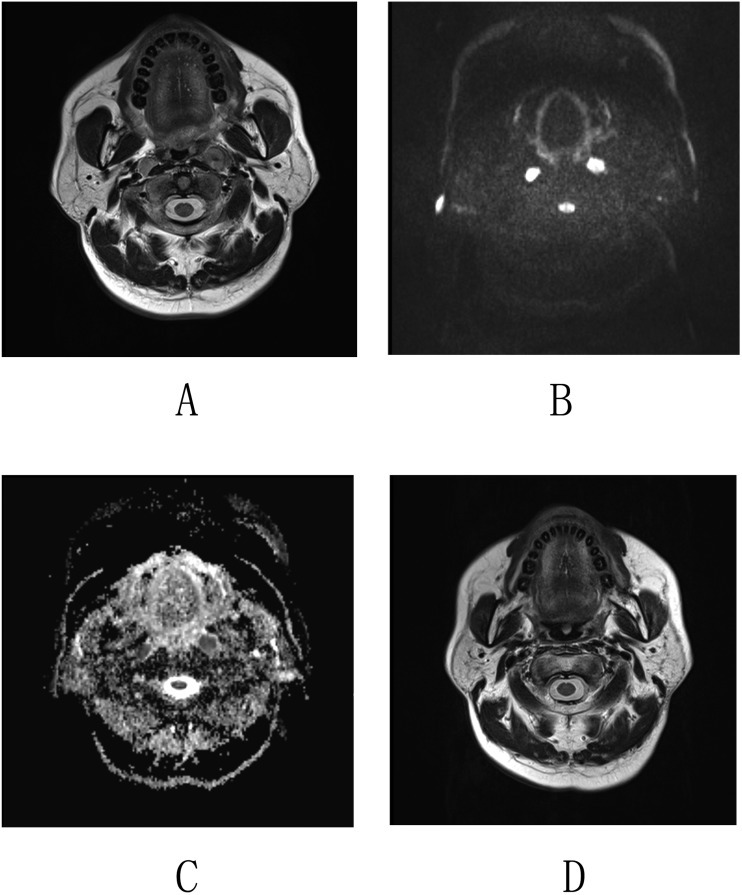
Figure 1.
Imaging by MRI and diffusion-weighted imaging (DWI) of nasopharyngeal carcinoma in a 52-year-old male. (a) Axial T2 weighted images revealed enlarged lymph nodes in both sides of the retropharyngeal space. (b, c) Corresponding DWI and apparent diffusion coefficient (ADC) map. The lymph nodes exhibit a high homogeneous signal intensity on the DWI map and a low homogeneous signal intensity on the ADC map. The mean ADC values of the retropharyngeal lymph nodes (RLNs) were 0.768 × 10−3 mm2 s−1 (left) and 0.832 × 10−3 mm2 s−1 (right), and the minimum ADC values were 0.622 × 10−3 mm2 s−1 (left) and 0.583 × 10−3 mm2 s−1 (right). (d) Axial T2 weighted images obtained at 6 months following radiation therapy show that both enlarged RLNs were resolved.
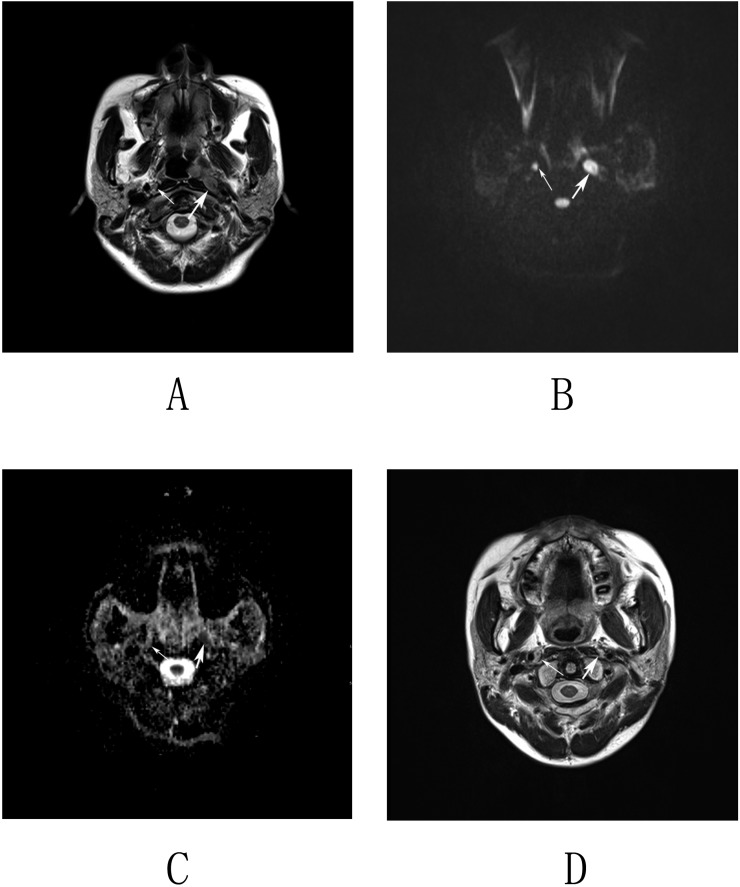
Figure 2.
Imaging by MRI and diffusion-weighted imaging (DWI) of nasopharyngeal carcinoma in a 48-year-old male. (a) Axial T2 weighted images revealed an enlarged lymph node in the left retropharyngeal space (thick arrow) and a lymph node with the short-axis diameter of 5 mm in the right retropharyngeal space (thin arrow). (b, c) Corresponding DWI and apparent diffusion coefficient (ADC) map. The left lymph node displays a high homogeneous signal intensity on the DWI map and a low homogeneous signal intensity on the ADC map (thick arrows). The right lymph node displays a slightly high but homogeneous signal intensity on both the DWI and ADC maps (thin arrows). The mean and minimum ADC values of the left retropharyngeal lymph nodes (RLNs) were 0.899 × 10−3 and 0.813 × 10−3 mm2 s−1. The mean and minimum ADC values of the right RLN were 1.312 × 10−3 and 1.21 × 10−3 mm2 s−1, respectively. (d) Axial T2 weighted images obtained at 12 months following radiation therapy show that the left lymph node was resolved, whereas the right lymph node remained stable.
DWI was equally effective in revealing the same 335 RLNs originally identified with MRI, and parameters were extracted from the imaging. The mean ADC was successfully calculated in the ADC map for all 335 RLNs. However, the minimum ADC values could be calculated in only 329/335 RLNs owing to the fact that 6 RLNs were too small to draw circular ROIs in 6 mm2. All of the parameters, as derived from DWI, are summarized for positive and negative RLNs in Table 1. On a morphological level, the mean values of the short-axial and long-axial diameters of the positive RLNs were significantly greater than those of the negative RLNs indicating that generally positive RLNs were larger than negative ones. In terms of a functional property, statistical analysis revealed that the mean and minimum ADC values of positive RLNs were significantly lower than those of negative RLNs demonstrating impaired diffusion in metastatic vs benign nodes (p = 0.000, < 0.05).
Table 1.
Parameter variation between positive and negative retropharyngeal lymph nodes
| Size and ADC criteria | Result | n | Mean | Standard deviation | t | p-value |
|---|---|---|---|---|---|---|
| Short-axis diameter | Negative | 102 | 3.85 | 1.52 | −11.401 | <0.001 |
| Positive | 233 | 7.62 | 4.50 | |||
| Long-axis diameter | Negative | 102 | 5.99 | 2.40 | −10.332 | <0.001 |
| Positive | 233 | 10.61 | 5.79 | |||
| Mean ADC | Negative | 102 | 1.24 | 0.43 | 10.664 | <0.001 |
| Positive | 233 | 0.77 | 0.16 | |||
| Minimum ADC | Negative | 96 | 1.28 | 0.75 | 8.208 | <0.001 |
| Positive | 233 | 0.67 | 0.14 |
ADC, apparent diffusion coefficient.
Size- vs ADC-based criteria
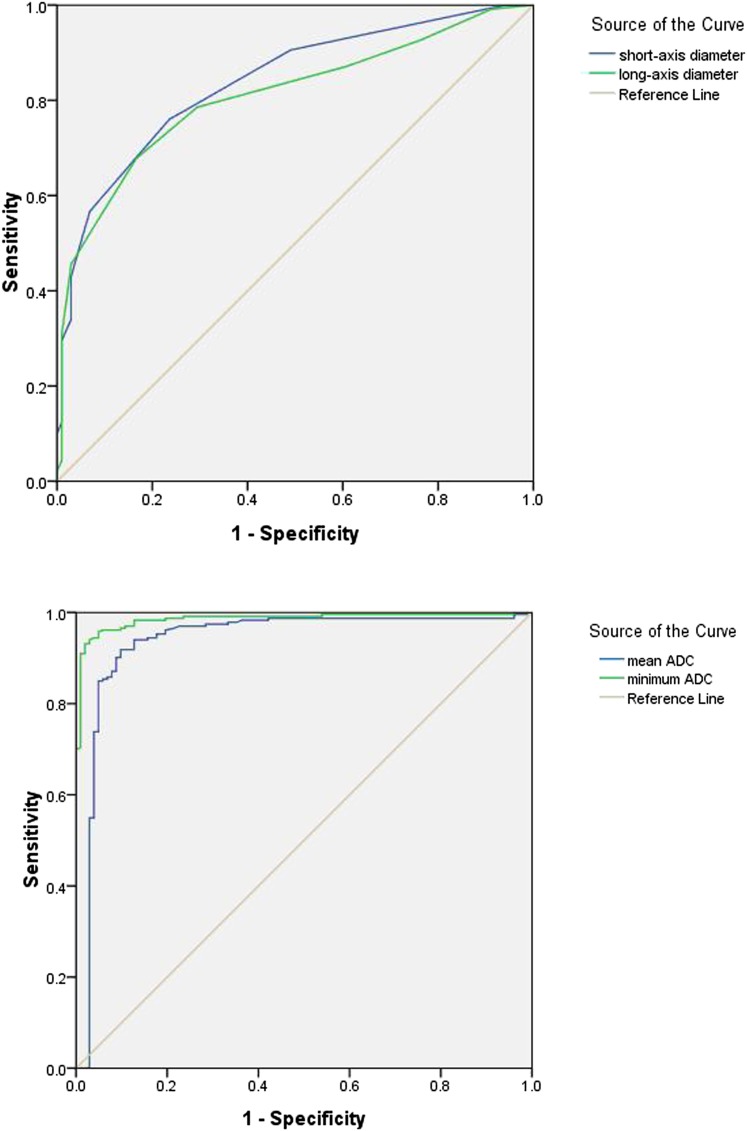
The data were subsequently analysed for numerical threshold values for the four parameters that could be applied to the diagnosis of metastatic vs non-metastatic nodes. ROC curve analysis revealed that the best performing thresholds were 4.5 mm for short-axial diameters, 6.5 mm for long-axial diameters (positive > axial diameter threshold > negative), 0.929 × 10−3 mm2 s−1 for mean ADC values and 0.89 × 10−3 mm2 s−1 for minimum ADC values (negative > ADC threshold > positive; Figure 3). The sensitivity and specificity for both size-based and ADC-based criteria at the threshold values derived from ROC curves indicated that ADC values overall would better predict RLN metastasis (Table 2). This result is highlighted in the ROC curves (Figure 3). Of all the criteria, however, the minimum ADC value was the most accurate for the diagnosis of the RLN metastases (AUC = 0.965). The sensitivity, specificity and accuracy of this criterion were 95.7%, 95.1% and 96.5%, respectively.
Figure 3.
The receiver operating characteristic (ROC) curve of size-based and apparent diffusion coefficient (ADC)-based criteria. The graph demonstrates that the ROC curve of ADC-based criteria is superior to size-based criteria.
Table 2.
Diagnostic performance of different criteria for the detection of retropharyngeal lymph nodes
| Size and ADC criteria | Cut-off point | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| Short-axis diameter | 4.50 | 0.760 | 0.765 | 0.838 |
| Long-axis diameter | 6.50 | 0.785 | 0.706 | 0.809 |
| Mean ADC | 0.929 | 0.918 | 0.902 | 0.938 |
| Minimum ADC | 0.8905 | 0.957 | 0.951 | 0.965 |
ADC, apparent diffusion coefficient.
As an illustration of the sensitivity and specificity of these thresholds, RLNs from the cohort were diagnosed as positive or negative with the short-axis diameter and minimum ADC threshold values. When the short-axis diameter of 4.5 mm was used as the threshold value (positive > 4.5 mm > negative), 56 positive RLNs in this study would have been falsely diagnosed as benign (negative). However, when the threshold for the minimum ADC value was applied to the RLNs, 53 of these false-negative nodes were diagnosed correctly as metastatic (positive).
Nodal necrosis and ENS had a specificity of 100% for the diagnosis of RLN metastases. Of the 233 positive RLNs, 22 (9.4%) and 41 (17.6%) exhibited nodal necrosis and ENS, respectively (as determined by T1WI and T2WI signal intensities from MRI); all were revealed to be malignant at the post-treatment follow-up MRI. When RLNs were diagnosed according to the threshold for the short-axial diameter, 2/22 and 7/41 of the RLNs with nodal necrosis and ENS, respectively, had a short-axial diameter <4.5 mm and would have been misdiagnosed as negative.
Discussion
RLN metastasis has been shown to be a significant independent predictor for overall survival, locoregional relapse-free survival and distant metastasis-free survival in patients with NPC.14 The correct diagnosis of RLNs is, thus, important in order to understand the course of the disease in response to therapy. Because of their anatomical location, RLNs are not amenable to evaluation using manual palpation or histological analysis, and as a result, the diagnosis of RLN metastasis in patients with NPC is made largely on the basis of imaging.5 Although MRI has proven to be an invaluable tool for distinguishing RLN and cervical lymph node metastasis,2,15,16 functional imaging, such as DWI, has been shown to facilitate diagnosis of lymph node metastasis in other malignant diseases.17–20 Here, we evaluated the utility of DWI in differentiating metastatic from benign RLN in NPC, compared the accuracy of size- and ADC-based criteria in RLN metastasis and determined whether DWI would at all improve the diagnostic accuracy of RLN metastasis.
Our basis for comparison of nodal assessment was MRI follow-up data as is currently in practice. As in previous studies, a node was considered to be positive for malignant involvement if it resolved after the patient completed RT or displayed stability in size after RT but progressed during the subsequent MRI. By contrast, the RLN was considered to be negative for malignant involvement if it showed stability in size after the completion of RT, and the patient remained disease free during the follow-up.6,21,22 In addition, a detailed approach for diagnosis of nodes where partial resolution had occurred was presented. The node was considered to be positive if it gradually decreased in size or progressed on subsequent MRI. A more complicated scenario of partial resolution appeared when a node showed stability in size but attenuated T2WI signal and contrast-enhanced T1WI following treatment. This clinical behaviour was attributed to chemoradiation fibrosis of metastatic nodes in the post-treatment phase, and these nodes were also diagnosed as positive.
The morphological parameter of size, assessed by the radiological criterion of a short-axis diameter of 5 mm or larger, has also often been used to evaluate RLN involvement. In a previous study, a minimal axial diameter of 6 mm or larger (with an accuracy of 87.5%) was reported to be the best size criterion for diagnosing RLN metastases.6 In our study, the threshold short-axis diameter was 4.5 mm, where sensitivity, specificity and accuracy were 76.0%, 76.5% and 83.8%, respectively. The ability to define a smaller threshold short-axis diameter that more accurately detects RLN may be owing to better tissue resolution with 3.0-T MRI than 1.5 T MRI used in previous studies. Nevertheless, this criterion even at the new resolution remains unsatisfactory in diagnostic performance for differentiating metastatic from non-metastatic lymph nodes.
Diagnostic performance improved when the functional data derived from DWI was used. Both the mean and minimum ADC values of positive RLNs derived from DWI were significantly lower than the values of negative RLNs. At best, when mean and minimum ADC values of 0.929 × 10−3 and 0.891 × 10−3 mm2 s−1 were chosen as the thresholds for differentiating positive RLNs from negative nodes, sensitivity of 91.8% and 95.7%, specificity of 90.2% and 95.1%, and accuracy of 93.8% and 96.5% were achieved, respectively. Relative to size criteria, however, the sensitivity, specificity and accuracy of both ADC criteria were greater. Furthermore, in practice, our results demonstrated that most of the false-negative RLNs (53/56) as determined by size criteria could be correctly diagnosed by ADC. Similar criteria were used to diagnose 153 enlarged pelvic nodes, including 66 metastatic and 87 follicular hyperplastic nodes, in patients with cervical carcinoma. The ADC values of these metastatic nodes were also found to be significantly lower than those of the hyperplastic nodes.23 On histopathological analysis, metastases were observed and tumour areas exhibited hypercellularity, enlarged cell size, enlarged nuclei, hyperchromatism and a high nuclear-to-cytoplasmic ratio. The consequence of this reorganization of the normal tissue architecture was proposed to reduce diffusion of water molecules in both extracellular and intracellular spaces, which could then lead to reduced ADC values.24
However, several issues regarding ADC influence the proper diagnosis of a metastatic lymph node. Firstly, metastatic lymph nodes may have areas of carcinoma interspersed within normal tissue. Although ADC values may differ between cancerous and non-cancerous areas within a single node, the mean ADC value of the metastatic lymph node may not be significantly lower than that of a non-metastatic node. Thus, the node would not be properly diagnosed. Secondly, necrosis itself is a factor that influences diffusion and as the amount of necrosis in an affected node increases, so does the ADC value. In this case, the ADC value begins to approach normal tissue values. Of the 233 positive RLNs in our study, 22 (9.4%) exhibited nodal necrosis, and the necrosis possibly led to an increase in the ADC value of the nodes. Finally, pathological analysis has shown that micronecrosis is common in metastatic lymph nodes.25 Consequently, even after visible necrotic areas were excluded in the determination of the ADC value, the micronecrosis may still influence the mean ADC value of the nodes. However, analysis of “hot spots” with minimum ADC values has been reported to be beneficial for detecting malignant nodes with only focal infiltration. This strategy was confirmed by the fact that accuracy of the minimum ADC in the differentiation of metastatic lymph nodes from non-metastatic ones was greater than that of all size-based criteria as well as the mean ADC in patients with uterine cervical cancer.26 In our study, the criterion that was based on a minimum ADC value was also the most accurate for the diagnosis of the RLN metastases (AUC = 0.985). The sensitivity, specificity and accuracy of this criterion were 95.7%, 95.1% and 96.5%, respectively.
Apart from the nodal size and the ADC value, central necrosis and ENS are other reliable criteria in the evaluation of RLN metastases. Central necrosis and ENS had a specificity of 100% for the diagnosis of RLN metastases, in accordance with the results of a previous study.6 However, central necrosis is usually not visible in small lymph nodes. One study reported that fewer than 2% of metastatic RLNs with a short-axial diameter of <6 mm had central necrosis, so that central necrosis combined with the size criterion led to only a slight improvement in the sensitivity of RLN metastasis detection.6 Our data revealed that, of the metastatic RLNs with nodal necrosis (n = 22) and ENS (n = 41), only 2/22 and 7/41 had short-axial diameters of <4.5 mm, respectively. Therefore, axial diameter led to limited improvement over the detection of RLN metastasis based on central necrosis and ENS and is in agreement with previous studies.
The weakness of our study is that radiological findings were not correlated with histopathology. The determination of the nature of the nodes was based solely on the findings with MRI, which may effectively perpetuate diagnostic errors rather than eliminate them. Moreover, some pathological presentations of RLNs will simply be difficult to evaluate. Not all micrometastases of RLNs, for example, can be detected by MRI, and/or they may not exhibit an obvious change in size in follow-up studies. By contrast, an enlarged RLN may be contiguous with a primary tumour, and it may not be possible to accurately draw the ROI to reflect only the node on the ADC map. Finally, for some small RLNs, the minimum ADC value cannot be determined because the circular ROI of 6 mm2 will also encompass normal surrounding tissues. Therefore, our radiological criteria need to be validated in subsequent studies.
In conclusion, ADC determinations from DWI are useful in differentiating metastatic from non-metastatic RLNs in patients with NPC and can provide supplementary clinical data that may help to guide therapeutic decisions.
References
- 1.Li WF, Sun Y, Mao YP, Chen L, Chen YY, Chen M, et al. Proposed lymph node staging system using the International Consensus Guidelines for lymph node levels is predictive for nasopharyngeal carcinoma patients from endemic areas treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2013; 86: 249–56. doi: 10.1016/j.ijrobp.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 2.Tang L, Mao Y, Liu L, Liang S, Chen Y, Sun Y, et al. The volume to be irradiated during selective neck irradiation in nasopharyngeal carcinoma: analysis of the spread patterns in lymph nodes by magnetic resonance imaging. Cancer 2009; 115: 680–8. doi: 10.1002/cncr.24049 [DOI] [PubMed] [Google Scholar]
- 3.Ho FC, Tham IW, Earnest A, Lee KM, Lu JJ. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis of clinical evidence. BMC Cancer 2012; 12: 98. doi: 10.1186/1471-2407-12-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang XS, Hu CS, Ying HM, Zhou ZR, Ding JH, Feng Y. Patterns of retropharyngeal node metastasis in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2009; 73: 194–201. doi: 10.1016/j.ijrobp.2008.03.067 [DOI] [PubMed] [Google Scholar]
- 5.Tang L, Li L, Mao Y, Liu L, Liang S, Chen Y, et al. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma detected by magnetic resonance imaging : prognostic value and staging categories. Cancer 2008; 113: 347–54. doi: 10.1002/cncr.23555 [DOI] [PubMed] [Google Scholar]
- 6.Zhang GY, Liu LZ, Wei WH, Deng YM, Li YZ, Liu XW. Radiologic criteria of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma treated with radiation therapy. Radiology 2010; 255: 605–12. doi: 10.1148/radiol.10090289 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Chen J, Shen J, Zhong J, Ye R, Liang B. Apparent diffusion coefficient values of necrotic and solid portion of lymph nodes: differential diagnostic value in cervical lymphadenopathy. Clin Radiol 2013; 68: 224–31. doi: 10.1016/j.crad.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 8.Wu LM, Xu JR, Liu MJ, Zhang XF, Hua J, Zheng J, et al. Value of magnetic resonance imaging for nodal staging in patients with head and neck squamous cell carcinoma: a meta-analysis. Acad Radiol 2012; 19: 331–40. doi: 10.1016/j.acra.2011.10.027 [DOI] [PubMed] [Google Scholar]
- 9.Perrone A, Guerrisi P, Izzo L, D'Angeli I, Sassi S, Mele LL, et al. Diffusion-weighted MRI in cervical lymph nodes: differentiation between benign and malignant lesions. Eur J Radiol 2011; 77: 281–6. doi: 10.1016/j.ejrad.2009.07.039 [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Tang LL, Chen L, Li WF, Mao YP, Liu LZ, et al. Promising treatment outcomes of intensity-modulated radiation therapy for nasopharyngeal carcinoma patients with N0 disease according to the seventh edition of the AJCC staging system. BMC Cancer 2012; 12: 68. doi: 10.1186/1471-2407-12-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo R, Sun Y, Yu XL, Yin WJ, Li WF, Chen YY, et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol 2012; 104: 294–9. doi: 10.1016/j.radonc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Mao YP, Xie FY, Liu LZ, Sun Y, Tian L, et al. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiother Oncol 2012; 104: 331–7. doi: 10.1016/j.radonc.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 13.Gebauer B, Bohnsack O, Riess H. Radiological evaluation of tumor response in oncological studies (tumor response evaluation). [In German.] Rofo 2011; 183: 695–703. doi: 10.1055/s-0029-1246074 [DOI] [PubMed] [Google Scholar]
- 14.Ma J, Liu L, Tang L, Zong J, Lin A, Lu T, et al. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: prognostic value and staging categories. Clin Cancer Res 2007; 13: 1445–52. [DOI] [PubMed] [Google Scholar]
- 15.King AD, Vlantis AC, Bhatia KS, Zee BC, Woo JK, Tse GM, et al. Primary nasopharyngeal carcinoma: diagnostic accuracy of MR imaging versus that of endoscopy and endoscopic biopsy. Radiology 2011; 258: 531–7. doi: 10.1148/radiol.10101241 [DOI] [PubMed] [Google Scholar]
- 16.Mao YP, Liang SB, Liu LZ, Chen Y, Sun Y, Tang LL, et al. The N staging system in nasopharyngeal carcinoma with radiation therapy oncology group guidelines for lymph node levels based on magnetic resonance imaging. Clin Cancer Res 2008; 14: 7497–503. doi: 10.1158/1078-0432.CCR-08-0271 [DOI] [PubMed] [Google Scholar]
- 17.Cho EY, Kim SH, Yoon JH, Lee Y, Lim YJ, Kim SJ, et al. Apparent diffusion coefficient for discriminating metastatic from non-metastatic lymph nodes in primary rectal cancer. Eur J Radiol 2013; 82: e662–8. doi: 10.1016/j.ejrad.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 18.Wu LM, Xu JR, Hua J, Gu HY, Zhu J, Hu J. Value of diffusion-weighted MR imaging performed with quantitative apparent diffusion coefficient values for cervical lymphadenopathy. J Magn Reson Imaging 2013; 38: 663–70. doi: 10.1002/jmri.24014 [DOI] [PubMed] [Google Scholar]
- 19.Luo N, Su D, Jin G, Liu L, Zhu X, Xie D, et al. Apparent diffusion coefficient ratio between axillary lymph node with primary tumor to detect nodal metastasis in breast cancer patients. J Magn Reson Imaging 2013; 38: 824–8. doi: 10.1002/jmri.24031 [DOI] [PubMed] [Google Scholar]
- 20.Usuda K, Sagawa M, Motono N, Ueno M, Tanaka M, Machida Y, et al. Advantages of diffusion-weighted imaging over positron emission tomography-computed tomography in assessment of hilar and mediastinal lymph node in lung cancer. Ann Surg Oncol 2013; 20: 1676–83. doi: 10.1245/s10434-012-2799-z [DOI] [PubMed] [Google Scholar]
- 21.Sharma M, Bartlett E, Yu E. Metastatic retropharyngeal lymph nodes in nasopharyngeal carcinoma: imaging criteria. Expert Rev Anticancer Ther 2010; 10: 1703–6. doi: 10.1586/era.10.159 [DOI] [PubMed] [Google Scholar]
- 22.Kato H, Kanematsu M, Watanabe H, Mizuta K, Aoki M. Metastatic retropharyngeal lymph nodes: comparison of CT and MR imaging for diagnostic accuracy. Eur J Radiol 2014; 83: 1157–62. doi: 10.1016/j.ejrad.2014.02.027 [DOI] [PubMed] [Google Scholar]
- 23.Chen YB, Liao J, Xie R, Chen GL, Chen G. Discrimination of metastatic from hyperplastic pelvic lymph nodes in patients with cervical cancer by diffusion-weighted magnetic resonance imaging. Abdom Imaging 2011; 36: 102–9. doi: 10.1007/s00261-009-9590-z [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Liao Q, Zhang Y, Yu C, Bai R, Sun H. Differential diagnosis of axillary inflammatory and metastatic lymph nodes in rabbit models by using diffusion-weighted imaging: compared with conventional magnetic resonance imaging. Korean J Radiol 2012; 13: 458–66. doi: 10.3348/kjr.2012.13.4.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimoto K, Edamitsu O, Meno S, Abe T, Honda N, Ogoh Y, et al. MR diagnosis for metastasis or non-metastasis of mediastinal and hilar lymph nodes in cases of primary lung cancer: detectability, signal intensity, and MR-pathologic correlation. [In Japanese.] Jpn J Radiol 1995; 55: 162–71. [PubMed] [Google Scholar]
- 26.Liu Y, Liu H, Bai X, Ye Z, Sun H, Bai R, et al. Differentiation of metastatic from non-metastatic lymph nodes in patients with uterine cervical cancer using diffusion-weighted imaging. Gynecol Oncol 2011; 122: 19–24. doi: 10.1016/j.ygyno.2011.03.023 [DOI] [PubMed] [Google Scholar]