Abstract
Purpose
The purpose of this study is to evaluate the role of regular postoperative surveillance to improve the prognosis of patients with breast cancer after curative surgery.
Materials and Methods
We retrospectively analyzed the medical records of 4,119 patients who received curative surgery for breast cancer at Samsung Medical Center between January 2000 and September 2008. Patients were divided into two groups (group I, regular postoperative surveillance; group II, control group) according to their post-therapy follow-up status for the first 5 years after surgery.
Results
Among the 3,770 patients selected for inclusion, groups I and II contained 3,300 (87%) and 470 (13%) patients, respectively. The recurrence rates at 5 years for groups I and II were 10.6% and 16.4%, respectively (hazard ratio, 0.85; 95% confidence interval [CI], 0.67 to 1.09; p=0.197). The 10-year mortality cumulative rates were 8.8% for group I and 25.4% for group II (hazard ratio, 0.28; 95% CI, 0.22 to 0.35; p < 0.001). In multivariate analysis for recurrence-free survival (RFS), age over 40 years (p < 0.001), histologic grade 1 (p < 0.001), and pathologic stage I (p < 0.001) were associated with longer RFS but not with follow-up status. Multivariate analysis for overall survival (OS) revealed that patients in group I showed significantly improved OS (hazard ratio, 0.29; 95% CI, 0.23 to 0.37; p < 0.001). Additionally, age over 40 years, histologic grade I, and pathologic stage I were independent prognostic factors for OS.
Conclusion
Regular follow-up for patients with breast cancer after primary surgery resulted in clinically significant improvements in patient OS.
Keywords: Breast neoplasms, Epidemiology, Recurrence
Introduction
Regular follow-up of patients after primary therapy for breast cancer is common practice. However, the best follow-up strategy for patients with breast cancer remains controversial. Statistically, within 5 years, approximately 30% of all patients treated with curative intent exhibit recurrence of disease [1]. Moreover, 60%-80% of all recurrences occur in the first 3 years of follow-up treatment [2]. For these reasons, many clinicians still have regular follow-up policies. Regular follow-up is considered to be effective if recurrences are detected at an early stage and also if the immediate treatment of recurrences offers a higher probability of cure or improved survival. Although regular follow-up is generally assumed to be beneficial, this hypothesis has not yet been proven by scientific evidence. Several retrospective studies have investigated the effects of extensive versus limited follow-up procedures; these studies have concluded that routine surveillance for the early detection of breast cancer does not improve the chances of survival [3-6]. Only two randomized controlled trials have attempted to address the importance of follow-up frequency [7,8]. However, these trials were insufficiently powered and were thus unable to provide clear answers regarding the relationship between follow-up frequency and survival. Therefore, we set out to identify the impact of regular postoperative surveillance on the survival of patients with early breast cancer. In contrast to many previous studies, our study also incorporated a large sample size and a long follow-up duration.
Materials and Methods
1. Study design and patients
A total of 4,119 patients who had received curative surgery for stage I-III invasive breast cancer between 2000 and 2008 were retrospectively reviewed from the breast cancer database of Samsung Medical Center (SMC). We excluded patients with stage IV disease at diagnosis, patients with carcinoma in situ, and patients who received neoadjuvant chemotherapy. After application of our exclusion criteria, a total of 3,770 patients were included in this study (Fig. 1). Management of all patients was based on the National Comprehensive Cancer Network guidelines. To detect local or distant recurrence, clinical follow-up was carried out every 3-6 months for the first 5 years after primary therapy and annually thereafter. Clinical follow-up included history-taking; physical examinations; laboratory tests, including carcinoembryonic antigen, cancer antigen 15-3, complete blood counts, and liver function tests; chest radiography; mammography; breast and abdominopelvic ultrasonography; and bone scans. In addition, a computed tomography (CT) scan, a magnetic resonance imaging (MRI), or a fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET)/CT scan was carried out if necessary.
Fig. 1.
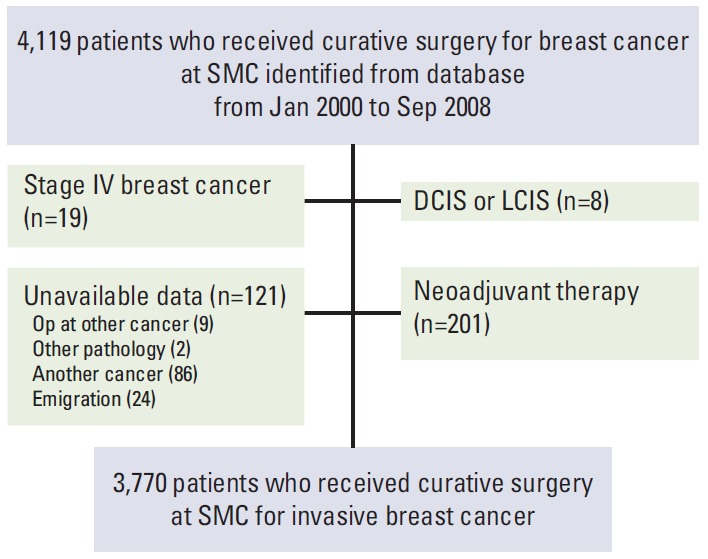
Patient cohort. SMC, Samsung Medical Center; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ; Op, operation.
2. Data collection
To obtain clinical data, the electronic medical records of all patients were reviewed. Clinical data gathered included patient characteristics and tumor subtype according to the status of immunohistochemistry (IHC) for estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor type 2 (HER2). ER and PgR positivity were defined as Allred scores of 3-8 by IHC using anti-ER antibodies (Novocastra, Newcastle upon Tyne, UK) and anti-PgR antibodies (Novocastra), respectively. HER2 status was evaluated using anti-HER2 antibodies (DAKO, Glostrup, Denmark) and/or fluorescence in situ hybridization (FISH). Grades 0 and 1 for HER2 by IHC were defined as negative; grade 3 was defined as positive. For patients classified as HER2 2+ by IHC, FISH was performed to confirm HER2 amplification. Triple negativity was defined as a lack of ER, PgR, and HER2 expression. To evaluate the effects of regular follow-up on survival, patients were divided into two groups according to their follow-up status. Patients in group I received regular follow-up for the first 5 years or until the first day of recurrence during the 5 years after curative surgery, whereas patients in group II did not receive regular follow-up.
3. Statistical analysis
Recurrence-free survival (RFS) was calculated as the time from the date of curative surgery to the first radiologic or clinical observation of disease recurrence. Recurrences were defined as locoregional evidence, contralateral recurrence, or distant metastasis. Seventy-five patients (16%) of group II revisited after recurrence of disease. In order to assess RFS of patients who had not followed-up since surgery in our institute, we defined RFS from date of curative surgery to the last visit day to our clinic (censored). Overall survival (OS) was defined as the time from curative surgery to death from any cause. A total of 3,770 patients were analyzed for OS using the survival data from the Office for National Statistics. Survival was estimated with Kaplan-Meier methodology and is expressed as a mean value with a range and a two-sided 95% confidence interval (CI). A two-sided log-rank test was used to compare survival between the two groups. Cox proportional hazards regression models were used to identify independent factors associated with RFS or OS. A forest plot was used to compare the overall hazard ratio with the hazard ratios obtained in the subgroups, defined on the basis of several potentially prognostic factors. p-values for interaction were obtained from a Cox regression analysis. All analysis was carried out using SPSS ver. 20.0 (IBM Co., Armonk, NY).
Results
1. Baseline patient characteristics
The general characteristics of the study population are summarized in Table 1.
Table 1.
Characteristics according to routine follow-up status (n=3,770 patients)
| Characteristic | Group I (n=3,300, 87%) | Group II (n=470, 13%) | p-value (χ2 test) |
|---|---|---|---|
| Age (mean±SD, yr) | 48±10 | 51±13 | 0.008 |
| ER and PgR status (n=3,749) | 0.013 | ||
| ER and/or PgR positive | 2,312 (70) | 299 (65) | |
| ER and PgR negative | 974 (30) | 164 (35) | |
| HER2+ (n=3,728) | 0.672 | ||
| Positive | 696 (21) | 103 (22) | |
| Negative | 2,570 (79) | 359 (78) | |
| TNBC (ER-/PR-/HER2-) (n=3,745) | 0.072 | ||
| Yes | 585 (18) | 99 (21) | |
| No | 2,697 (82) | 364 (79) | |
| Type of surgery (n=3,770) | 0.945 | ||
| MRM | 1,459 (44) | 207 (44) | |
| BCS | 1,841 (56) | 263 (56) | |
| Histopathologic type (n=3,770) | 0.691 | ||
| IDC | 2,756 (84) | 389 (83) | |
| Others | 544 (16) | 81 (17) | |
| Nuclear grade (n=3,631) | 0.110 | ||
| I | 459 (14) | 61 (13) | |
| II | 1,427 (45) | 185 (41) | |
| III | 1,291 (41) | 208 (46) | |
| Histologic grade (n=3,417) | 0.141 | ||
| I | 584 (20) | 80 (19) | |
| II | 1,304 (43) | 162 (39) | |
| III | 1,113 (37) | 174 (42) | |
| Tumor size (n=3,769) | 0.396 | ||
| T1 | 1,913 (58) | 264 (56) | |
| T2 | 1,263 (38) | 187 (40) | |
| T3 | 113 (3) | 16 ⑶ | |
| T4 | 10 (0) | 3 (0) | |
| Nodal status (n=3,768) | 0.646 | ||
| N0 | 2,002 (61) | 297 (63) | |
| N1 | 867 (26) | 115 (25) | |
| N2 | 274 (8) | 39 (8) | |
| N3 | 156 (5) | 18 ⑷ | |
| Pathologic stage (n=3,770) | 0.831 | ||
| I | 1,362 (41) | 195 (42) | |
| II | 1,469 (45) | 213 (45) | |
| III | 469 (14) | 62 (13) | |
| Adjuvant chemotherapy (n=3,769) | < 0.001 | ||
| Done | 2,647 (80) | 264 (56) | |
| None | 653 (20) | 205 (44) | |
| Adjuvant radiotherapy (n=3,769) | < 0.001 | ||
| Done | 2,285 (69) | 226 (48) | |
| None | 1,015 (31) | 243 (52) | |
| Adjuvant hormone therapy (n=3,769) | < 0.001 | ||
| Done | 2,261 (69) | 217 (46) | |
| None | 1,039 (31) | 252 (54) |
SD, standard deviation; ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TNBC, triple negative breast cancer; MRM, modified radical mastectomy; BCS, breast conserving surgery; IDC, invasive ductal carcinoma.
Among the 3,770 patients included in the study, 3,300 (87%) were classified in the regular follow-up group (group I) for the first 5 years. The remaining 470 patients were lost for follow-up after adjuvant treatments following surgery; these patients had all also been treated for metastatic breast cancer at SMC (group II). Compared with the patients in group II, the patients in group I were younger (48 years vs. 51 years, p=0.008), had a more favorable hormone status (ER and/or PgR positive, p=0.013), and had received more adjuvant therapy (adjuvant chemotherapy, p < 0.001; adjuvant radiotherapy, p < 0.001; adjuvant hormone therapy, p < 0.001). All other factors, including HER2 status, presence of triple negative breast cancer, type of surgery, pathologic type, nuclear grade, histologic grade, tumor size, nodal status, and pathologic stage were not significantly different between the two groups.
2. Survival outcomes
The median follow-up duration of the study was 7.1 years (range, 0 to 13.7 years). During the follow-up period of the 3,770 patients, recurrence occurred in 571 patients (15%): locoregional recurrence in 62 patients (11%), contralateral recurrence in 67 patients (12%), and distant metastasis in 442 patients (77%). Death occurred in 352 patients (9%). RFS at 5 years was 88%. It was particularly noteworthy that the rate of distant metastasis was significantly higher in group II compared with group I (93% vs. 75%, p=0.003).
The 5-year recurrence rates for groups I and II were 10.6% and 16.4%, respectively. No significant benefit in RFS was noted for group I over group II (hazard ratio, 0.85; 95% CI, 0.67 to 1.09; p=0.197) (Fig. 2A). On the other hand, the 10-year mortality cumulative rates (8.8% for group I and 25.4% for group II) revealed that regular follow-up provided a significant benefit to patient survival (hazard ratio, 0.28; 95% CI, 0.22 to 0.35; p < 0.001) (Fig. 2B). Among the patients with recurrence, median OS was 11 months in group I and 4 months in group II (hazard ratio, 0.19; 95% CI, 0.14 to 0.25; p < 0.001) (Fig. 2C). Clinical and pathological features such as age > 40 years, histologic grade I, pathologic stage I, and hormone receptor (HR) status (HR+/HER2–) were also associated with good prognosis for both RFS and OS (Appendices 1 and 2). Multivariate analysis revealed that patients in group I showed significantly better OS (hazard ratio, 0.29; 95% CI, 0.23 to 0.37; p < 0.001), which was associated with a 70% reduction in the risk of death; however, this clinical benefit did not translate into a benefit for RFS (hazard ratio, 0.88; 95% CI, 0.68 to 1.14; p=0.329). Instead, the risk of recurrence and death correlated with age, histologic grade, and pathologic stage. In contrast to the univariate analysis results, HR status (HR+/HER2–) was not an independent prognostic factor (Tables 2 and 3).
Fig. 2.
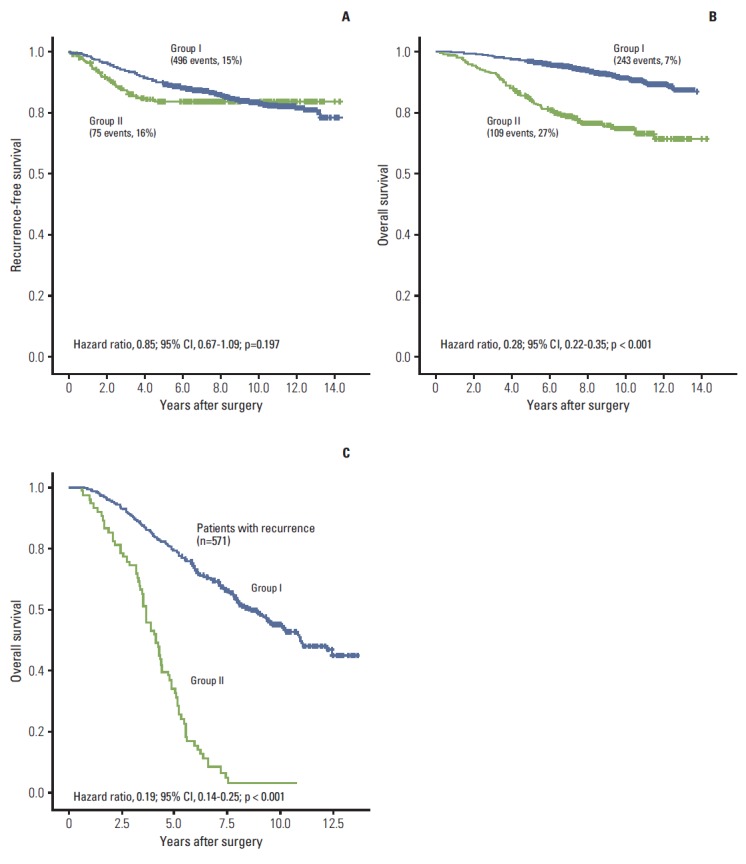
Kaplan-Meier curves for recurrence-free survival (A), overall survival (B), and overall survival for patients with recurrence (C) according to follow-up status. CI, confidence interval.
Table 2.
Cox-regression multivariate analysis for relapse
| Variable | Significance (p-value) | Hazard ratio | 95% CI for Exp(B) |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Regular follow-up for 5 yr | 0.329 | 0.88 | 0.68 | 1.14 |
| Age > 40 yr | < 0.001 | 0.58 | 0.48 | 0.70 |
| Histologic grade I (vs. II/III) | < 0.001 | 0.44 | 0.32 | 0.62 |
| Staging I (vs. II/III) | < 0.001 | 0.51 | 0.41 | 0.63 |
| HR+ and HER2- (vs. the others) | 0.156 | 0.88 | 0.74 | 1.05 |
CI, confidence interval; HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Table 3.
Cox-regression multivariate analysis for overall survival
| Variable | Significance (p-value) | Hazard ratio | 95% CI for Exp(B) |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Routine follow-up for 5 yr | < 0.001 | 0.29 | 0.23 | 0.37 |
| Age > 40 yr | 0.001 | 0.67 | 0.52 | 0.85 |
| Histologic grade I (vs. II/III) | < 0.001 | 0.23 | 0.13 | 0.42 |
| Staging I (vs. II/III) | < 0.001 | 0.44 | 0.33 | 0.58 |
| HR+ and HER2- (vs. the others) | 0.105 | 0.83 | 0.66 | 1.04 |
CI, confidence interval; HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
3. Subgroup analysis of OS
We also investigated whether any patient subgroup reaped a greater benefit from regular follow-up. However, OS benefits seemed to be consistent across all clinical subgroups, irrespective of age, hormone status, pathologic type, histologic grade, and pathologic stage (Fig. 3).
Fig. 3.
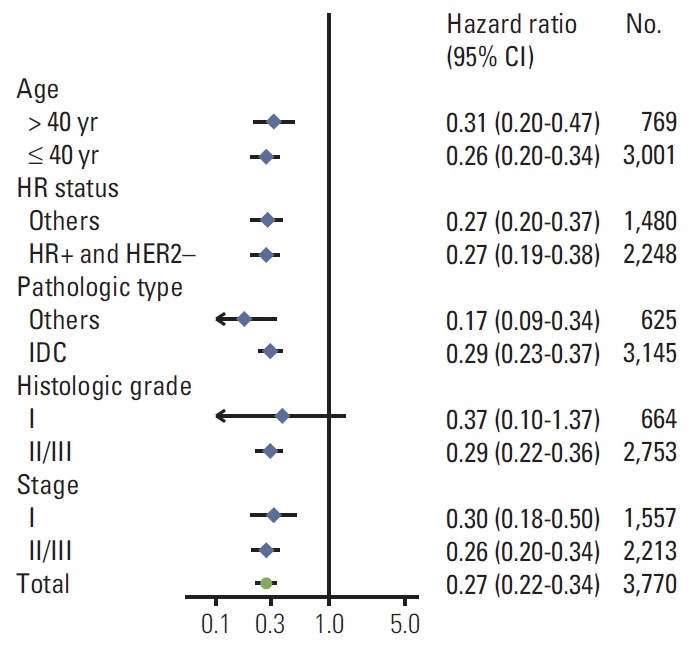
Subgroup analysis of overall survival. CI, confidence interval; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; IDC, invasive ductal carcinoma.
Discussion
One of the important goals of surveillance is improvement of patient survival. However, the precise effect of early detection of curable recurrence on OS remains questionable, and controversy persists regarding the benefits of regular follow-up. This retrospective study shows that regular follow-up improves the OS of patients with breast cancer who received curative surgery.
Routine surveillance for the early detection of breast cancer recurrence has been suggested to not actually improve patient chances of survival [9-11]. One meta-analysis estimated that 40% of all isolated locoregional recurrences were diagnosed in asymptomatic patients during routine visits. Unfortunately, none of these studies have investigated the relationship between the survival benefit and the way in which the recurrence was diagnosed [12]. On the other hand, another recent meta-analysis demonstrated that early detection of recurrence by routine follow-up does improve the survival of patients with breast cancer [13]. A similar effect was seen in this study. The 10-year mortality cumulative rates were 8.8% for the regular follow-up group and 25.4% for the group whose patients had been lost for follow-up. Survival analysis using the Cox proportional hazards method suggested that survival is better when patients receive regular follow-up examinations (hazard ratio, 0.29; 95% CI, 0.23 to 0.37; p < 0.001).
Most previous reports have not been able to demonstrate the effectiveness of routine regular follow-up using a risk stratification model for recurrence. It is true that not all patients have the same risk of developing recurrence after primary treatment of breast cancer [14,15]. We found that young age, a higher tumor stage, and a higher tumor grade were all independent risk factors for recurrence (p ≤ 0.001). Consequently, regular surveillance of these groups of patients would likely be more effective, mainly due to the increased risk of recurrence in these groups. However, the overall benefit of regular follow-up was maintained across all subgroups.
Our study does have some limitations which restrict the conclusions that can be drawn from it. Firstly, our study was a retrospective single center cohort study. Thus, selection bias could be a confounding variable. Secondly, the patients in group II might have poor compliance; if so, comparing group I with group II may be an inappropriate way to evaluate the effect of regular surveillance on long-term outcomes after or during adjuvant endocrine treatment. For example, fewer patients in group II received adjuvant treatments than in group I, as shown in Table 1. Thus, the worse outcome in group II compared with group I, in terms of OS, may be closely related to the number of patients receiving treatment during the adjuvant systemic treatment period. Thirdly, no plausible explanation can describe the discrepancy between RFS and OS observed in this study (Fig. 2). Crossed Kaplan-Meier survival curves in RFS may imply that late recurrence beyond 5 years does not correlate with long-term survival; however, this hypothesis needs to be better defined. Fourthly, this study could not analyze whether different frequencies of visits to clinic would be of particular value in survival outcomes. Lastly, our study could not evaluate the relationship between surveillance method and recurrence for patients with breast cancer following surgery.
Nonetheless, we focused on survival according to regular follow-up status, rather than on survival according to symptom status at the time of recurrence. This is one strength of the present study, since it avoids a time bias (slow-growing tumors are preferentially detected early since they are detectable for a longer time). Additionally, we avoided lead-time bias by calculating survival as the interval from the curative surgery to death.
Our study was not able to verify whether stratified surveillance according to different risks of recurrence could improve long-term clinical outcomes. Several studies have concluded that early detection of local disease recurrence requires both clinical examination and mammography [16,17]. However, no evidence has yet shown that intensive surveillance programs that include tumor marker assessments, bone scans, CT scans, MRI scans, FDG-PET/CT scans, or ultrasounds in asymptomatic patients confer any survival advantage [6,18]. Thus, future prospective comparative studies on cost-effective strategies for the follow-up of patients after primary treatment are warranted.
Conclusion
Our findings indicate that regular surveillance may have a beneficial impact on survival for patients with breast cancer.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1234).
Appendix 1. Cox-regression univariate analysis for relapse
| Variable | Significance (p-value) | Hazard ratio | 95% CI for Exp(B) |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Routine follow-up for 5 yr | 0.197 | 0.85 | 0.67 | 1.09 |
| Age > 40 yr | < 0.001 | 0.55 | 0.46 | 0.66 |
| Histologic grade I (vs. II/III) | < 0.001 | 0.34 | 0.24 | 0.46 |
| Staging I (vs. II/III) | < 0.001 | 0.44 | 0.37 | 0.54 |
| HR+ and HER2- (vs. the others) | < 0.001 | 0.70 | 0.59 | 0.82 |
CI, confidence interval; HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Appendix 2. Cox-regression univariate analysis for overall survival
| Variable | Significance (p-value) | Hazard ratio | 95% CI for Exp(B) | |
|---|---|---|---|---|
| Lower | Upper | |||
| Routine follow-up for 5 yr | < 0.001 | 0.28 | 0.22 | 0.35 |
| Age > 40 yr | < 0.001 | 0.62 | 0.49 | 0.78 |
| Histologic grade I (vs. II/III) | < 0.001 | 0.17 | 0.09 | 0.29 |
| Staging I (vs. II/III) | < 0.001 | 0.36 | 0.28 | 0.47 |
| HR+ and HER2- (vs. others) | < 0.001 | 0.59 | 0.48 | 0.73 |
CI, confidence interval; HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Jacobs HJ, van Dijck JA, de Kleijn EM, Kiemeney LA, Verbeek AL. Routine follow-up examinations in breast cancer patients have minimal impact on life expectancy: a simulation study. Ann Oncol. 2001;12:1107–13. doi: 10.1023/a:1011624829512. [DOI] [PubMed] [Google Scholar]
- 2.Schapira DV, Urban N. A minimalist policy for breast cancer surveillance. JAMA. 1991;265:380–2. [PubMed] [Google Scholar]
- 3.The GIVIO Investigators Impact of follow-up testing on survival and health-related quality of life in breast cancer patients: a multicenter randomized controlled trial. JAMA. 1994;271:1587–92. doi: 10.1001/jama.1994.03510440047031. [DOI] [PubMed] [Google Scholar]
- 4.Joseph E, Hyacinthe M, Lyman GH, Busch C, Demps L, Reintgen DS, et al. Evaluation of an intensive strategy for follow-up and surveillance of primary breast cancer. Ann Surg Oncol. 1998;5:522–8. doi: 10.1007/BF02303645. [DOI] [PubMed] [Google Scholar]
- 5.Palli D, Russo A, Saieva C, Ciatto S, Rosselli Del Turco M, Distante V, et al. Intensive vs clinical follow-up after treatment of primary breast cancer: 10-year update of a randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA. 1999;281:1586. doi: 10.1001/jama.281.17.1586. [DOI] [PubMed] [Google Scholar]
- 6.Rosselli Del Turco M, Palli D, Cariddi A, Ciatto S, Pacini P, Distante V. Intensive diagnostic follow-up after treatment of primary breast cancer: a randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA. 1994;271:1593–7. doi: 10.1001/jama.271.20.1593. [DOI] [PubMed] [Google Scholar]
- 7.Gulliford T, Opomu M, Wilson E, Hanham I, Epstein R. Popularity of less frequent follow up for breast cancer in randomised study: initial findings from the hotline study. BMJ. 1997;314:174–7. doi: 10.1136/bmj.314.7075.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokko R, Hakama M, Holli K. Follow-up cost of breast cancer patients with localized disease after primary treatment: a randomized trial. Breast Cancer Res Treat. 2005;93:255–60. doi: 10.1007/s10549-005-5199-2. [DOI] [PubMed] [Google Scholar]
- 9.Churn M, Kelly V. Outpatient follow-up after treatment for early breast cancer: updated results after 5 years. Clin Oncol (R Coll Radiol) 2001;13:187–94. doi: 10.1053/clon.2001.9251. [DOI] [PubMed] [Google Scholar]
- 10.Grosse A, Schreer I, Frischbier HJ, Maass H, Loening T, Bahnsen J. Results of breast conserving therapy for early breast cancer and the role of mammographic follow-up. Int J Radiat Oncol Biol Phys. 1997;38:761–7. doi: 10.1016/s0360-3016(97)00062-x. [DOI] [PubMed] [Google Scholar]
- 11.te Boekhorst DS, Peer NG, van der Sluis RF, Wobbes T, Ruers TJ. Periodic follow-up after breast cancer and the effect on survival. Eur J Surg. 2001;167:490–6. doi: 10.1080/110241501316914849. [DOI] [PubMed] [Google Scholar]
- 12.de Bock GH, Bonnema J, van der Hage J, Kievit J, van de Velde CJ. Effectiveness of routine visits and routine tests in detecting isolated locoregional recurrences after treatment for early-stage invasive breast cancer: a meta-analysis and systematic review. J Clin Oncol. 2004;22:4010–8. doi: 10.1200/JCO.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 13.Lu WL, Jansen L, Post WJ, Bonnema J, Van de Velde JC, De Bock GH. Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: a meta-analysis. Breast Cancer Res Treat. 2009;114:403–12. doi: 10.1007/s10549-008-0023-4. [DOI] [PubMed] [Google Scholar]
- 14.Elkhuizen PH, van de Vijver MJ, Hermans J, Zonderland HM, van de Velde CJ, Leer JW, et al. Local recurrence after breast-conserving therapy for invasive breast cancer: high incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys. 1998;40:859–67. doi: 10.1016/s0360-3016(97)00917-6. [DOI] [PubMed] [Google Scholar]
- 15.Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. Breast Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. J Clin Oncol. 2001;19:1688–97. doi: 10.1200/JCO.2001.19.6.1688. [DOI] [PubMed] [Google Scholar]
- 16.Mellink WA, Holland R, Hendriks JH, Peeters PH, Rutgers EJ, van Daal WA. The contribution of routine follow-up mammography to an early detection of asynchronous contralateral breast cancer. Cancer. 1991;67:1844–8. doi: 10.1002/1097-0142(19910401)67:7<1844::aid-cncr2820670705>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 17.Voogd AC, van Tienhoven G, Peterse HL, Crommelin MA, Rutgers EJ, van de Velde CJ, et al. Local recurrence after breast conservation therapy for early stage breast carcinoma: detection, treatment, and outcome in 266 patients. Dutch Study Group on Local Recurrence after Breast Conservation (BORST) Cancer. 1999;85:437–46. doi: 10.1002/(sici)1097-0142(19990115)85:2<437::aid-cncr23>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Rojas MP, Telaro E, Russo A, Moschetti I, Coe L, Fossati R, et al. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2005;(1):CD001768. doi: 10.1002/14651858.CD001768.pub2. [DOI] [PubMed] [Google Scholar]


