Abstract
Objectives:
To verify the use of a single coupling agent as a reference to obtain the elasticity index (EI) ratios and to investigate the EI ratios of the masseter muscles of healthy volunteers.
Methods:
Muscle phantoms with known elasticity (20, 40 and 60 kPa in the Young's modulus) were examined by strain-type sonoelastography using a coupling agent as the reference. Eight examiners tested soft (with 7 kPa) and hard (with 40 kpa) reference coupling agents separately. The correlation coefficients were determined between the EI ratio and Young's modulus of muscle phantoms. The interclass correlation coefficients were calculated for inter- and intraexaminer agreement.
Results:
Strong correlations were found between the EI ratios and Young's modulus for both soft and hard references. The variations of the EI ratios were larger with soft coupling agents than those with hard coupling agents, and they increased in phantoms with 60 kPa elasticity. There were no differences in the EI ratios of the masseter muscle at rest between males and females or between the right and left sides. The ratio increased during clenching.
Conclusions:
The hard reference coupling agent was suitable for obtaining EI ratio of the masseter muscle. No differences were found in the EI ratios of the masseter muscle either between sexes or between the right and left sides at rest, and the ratios increased with the widening of their variations during clenching.
Keywords: ultrasonography, elasticity imaging techniques, masseter muscle
Introduction
Hardness or tenderness of the skeletal muscle, which can be measured by a commercially available hardness metre, is known to be an effective index for evaluating patients with myalgia in the field of orthopaedics or sports medicine.1–4 Additionally, in cases of temporomandibular disorder with myofascial pain, the hardness of the masseter muscle is frequently found to be increased, according to the measurements obtained by a hardness metre.5–7 However, the hardness metre can measure only certain points, and the results might not reflect the total muscle change. In this regard, sonoelastography is superior to hardness metre because it can depict a wide area of the target muscle, showing an elasticity map superimposed onto a B-mode image.
Sonoelastography enables us to measure tissue elasticity as an index of muscle hardness.6,8–11 In a strain-type sonoelastography, the tissue displacement is measured under the external freehand compression with a sonographic probe, and the muscle strain is expressed as the elasticity index (EI), which was originally developed to be used in conjunction with the device applied in the present study. The EI is defined as the strain values of the target area compared with the average strain value of the whole area of interest (for which it is assigned a value of 1). When the strain values are smaller than the average, the EIs are assigned a value of 0–1, when larger, they are assigned a value of 1–6. According to the EIs measured, sonoelastography are expressed as colour-coded images superimposed on B-mode images. In the device used, the colours reflect the relative hardness of the tissues in ascending order from red, yellow, green to blue. Among the several weaknesses of strain-type sonoelastography described, the EIs are known to vary depending on the compression force. In our previous studies, therefore, the EI of subcutaneous fat tissue was used as a reference.6,9,10 The EI ratio of the masseter muscle was defined as the EI of the masseter muscle divided by that of the subcutaneous fat tissue. This method might be useful for the comparison of acquired values among examinations within a person, whereas it might not be suitable for comparing values between individuals or for obtaining the average value of a certain group because the strain values of subcutaneous fat would vary individually. Therefore, an acoustic coupling agent (also called a standoff pad), which is usually applied to the maxillofacial sonographic examinations, could potentially be a reference. Although several techniques have been proposed with the use of reference materials,1,2,12 they have been developed for elbow or leg muscles, and their application to the maxillofacial muscles, which are relatively small and have curved surfaces, is difficult.
The first aim of the present study was to verify experimentally the use of a single coupling agent as a reference for obtaining EI ratios. The second aim was to investigate the EI ratios of the masseter muscles of healthy volunteers.
Methods and materials
Phantom study
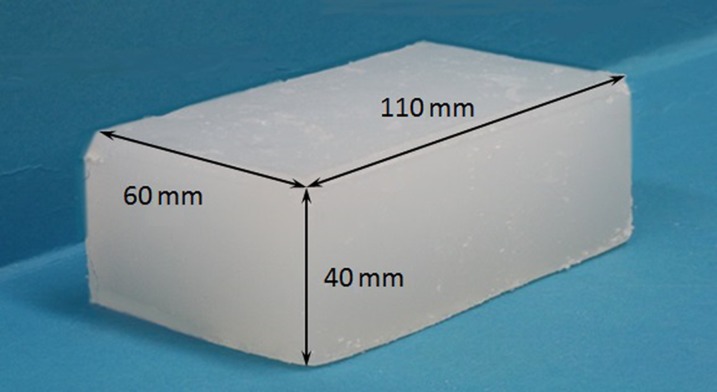
For the experimental study, the muscle phantoms with known elasticity, which were specially fabricated from a low-molecular-weight gel containing agar, talc and thickening agents, were provided by a company (OST Co., Ltd., Chiba, Japan). The phantoms were rectangular in shape (110, 60 and 40 mm in length, width and thickness, respectively) with three different elasticities (20, 40 and 60 kPa) expressed in the Young's modulus (Figure 1).
Figure 1.
Muscle phantom.
Two coupling agents with different elasticity (7 and 40 kPa), one of which was simultaneously used as a reference in examinations, were also fabricated from the same materials as the muscle phantoms (OST Co., Ltd.). They were 110, 30 and 5 mm in length, width and thickness, respectively. The coupling agents with 7 and 40 kPa elasticities were assigned as the soft reference and hard reference coupling agents, respectively.
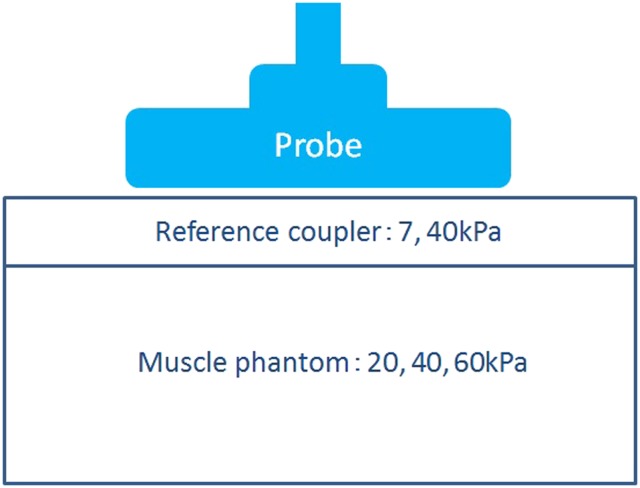
The phantoms were examined with a sonographic machine (Logiq E9; GE Healthcare, Tokyo, Japan) equipped with a 4.5–15 MHz wide-band width linear active matrix transducer (ML6-15-D). Sonograms were obtained using a multiple focus technique with a focal range of 0.5–2.0 cm and an image depth of 4.0 cm. The other settings were gain of 55 dB and dynamic range of 66 dB. A coupling was set on the phantom during the examinations, and an adequate compression pressure, which could be monitored on the display of the device, was given freehand through the probe (Figure 2).
Figure 2.
Acquisition method of the elastogram. A reference coupling is set on the muscle phantom, and an adequate compression pressure is given freehand through the probe.
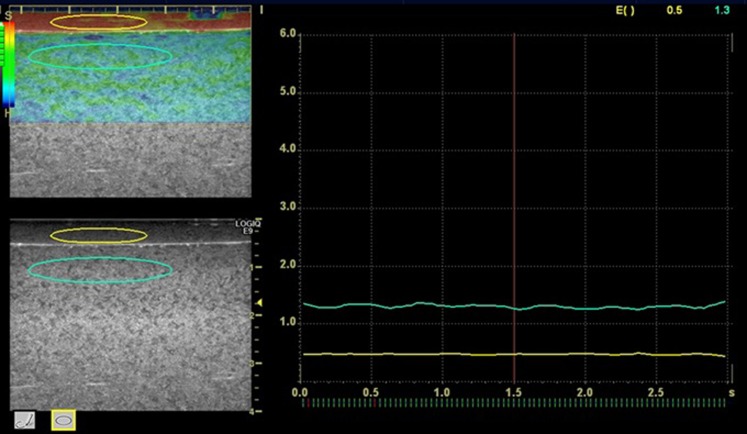
On the sonoelastography acquired, the regions of interest (ROIs) were set in the areas corresponding to the phantom and reference coupling agent, and the EIs were measured using the software Elasto Q Analysis (BT11; GE Healthcare, Tokyo, Japan) provided with the device. The ROIs were elliptical in shape and 3 × 20 and 5 × 30 mm in size for the reference and phantom, respectively (Figure 3). A preliminary test was performed regarding the stability of phantom EIs according to the location of ROIs. Two locations, just below the reference coupling agent and mid portion of the muscle phantoms, were compared. Consequently, the ROI was set just below the reference coupling agent because no difference was found between the locations tested. The EI ratio was calculated as the EI of the masseter phantom divided by the EI of the reference coupling agent. Five examiners with more than 3 years' experience in sonographic examination evaluated every phantom with each reference coupling agent twice. To confirm inter- and intraexaminer agreements, interclass correlation coefficients (ICCs) were calculated.
Figure 3.
Acquisition method of elasticity index. Elliptical regions of interest are set in the reference (3 × 20 mm) and the muscle phantom (5 × 30 mm).
Study with volunteers
The present study was performed with the approval of the ethics committee of Aichi-Gakuin University School of Dentistry, Nagoya, Japan (No. 217). 25 healthy volunteers (10 females and 15 males) who were sufficiently informed about the aim and method of the present study and who gave written consent to participate were enrolled in the study and underwent the described measurements. The mean age was 30.8 (±9.8) years, ranging from 20 to 53 years. All the volunteers had normal occlusions, with asymptomatic temporomandibular joints and masseter muscles, and had no pathologies in the maxillofacial regions. The participants lay supine on a bed and were instructed to turn their heads so that the side to be examined would be facing upwards and to relax their masseter muscle. The same sonographic device and software used for the phantom study was used to examine the volunteers. Both sides of the masseter muscle were scanned perpendicular to the anterior border of the muscle and to the surface of the underlying mandibular ramus at 15 mm above the inferior border of the mandible. Based on the result of the phantom study, the hard reference coupling agent was set on the volunteer's skin surface and used like an acoustic standoff pad in an ordinary sonographic examination. The scan was obtained at rest and while the volunteer clenched the mandible with the maximum force. The EI of the reference coupling agent and the masseter muscle were measured on sonoelastogram, and the EI ratios were calculated as the EI of the masseter muscle divided by the EI of the reference coupling agent.
Statistical analyses
Statistical analyses were performed using statistical analysis software (Stat View v. 4.0; Abacus Concept, Cary, NC). The Pearson correlation coefficient was applied to evaluate between the EI ratio and Young's modules. The values with 0 < r ≤ 0.2 were regarded as no correlation, 0.2 < r ≤ 0.4 indicated a weak correlation, 0.4 < r ≤ 0.7 indicated a correlation and r > 0.7 indicated a strong correlation. The Student's t-test was applied to evaluate the sex-related differences and laterality of the EI ratios. Inter- and intraexaminer reliability were evaluated with ICCs indicating poor (0–0.20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80) and excellent (0.80–1.00) agreements. All differences were regarded as significant for values of p < 0.05.
Results
Phantom study
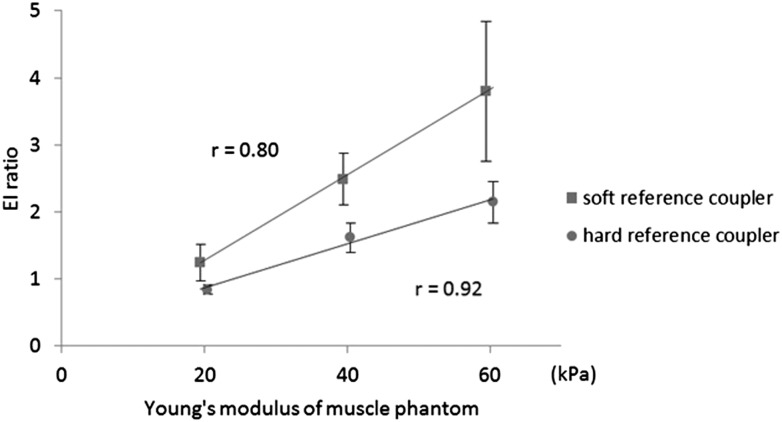
Strong correlations were found between Young's modules and EI ratios of the phantoms based on the calculations using both soft and hard reference coupling agents, with the correlation coefficients of 0.80 and 0.92, respectively. The variations of EI ratios calculated based on the soft coupling agent were larger than those on the hard coupling agent, especially in the hard phantom with 60-kPa elasticity (Figure 4).
Figure 4.
The relationship between the elasticity index (EI) ratio and the Young's modulus of the muscle phantom.
The ICCs for interexaminer agreement for two examinations were averaged as 0.81 and 0.89 for soft and hard reference couplings, respectively. The ICCs for intraexaminer agreement for five examiners were averaged as 0.74 and 0.86 for soft and hard reference couplings, respectively. Using the hard coupling, excellent agreements were obtained according to both the inter- and intraexaminer reliability.
Study with volunteers
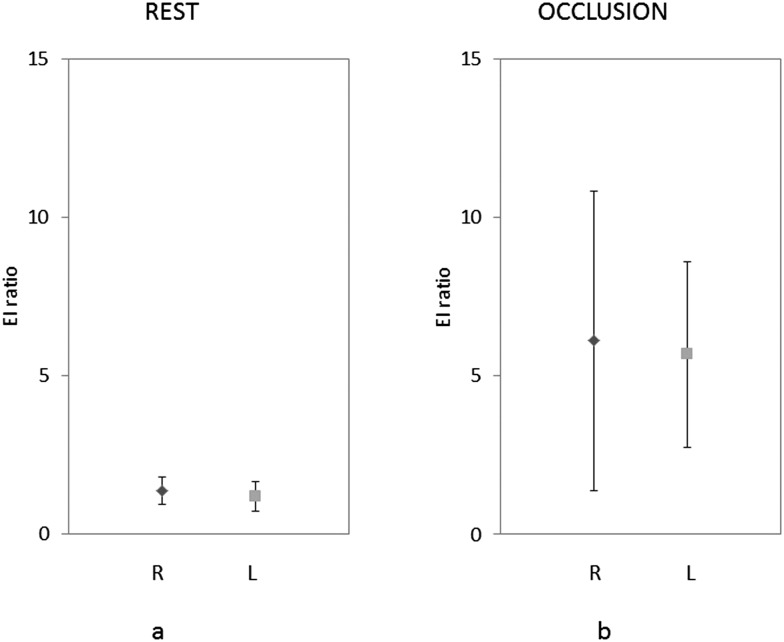
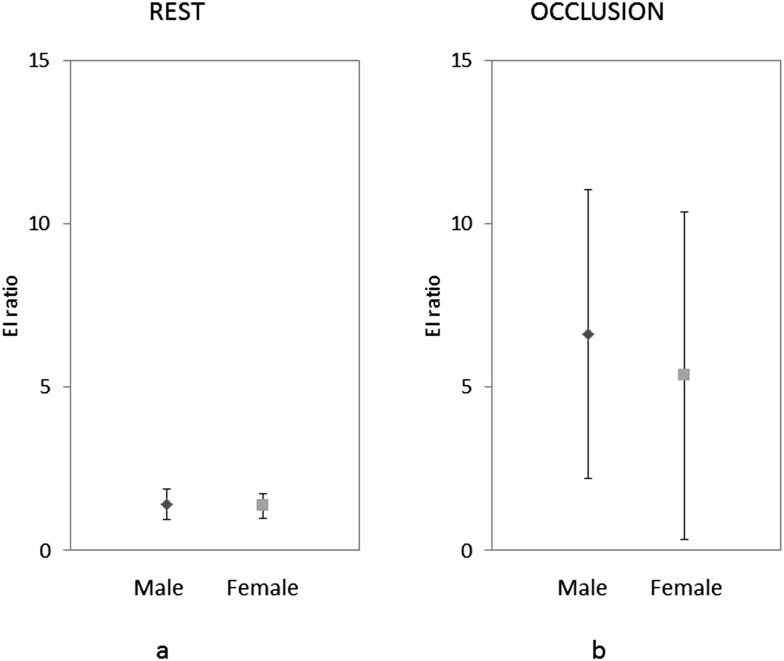
No significant differences were found in the EI ratios of the masseter muscle of healthy volunteers between males and females and between the right and left sides (Figure 5). However, there was a significant difference between the EI ratios at rest and maximum clenching positions. The EI ratios increased and showed wide variation with clenching (Figure 6).
Figure 5.
Elasticity index (EI) ratio of the right and left sides at rest (a) and occlusion with maximum clenching force (b). L, left side; R, right side.
Figure 6.
Elasticity index (EI) ratio of the right side in male and female at rest (a) and during occlusion with maximum clenching force (b).
Discussion
Although sonoelastography is known to be effective and is frequently used to obtain muscle elasticity measurements, a strain-type device with the use of freehand compression has some weaknesses. The compression force cannot be standardized, and the strain values obtained are relative values.6,10 To reduce these problems affecting the validity of strain values, subcutaneous fat6,8–10 or some materials1,2,12 are used as references. An experimental study clarified that the location of the reference areas did not affect the strain ratio, which corresponded to the EI ratio in the present study when the objective area was surrounded by a homogeneous structure in which the reference ROIs were set.13 This condition, however, is rarely found, especially in in vivo examinations of skeletal muscles. Although subcutaneous fat with enough thickness might be a suitable reference when the strain values (the EIs) can be measured and can be used for comparison of values of an individual, it is not adequate for comparing such values between individuals. Application of a commercially available standoff pad can be a potential reference material. However, they usually appear so lucent that the EIs cannot be measured.2 A material with enough strain values for measurement was applied to the evaluation of the elbow muscles before and after exercise in a previous study.2 However, this material was overly large for its application to the masseter muscle. Chino et al1 and Akagi et al14 described an interesting method to quantify the muscle hardness through the simultaneous use of two references with known elasticity. Their specially fabricated references had two different elasticities as expressed in the Young's modulus. They determined the average elasticity of the gastrocnemius muscle (GM) according to the Young's modulus, which resulted in approximately 30 kPa. In the initial trial, we followed and adapted their method to measure the masseter muscle elasticity. However, it was difficult to apply this method to the masseter muscle because it is a small and curved muscle. Therefore, we decided to use separately the references that they used simultaneously.
Strong correlations were found between the Young's modulus and the EI ratios of muscle phantoms using each reference coupling agent. Therefore, the resulting EI ratios could be compared among individuals or between the two or more values obtained at different times for the same individual when an identical reference coupling agent is applied to each measurement. The EI ratios showed relatively small variations when the hard reference coupling agent was applied to measurements. This was supported by the results of the inter- and intra-examiner agreements. The EI ratios varied widely in the phantom with high elasticity. However, these results could be verified within the range of the phantoms' elasticity, namely, from 20 to 60 kPa. As shown in previous reports,6,10 as well as in the present volunteer study, the elasticity of the masseter muscle increased during its contraction at the maximum force or after a continuous low-level contraction.15,16 In such situations, the muscle hardness surpassed that of the range. This relationship should be confirmed with phantoms harder than 60 kPa in a future study. Despite this limitation, the hard reference coupling agent appeared to be appropriate for the EI ratio acquisition, especially during the muscle contraction. Although the ROIs were set roughly just below the reference coupling agent with the same size in all measurements based on the preliminary test, it may be necessary to determine the location and size strictly as factors affecting the reliability of EI ratio.17,18 The elasticity of the reference coupling agent was observed to vary over time because of dehydration of its material. In this regard, this factor does not affect the results of the present study because it was performed in a period shorter than 3 months, which was the timeframe recommended by the manufacturer. However, this change should be investigated in a future study for its clinical application.
Skeletal muscle elasticity, which is often measured by MR elastography (MRE) or sonoelastography and is reported as the elastic modulus expressed in kilopascal, showed wide variations. In an MRE study, in which the elasticity was often reported in terms of the shear moduli instead of the Young's moduli, the GM hardness was reported to be 9.9 ± 6.8 kPa,19 while the hardness values of the vastus lateral and medial muscles were 3.73 ± 0.85 and 3.91 ± 1.15 kPa, respectively. A review by Ringleb et al20 showed that the reported hardness of the lateral GM varied from 9.9 to 22.0 kPa with MRE. Based on a shear-wave sonoelastography study by Arda et al,21 where the moduli used were probably the Young's moduli, the GM elasticity values were reported to be 11.4 ± 4.1 and 11.0 ± 4.0 kPa in males and females, respectively. Conversely, Chino et al1,3 stated that the hardness of the GM was approximately 30 kPa based on the Young's moduli by using strain-type sonoelastography. A member of the same research group reported a similar hardness of the GM using shear-wave type sonoelastography (27.6 ± 7.3 and 33.4 ± 6.3 kPa of the lateral and medial GM, respectively) based on the Young's modulus.14 Even after considering that the Young's moduli can be three-folds of the shear moduli,1,2,8 the variations were still relatively wide. There are several possible explanations for this discrepancy; anisotropic properties in the human skeletal muscle may be a cause in addition to the differences in modulus and devices used for the measurements.18,22–24 With the use of the MRE, the shear moduli measured in the direction parallel to the muscle fibre were greater than those measured in the direction perpendicular to the fibre, whereas the values themselves were very different from those reported in earlier studies.25 In the present study, therefore, we did not express the masseter muscle elasticity in kilopascal using the Young's moduli because the aim of present study was to verify the correlation between the EI ratios and the elasticity of the muscle phantoms. The use of EI ratio was considered sufficient to make comparisons of the hardness of the masseter muscle between individuals. However, if it would be expressed in kilopascal, the acquisition conditions, such as the reference elasticity and the kind of elastic moduli, should be clearly described together with the device used.
Regarding the masseter muscle, as far as we know, only one report expressed its elasticity as 10.4 ± 3.7 kPa probably using the Young's modulus and a shear-wave device.21 When the conversion curve, obtained based on the present phantom study with the hard reference coupling agent, was applied to the results of the volunteer study, the average elasticities were 38.25 and 89.21 kPa at rest and maximum contraction, respectively. A previous study did not find differences in hardness of healthy subjects based on sex.21 In our previous report,6 with the use of subcutaneous fat tissue as reference, the EI ratios of the masseter muscle were compared between sexes and between the right and left sides, and we found no differences in both comparisons. Ariji et al6 applied a muscle hardness metre, by which the hardness was expressed as newton per square metre at a point of the target muscle, to the masseter muscle in healthy volunteers. They did not find any sex-related or side differences either. Thus, the results of the present study support these results of earlier studies. At the maximum contraction, the elasticity of the masseter muscle increased and the variation became large. We consider that these variations are mainly attributed to the differences among individuals instead of measurement error generated during the use of the reference coupling agent given that the hard coupling agent used for these measurements presented small variations. Future studies should be conducted to clarify factors contributing the individual differences, such as age, sex, dentition and occlusion force, among others, and to compare them with the results obtained by shear-wave sonoelastography.
In conclusion, a hard coupling agent with 40 kPa elasticity appeared to be a suitable reference tool for obtaining the EI ratio of the masseter muscle. The EI ratios of the masseter muscle were not different between sexes or between the right and left sides at rest, and they increased during maximum contraction.
Acknowledgments
Acknowledgment
The authors thank all the volunteers who participated in the present study for their co-operation.
References
- 1.Chino K, Akagi R, Dohi M, Fukashiro S, Takahashi H. Reliability and validity of quantifying absolute muscle hardness using ultrasound elastography. PLoS One 2012; 7: e45764. doi: 10.1371/journal.pone.0045764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niitsu M, Michizaki A, Endo A, Takei H, Yanagisawa O. Muscle hardness measurement by using ultrasound elastography: a feasibility study. Acta Radiol 2011; 52: 99–105. doi: 10.1258/ar.2010.100190 [DOI] [PubMed] [Google Scholar]
- 3.Chino K, Akagi R, Dohi M, Takahashi H. Measurement of muscle architecture concurrently with muscle hardness using ultrasound strain elastography. Acta Radiol 2014; 55: 833–9. doi: 10.1177/0284185113507565 [DOI] [PubMed] [Google Scholar]
- 4.Shinohara M, Sabra K, Gennisson JL, Fink M, Tanter M. Real-time visualization of muscle stiffness distribution with ultrasound shear wave imaging during muscle contraction. Muscle Nerve 2010; 42: 438–41. doi: 10.1002/mus.21723 [DOI] [PubMed] [Google Scholar]
- 5.Kashima K, Igawa K, Maeda S. Sakoda S. Analysis of muscle hardness in patients with masticatory myofascial pain. J Oral Maxillofac Surg 2006; 64: 175–9. [DOI] [PubMed] [Google Scholar]
- 6.Ariji Y, Gotoh A, Hiraiwa Y, Kise Y, Nakayama M, Nishiyama W, et al. Sonographic elastography for evaluation of masseter muscle hardness. Oral Radiol 2013; 29: 64–9. [Google Scholar]
- 7.Hiraiwa Y, Ariji Y, Kise Y, Sakuma S, Kurita K, Ariji E. Efficacy of massage treatment technique in masseter muscle hardness: robotic experimental approach. Cranio 2013; 31: 291–9. [DOI] [PubMed] [Google Scholar]
- 8.Drakonaki EE, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol 2012; 85: 1435–45. doi: 10.1259/bjr/93042867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umemoto T, Ueno E, Matsumura T, Yamakawa M, Bando H, Mitake T, et al. Ex vivo and in vivo assessment of the non-linearity of elasticity properties of breast tissues for quantitative strain elastography. Ultrasound Med Biol 2014; 40: 1755–68. doi: 10.1016/j.ultrasmedbio.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 10.Gotoh A, Ariji Y, Hasegawa T, Nakayama M, Kise Y, Matsuoka M, et al. Sonographic elastography for assessing changes in masseter muscle elasticity after low-level static contraction. Oral Radiol 2013; 29: 140–5. [Google Scholar]
- 11.Ariji Y, Nakayama M, Nishiyama W, Ariji E. Applications of sonographic elastography to the oral and maxillofacial region. Sci Med 2014; 2: 1049. [Google Scholar]
- 12.Yanagisawa O, Niitsu M, Kurihara T, Fukubayashi T. Evaluation of human muscle hardness after dynamic exercise with ultrasound real-time tissue elastography: a feasibility study. Clin Radiol 2011; 66: 815–19. doi: 10.1016/j.crad.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 13.Imaizumi A, Sasaki Y, Sakamoto J, Kamio T, Nishikawa K, Otonari-Yamamoto M, et al. Effects of compression force on elasticity index and elasticity ratio in ultrasound elastography. Dentomaxillofac Radiol 2014; 43: 20130392. doi: 10.1259/dmfr.20130392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akagi R, Chino K, Dohi M, Takahashi H. Relationships between muscle size and hardness of the medial gastrocnemius at different ankle joint angles in young men. Acta Radiol 2012; 53: 307–11. doi: 10.1258/ar.2011.110481 [DOI] [PubMed] [Google Scholar]
- 15.Ariji Y, Nakayama M, Taguchi A, Gotoh A, Kise Y, Katsumata A, et al. Intramuscular changes of soft and hard areas after low-level static contraction of the masseter muscle and the correlations with muscle hardness and increase in water content: evaluations with sonographic elastography and magnetic resonance imaging. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116: 354–61. doi: 10.1016/j.oooo.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 16.Ariji Y, Katsumata A, Hiraiwa Y, Izumi M, Sakuma S, Shimizu M, et al. Masseter muscle sonographic features as indices for evaluating efficacy of massage treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110: 517–26. doi: 10.1016/j.tripleo.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 17.Kot BC, Zhang ZJ, Lee AW, Leung VY, Fu SN. Elastic modulus of muscle and tendon with shear wave ultrasound elastography : variations with different technical settings. PLoS One 2012; 7: e44348. doi: 10.137/joumal.pone.004348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muraki S, Fukumoto K, Fukuda O. Prediction of the muscle strength by the muscle thickness and hardness using ultrasound muscle hardness meter. Springerplus 2013; 2: 457. doi: 10.1186/2193-1801-2-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uffmann K, Maderwald S, Ajaj W, Galban CG, Mateiescu S, Quick HH, et al. In vivo elasticity measurements of extremity skeletal muscle with MR elastography. NMR Biomed 2004; 17: 181–90. [DOI] [PubMed] [Google Scholar]
- 20.Ringleb SI, Bensamoun SF, Chen Q, Manduca A, An KN, Ehman RL. Applications of magnetic resonance elastography to healthy and pathologic skeletal muscle. J Magn Reson Imaging 2007; 25: 301–9. [DOI] [PubMed] [Google Scholar]
- 21.Arda K, Ciledag N, Aktas E, Aribas BK, Köse K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol 2011; 197: 532–6. doi: 10.2214/AJR.10.5449 [DOI] [PubMed] [Google Scholar]
- 22.Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat 2010; 23: 497–511. doi: 10.1002/ca.21006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akagi R, Takahashi H. Acute effect of static stretching on hardness of the gastrocnemius muscle. Med Sci Sports Exerc 2013; 45: 1348–54. doi: 10.1249/MSS.0b013e3182850e17 [DOI] [PubMed] [Google Scholar]
- 24.Akagi R, Takahashi H. Effect of a 5-week static stretching program on hardness of the gastrocnemius muscle. Scand J Med Sci Sports 2014; 24: 950–7. doi: 10.1111/sms.12111 [DOI] [PubMed] [Google Scholar]
- 25.Green MA, Sinkus R, Gandevia SC, Herbert RD, Bilston LE. Measuring changes in muscle stiffness after eccentric exercise using elastography. NMR Biomed 2012; 25: 852–8. doi: 10.1002/nbm.1801 [DOI] [PubMed] [Google Scholar]