Abstract
Purpose
Apurinic/apyrimidinic endonuclease 1/redox factor-1 (APE1/Ref-1) is a multifunctional protein that shows elevated expression in a number of cancers. We attempted to determine whether serum APE1/Ref-1 is elevated in patients with bladder cancer.
Materials and Methods
Serum APE1/Ref-1 levels were determined using enzyme-linked immunosorbent assay in serum from patients with bladder cancer who had not received chemotherapy or radiotherapy (n=51) and non-tumor controls (n=55). The area under the receiver operating characteristic area under the curve was applied to determine the correlation between clinical factors and the serum levels of APE1/Ref-1.
Results
Serum levels of APE1/Ref-1 in bladder cancer patients were significantly elevated compared to those of the control group (3.548±0.333 ng/100 μL [n=51] for bladder cancer vs. 1.547±0.319 ng/100 μL [n=55] for the control group), with a sensitivity and specificity of 93% and 59%, respectively. Serum APE1/Ref-1 levels are associated with tumor stage, grade, muscle invasion, and recurrence.
Conclusion
Serum APE1/Ref-1 might be useful as a potential serologic biomarker for bladder cancer.
Keywords: Apurinic/apyrimidinic endonuclease 1/redox factor-1, Urinary bladder neoplasms, Biological markers, Enzyme-linked immunosorbent assay
Introduction
Bladder cancer is the second most common malignancy of all genitourinary tumors in the United States [1] and Korea [2]. Recurrence of bladder cancer is common, and is reported to occur in 50%-70% of cases, and approximately 10%-15% of patients will experience disease progression [3]. Detection of bladder cancer when it is still at an early stage is important for effective treatment, and currently the standard approach in detection of bladder cancer is urethrocystoscopy and urine cytology [3,4]. However, due to its invasiveness and cost, cystoscopy is unsuitable for screening. Serial cystoscopies may cause discomfort and distress to patients for life-long surveillance. Urine cytology has a reasonable sensitivity for detection of high-grade bladder cancer; however, it is restricted in its capacity for detection of low-grade urothelial carcinoma, with sensitivity and specificity of 8.5% and 50%, respectively [5,6]. Unfortunately, biomarkers for bladder cancer that can provide reliable information about diagnosis, progression, and surveillance have not been clearly established. Development of a bladder tumor biomarker with high sensitivity and specificity would be a useful adjunct tool for detection or follow-up of cases of bladder cancer.
A bladder cancer usually originates from cells lining the bladder—transitional cells, which could be exposed to several cytokines during chronic inflammation and DNA damage [7,8]. The urinary bladder is a sac which acts as a reservoir containing metabolites, waste products, and several releasing proteins from the cells. Interestingly, it was reported that redox regulating and DNA repair protein such as Apurinic/apyrimidinic endonuclease 1/redox factor-1 (APE1/Ref-1) was increased with occurrence of cancer [8,9]. APE1/Ref-1 was secreted into the bloodstream in response to inflammatory signals [10,11].
APE1/Ref-1 is a multifunctional protein involved in both base excision DNA repair and transcriptional regulation [12]. There is growing evidence linking heterogeneous APE1/Ref-1 expression to a wide range of pathological conditions, including metabolic and differentiation disorders such as cancers [13]. APE1/Ref-1 is mainly localized in the nucleus, but cytoplasmic and mixed nuclear/cytoplasmic localization of APE1/Ref-1 has been reported in several tumor types [9,14]. Active secretion of APE1/Ref-1 into the circulation also occurs in response to lipopolysaccharides [10] or intracellular acetylation [11], so that it could act as a serologic biomarker. APE1/Ref-1 secretion from cells is also supported by the presence of auto-antibody against APE1/Ref-1 in blood of lung cancer patients [15].
Because tumor biomarkers may originate from tumors or adjacent tissue in response to tumor growth, we hypothesized that serum APE1/Ref-1 may serve as a biomarker for bladder cancer. Therefore, the aim of the current study was to determine whether serum APE1/Ref-1 levels are elevated in bladder cancer patients.
Materials and Methods
1. Study population
Samples were obtained from 106 consecutive patients. All analyses were performed within 6 months of collection. The study groups were classified as either non-cancer controls (n=55), consisting of individuals with no evidence of malignancy, or patients with operable bladder cancer (n=51). All samples were obtained from the archives of the Department of Urology, Chungnam National University from 2010-2011. This study was approved by the Chungnam National University Hospital Institutional Review Board, and all participants signed informed consent forms. In the control group, we included patients with benign prostate hyperplasia (n=22), a urinary tract stone (n=18), trauma (n=11), and urethral stricture (n=4). We excluded patients with chronic or recurrent urinary tract infections, unevaluated gross hematuria, and malignancy. In the bladder cancer group, postoperative histological confirmation of urothelial cell carcinoma, including grade and stage, was recorded. No chemotherapy or radiotherapy was administered prior to surgery. Thirty-eight patients underwent transurethral resection of bladder cancer and 13 patients underwent radical or partial cystectomy. Blood (8-10 mL) was drawn from an antecubital vein in patients with bladder cancer or healthy control subjects using a 22-G needle and placed in a vacuum tube. Serum was separated by centrifugation at 3,000 rpm for 10 minutes at room temperature, and then re-centrifuged at 5,000 rpm for 5 minutes to obtain cell-free serum, which was stored in liquid nitrogen until use.
The clinical and pathological characteristics of the bladder cancer subjects, which were divided according to nonmuscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC), are shown in Table 1. Clinical staging of bladder cancer was performed using cystoscopy, computed tomography, bone scanning, and simple chest radiography. All tumor tissues were analyzed by a single genitourinary pathologist according to a standardized protocol. Pathological staging was recorded in accordance with the 2003 TNM classification and the tumors were assigned a grade according to the World Health Organization classification [16,17]. Tumors were thus classified as Ta (noninvasive papillary carcinoma), T1 (invasive up to the subepithelial connective tissue), T2 (invading the muscle), T3 (invading the perivesical fat), and T4 (invading an adjacent organ). Tumors were also graded based on the likelihood of recurrence and progression into grade I (tumors may recur but have only a low risk of progression), grade II (tumors are more likely to recur and progress compared with grade I), and grade III (tumors are very likely to recur and progress). Bladder cancer patients were followed-up, and cystoscopy and urine cytology were performed every 3 months for the first year, every 6 months for the second year, and annually thereafter. Recurrence was defined as transitional cell carcinomas with lower or equivalent pathologic stage after treatment. For MIBC patients who had undergone partial cystectomy or radiation treatment, reappearance of bladder cancer including Ta and carcinoma in situ was considered recurrence.
Table 1.
Clinico-pathological characteristics of patients with bladder cancer
| Chatacteristic | Control | Bladder cancer |
||
|---|---|---|---|---|
| NMIBC | MIBC | Total | ||
| No. | 55 | 38 | 13 | 51 |
| Age (mean) | 58.7 | 68.4 | 64.1 | 67.3 |
| Gender | ||||
| Male | 53 (96.4) | 36 (94.7) | 11 (84.6) | 47 (92.2) |
| Female | 2 (3.6) | 2 (5.2) | 2 (15.4) | 4 (7.8) |
| Median follow-up (mo) | 26.5 | 31.7 | 24.5 | 27.4 |
| Median tumor size (cm) | NA | 2.2 | 3.9 | 2.8 |
| No. of tumor multiplicities | NA | 19 (50.0) | 6 (46.1) | 25 (49.0) |
| Tumor stage | NA | |||
| Ta | 21 (55.3) | - | 21 (41.2) | |
| T1 | 17 (44.7) | - | 17 (33.3) | |
| T2 | - | 10 (76.9) | 10 (19.6) | |
| T3-4 | - | 3 (23.1) | 3 (5.9) | |
| Tumor grade I | NA | |||
| I | 16 (42.1) | 0 | 16 (31.4) | |
| II | 10 (26.3) | 2 (15.4) | 12 (23.5) | |
| III | 12 (31.6) | 11 (84.6) | 23 (45.1) | |
| No recurrence | NA | 21 (55.3) | 10 (76.9) | 31 (60.8) |
| Recurrence | 17 (44.7) | 3 (23.1) | 20 (39.2) | |
Values are presented as number (%). NMIBC, non muscle invasive bladder cancer; MIBC, muscle invasive bladder cancer; NA, non-applicable.
2. Serum APE1/Ref-1 measurements
A sandwich enzyme-linked immunosorbent assay (ELISA) was used to quantify serum APE1/Ref-1 levels. Briefly, 96-microwell plates (Nunc, Penfield, NY) were precoated overnight with 100 μL of a 1:1,000 dilution of a rabbit anti-APE1/Ref-1 antibody (Abcam, Cambridge, UK) in coating buffer (0.5 M carbonate buffer, pH 9.6) in each well. After blocking with blocking buffer (5% bovine serum albumin in phosphate buffered saline [PBS] containing 0.05% Tween 20 [PBS-T]) at room temperature for 60 minutes, 100 μL of sample was added to the wells and the plates were incubated at 4°C for 90 minutes, and then washed five times with PBS-T. This was followed by addition of 100 μL of a 1:1,000 dilution of mouse anti-APE1/Ref-1 antibody (Abcam), and further incubation at room temperature for 2 hours. The plate was then washed seven times with PBS-T, and 100 μL of horseradish peroxidase–conjugated secondary antibody (1:5,000) was added, followed by incubation at room temperature for 30 minutes. After further washing, 100 μL of freshly prepared tetramethyl benzidine substrate was added to the wells. The color development reaction was stopped by addition of 100 μL of 2.5 M H2SO4, and the absorbance was measured at 450 nm using an automatic microtiter plate reader (Sunrise Xfluor 4, Tecan Systems Inc., San Jose, CA). Each sample was assayed in duplicate, and mean values were determined. To establish a standard curve, purified recombinant human APE1/Ref-1 (1 mg/mL) was serially diluted (5-fold) and used in a concentration series from 0.16-20 ng/100 μL.
3. Preparation of recombinant human APE1/Ref-1 (rhAPE1/Ref-1)
Human full length APE1/Ref-1 DNA was inserted into the pET28b expression vector (Novagen, Gibbstown, NJ), containing a 6-histidine tag for easy purification [10]. pET28b-APE1/Ref-1 plasmids were then transformed into the BL21(DE3) strain of Escherichia coli. Following induction with isopropyl β-D-1-thiogalactopyranoside (IPTG), the cells were sonicated in lysis buffer (100 mM NaCl, 20 mM HEPES), and the recombinant protein was purified on a nickel-nitrilotriacetic acid agarose column (Qiagen, Valencia, CA). After washing, the isolated APE1/Ref-1 protein was eluted with 250 mM imidazole buffer followed by desalting on a PD-10 column (Amersham Pharmacia Biotech, Liverpool, UK) in PBS, and frozen in 10% glycerol at −80°C. This protein was subsequently used for the standard curve in the APE1/Ref-1 ELISA.
4. Immunohistochemical staining
Imunohistochemical staining of APE1/Ref-1 was determined in paraffin-embedded sections of bladder tissues, which were stored in the tissue bank of Chungnam National Hospital. Tissue specimens were cut into 4-μm sections from randomly selected tumor and non-tumor blocks. Distant, apparently normal tissue samples from the same patient were used as the non-tumor blocks. A monoclonal antibody against human APE1/Ref-1 was used at a 1:600 dilution (Novus Biological, Littleton, CO). Staining was developed using 3, 3′diaminobenzidine (DAKO, Carpinteria, CA). The sections were lightly counterstained with hematoxylin and mounted using Immu-Mount (Thermo Shandon, Midland, Canada). Negative control slides were incubated with the mouse IgG1 negative control reagent provided with the kit for evaluation of nonspecific staining.
5. Immunoblotting
Tumor tissues from bladder cancer patients were obtained from the tissue bank of Chungnam National Hospital. Distant, apparently normal tissue samples from the same patient were used as the non-tumor tissues. Tissues were suspended in PBS containing protease and phosphatase inhibitors, cut into small pieces, and homogenized using a polytron. The homogenates were centrifuged at 14,000 rpm for 40 minutes and the resultant supernatant fractions were used for immunoblotting. The proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane. After blocking with 5% skimmed milk powder, the blots were incubated with anti APE1/Ref-1 antibody (1:1,000, Abcam). Immunoreactive bands were visualized by enhanced chemiluminescence. Each membrane was stripped and re-probed with an anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody to ensure equivalent protein loading. Immunoblotting for each protein was performed at least twice using independently prepared lysates. Changes in protein levels were quantified by densitometric scanning of the immunoreactive band and normalized against GAPDH loading control.
6. Statistical analysis
Values are expressed as the mean±SE. A statistical evaluation was performed using a one-way analysis of variance and post-hoc analysis. SPSS ver. 18.0 (SPSS Inc., Chicago, IL) was used for evaluation of the data. Differences were considered statistically significant if the null hypothesis could be rejected with > 95% confidence interval (p < 0.05). Nonparametric receiver operating characteristic (ROC) curves were generated by plotting the sensitivity value against the false-positive rate (1–specificity).We assessed the potential predictive value of APE1/Ref-1 for bladder cancer as well specific cancer stages and grades, and recurrence, by calculating the area under the curve (AUC). We estimated the sensitivity and specificity of APE1/Ref-1 at the optimal cutoff value (Youden index) to maximize the sum of sensitivity and specificity [18].
Results
1. Serum APE1/Ref-1 levels are elevated in bladder cancer patients
Serum levels of APE1/Ref-1 in bladder cancer patients were significantly elevated compared to those of the control group (3.548±0.333 ng/100 μL [n=51] for bladder cancer vs. 1.547±0.319 ng/100 μL [n=55] for the control group) (Fig. 1A). The US National Cancer Institute recommends use of ROC curve for evaluation of the performance of potential cancer detection markers [19]. Serum samples from 55 non-cancer controls and 51 patients with bladder cancer were used for the ROC curve analysis to determine the sensitivity and specificity of APE1/Ref-1 in detection of bladder cancer. The ROC curve for all tumors resulted in an AUC of 0.824 (95% confidence interval, 0.74 to 0.90) (Fig. 1B).
Fig. 1.
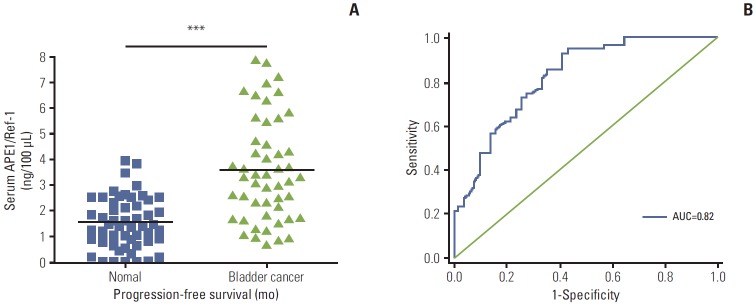
Serum apurinic/apyrimidinic endonuclease 1/redox factor-1 (APE1/Ref-1) expression is elevated in bladder cancer. (A) Serum APE1/Ref-1 was assayed using an enzyme-linked immunosorbent assay. The results are presented as a scatter plot. Each dot represents one patient (n=55 for non-cancer controls, n=51 for bladder cancer). ***p < 0.01 (compared with the control group). (B) Receiver operating curves of APE1/Ref-1 in bladder cancer detection. The area under curve (AUC) for detection of all cancer by APE1/Ref-1 was 0.82.
The ROC is drawn through points representing different decision cut-off values. The range of sensitivity and specificity at these different values was 0.51-0.95 and 0.51-0.86, respectively (Table 2). The sensitivity and specificity of APE1/Ref-1 were estimated at the optimal cutoff value in order to maximize the sum of sensitivity and specificity [18]. On this basis, the optimal combination of sensitivity and specificity were determined to be 0.93 and 0.59, using a cut-off value of 2.83 ng/100 μL (Table 2).
Table 2.
Receiver operating characteristic analysis of APE1/Ref-1 measurements in patients with bladder cancer
| Cutoff (ng/100 μL) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| 1.39 | 51 | 86 | 79 | 64 |
| 1.46 | 55 | 86 | 80 | 65 |
| 1.55 | 56 | 84 | 78 | 66 |
| 1.67 | 62 | 80 | 76 | 68 |
| 1.76 | 64 | 76 | 73 | 68 |
| 1.90 | 67 | 75 | 73 | 69 |
| 1.99 | 69 | 75 | 73 | 71 |
| 2.07 | 71 | 75 | 74 | 72 |
| 2.17 | 73 | 73 | 73 | 73 |
| 2.31 | 75 | 71 | 72 | 74 |
| 2.39 | 76 | 67 | 70 | 74 |
| 2.51 | 84 | 65 | 70 | 80 |
| 2.55 | 85 | 61 | 69 | 81 |
| 2.69 | 91 | 59 | 69 | 87 |
| 2.83 | 93 | 59 | 69 | 89 |
| 2.92 | 93 | 57 | 68 | 89 |
| 3.02 | 95 | 55 | 68 | 91 |
| 3.10 | 95 | 53 | 67 | 91 |
| 3.22 | 95 | 51 | 66 | 90 |
APE1/Ref-1, apurinic/apyrimidinic endonuclease 1/redox factor-1; PPV, positive predictive values; NPV, negative predictive values.
2. Serum APE1/Ref-1 levels are associated with tumor grade, stage, muscle invasion, and recurrence
The grade of bladder cancer provides important prognostic information and can help guide treatment, and is based on the degree of cellular abnormality. We found that serum APE1/Ref-1 levels were associated with the grade of bladder cancer (Fig. 2A). In particular, the mean values of serum APE1/Ref-1 levels of patients with grade II and III bladder cancer (2.719±0.465 ng/100 μL for grade II, n=12; 4.891±0.389 ng/100 μL for grade III, n=23) were significantly greater than those of the control group (1.547±0.319 ng/100 μL, n=55). The ROC-AUC for grade I, grade II, and grade III cancers were 0.70, 0.71, and 0.96, respectively (Fig. 2B), suggesting that serum APE1/Ref-1 may serve as a predictor of tumor grade.
Fig. 2.
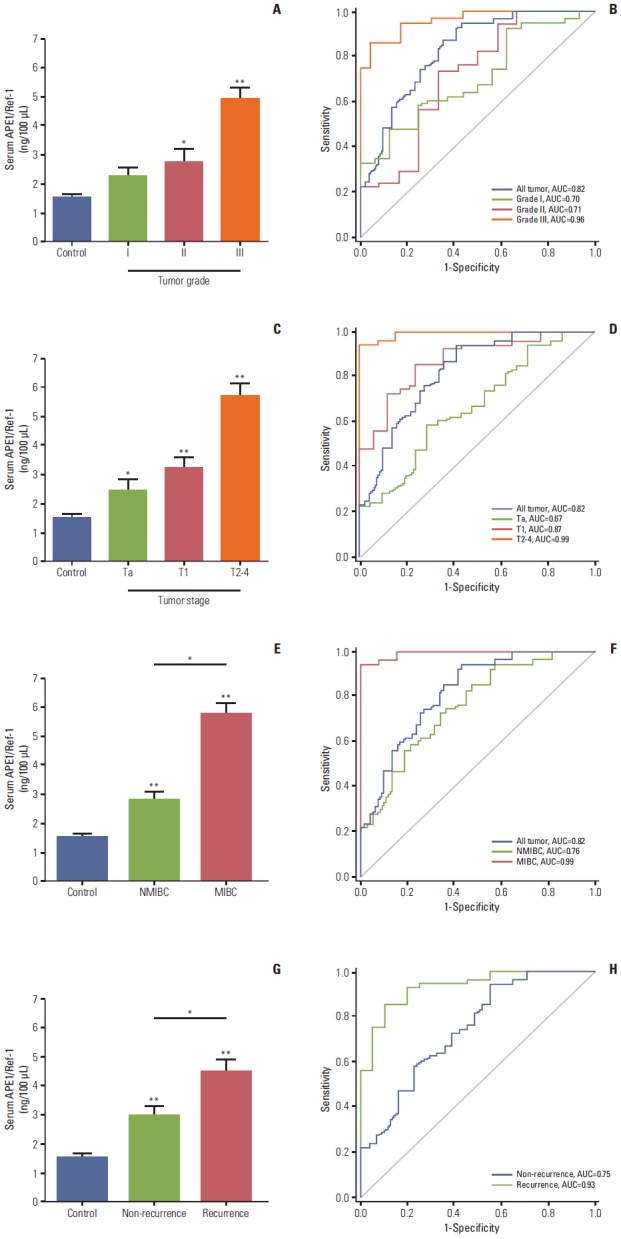
Serum apurinic/apyrimidinic endonuclease1/redox factor-1 (APE1/Ref-1) levels are associated with bladder tumor grade, stage, muscle invasion, and recurrence. Serum APE1/Ref-1 levels were assayed using an enzyme-linked immunosorbent assay. (A) Each bar shows the mean±standard error (SE) (n=16 for grade I, n=12 for grade II, n=23 for grade III, and n=55 for non-cancer controls). *p < 0.05 vs. control, **p < 0.01 vs. control. (B) Receiver operating curves for APE1/Ref-1 detection of different bladder tumor grades. (C) Serum APE1/Ref-1 levels are elevated in patients with higher stage tumors. Each bar shows the mean±SE (n=21 for stage Ta, n=17 for stage T1, n=13 for stage T2-3, and n=55 for non-cancer controls). *p < 0.05 vs. control, **p < 0.01 vs. control. (D) Receiver operating curves for APE1/Ref-1 detection of different bladder tumor stages. (E) Serum APE1/Ref-1 levels are higher in patients with muscle invasive bladder cancer. Each bar shows the mean±SE (n=38 for nonmuscle invasive bladder cancer [NMIBC], n=13 for muscle invasive bladder cancer [MIBC], and n=55 for non-cancer controls). *p < 0.05, **p < 0.01 vs. control. (F) Receiver operating curves for APE1/Ref-1 detection of NMIBC and MIBC. (G) Serum APE1/Ref-1 levels are higher in patients with recurrent tumors. Each bar shows the mean±SE (n=31 for non-recurrence, n=20 for recurrence, and n=55 for non-cancer controls). *p < 0.05, **p < 0.01 vs. control. (H) Receiver operating curves for APE1/Ref-1 detection of recurrent bladder tumors.
In addition to grade, bladder cancer is also staged by depth of invasion into the bladder wall. Thus, we attempted to determine whether serum APE1/Ref-1 is also associated with the tumor stage (Fig. 2C). Serum APE1/Ref-1 levels were significantly elevated among patients with Ta (2.450±0.365 ng/100 μL), T1 (3.251±0.305 ng/100 μL), and T2-T3 (5.711±0.437 ng/100 μL) bladder tumors compared to the control groups. The ROC AUC for Ta, T1, and T2-T4 were 0.67, 0.87, and 0.99, respectively (Fig. 2D). In addition, we evaluated the change of serum APE1/Ref-1 in NMIBC and MIBC. Serum APE1/Ref-1 levels in MIBC (5.711±0.437 ng/100 μL) were significantly elevated, compared with NMIBC (2.801±0.249 ng/100 μL) (Fig. 2E). The ROC-AUC for NMIBC and MIBC were 0.76 and 0.99, respectively (Fig. 2F).
Next, we attempted to determine whether serum APE1/Ref-1 levels were also elevated in bladder cancer recurrence. Among the 51 patients with bladder cancer, 20 patients (39%) had recurrent bladder cancer. The mean serum APE1/Ref-1 level titer in these patients was (4.480±0.430 ng/100 μL), significantly greater than in patients with non-recurrent tumors (2.940±0.324 ng/100 μL) (Fig. 2F). In results of ROC analysis, the AUC for non-recurrent tumors was 0.75, and the AUC for recurrent tumors was 0.93 (Fig. 2G), suggesting a high predictive value of serum APE1/Ref-1 for recurrent bladder tumors. Serum APE1/Ref-1 levels in patients with multifocal tumors did not differ significantly from those who had single tumors (data not shown).
3. APE1/Ref-1 is up-regulated in bladder cancer
To determine whether APE1/Ref-1 protein was differentially expressed in bladder cancer, immunoblotting for APE1/Ref-1 was performed in non-tumor tissues and tumor tissues of bladder cancer (Fig. 3A). Differences in protein level were quantified by densitometric scanning of the immunoreactive bands and normalized against the GAPDH loading control. This revealed that APE1/Ref-1 protein was expressed at a far higher level in tumor tissue (T) compared to non-tumor tissue as normal (N) (Fig. 3A). APE1/Ref-1 protein expression in tumor and non-tumor tissues of bladder cancer was visualized using immunohistochemistry (Fig. 3B). APE1/Ref-1 in non-tumor tissues and tumor tissues were mainly localized in the nucleus. However, nuclear expression of APE1/Ref-1 was significantly increased in tumor tissues. In addition, mononuclear cells including monocytes and lymphocytes were infiltrated close to tumor regions.
Fig. 3.
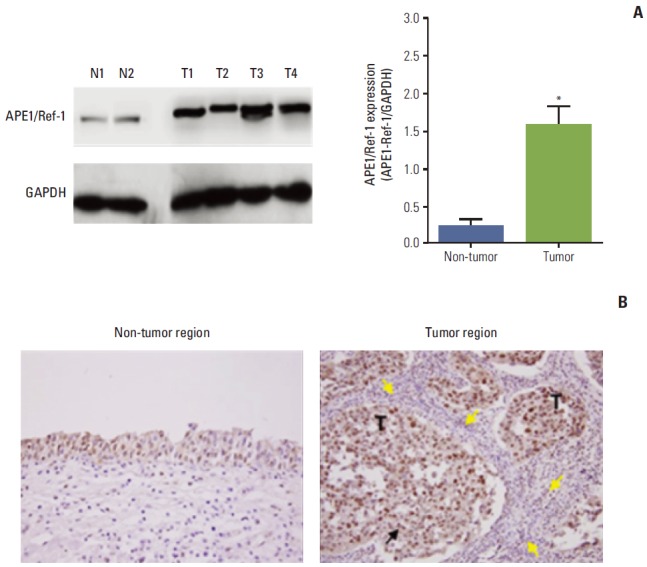
Apurinic/apyrimidinic endonuclease1/redox factor-1 (APE1/Ref-1) expression is elevated in bladder cancer. (A) Representative immunoblot for APE1/Ref-1 in bladder tissues of bladder cancer patients. N, non-tumor tissue as normal tissue; T, tumor tissue. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Each bar shows the mean±standard error (n=2 for non-tumor, n=4 for tumor tissues). *p < 0.05 vs. non-tumor regions. (B) Immunohistochemical staining for APE1/Ref-1 in bladder cancer. Positive cells are stained brown (black arrow). APE1/Ref-1 expression was clearly elevated in the tumor region, compared with non-tumor regions of bladder tissues. Mononuclear cells were infiltrated close to tumor regions (yellow arrows) (✕400). T, tumor cells.
Discussion
In this report, we describe a new potential diagnostic marker for bladder cancer. In clinical practice, the differential diagnosis of bladder cancer grade using non-invasive techniques is difficult. Our study demonstrates for the first time that APE1/Ref-1 protein is detectable in the serum of patients with bladder cancer, and is associated with tumor stage, grade, and recurrence.
The underlying mechanism for serum APE1/Ref-1 existence was completely uncovered. In the current study, our data showed increased expression of APE1/Ref-1 in tumor tissues and mononuclear cell infiltration close to tumor tissues. APE1/Ref-1 can be secreted from tumor cells itself and its secretion can also be evoked by cellular signaling between tumor and inflammatory cells. A previous study showed that intense inflammation occurs in patients with invasive bladder carcinoma, and it is likely that metalloproteinase-9 is derived from inflammatory cells infiltrating tumors [20]. In addition, nitric oxide (NO) is elevated in the urine of patients with bladder cancer. NO influences the subcellular localization of APE1/Ref-1 by nitrosation, and may similarly lead to extracellular translocation of APE1/Ref-1 [21]. In previous reports we showed that APE1/Ref-1 was secreted into the circulation in response to lipopolysaccharide, which can induce NO and tumor necrosis factor α production [10,11]. Thus, the release of APE1/Ref-1 by tumor cells into the circulation might be a result of tumor-mediated inflammation.
For measurement of secretory APE1/Ref-1 into blood, we developed a new ELISA assay and obtained ROC curve by applying to human serum samples. The ROC curve is used to evaluate the usefulness of a biomarker for classifying disease status. We used ROC curve based on the Youden index, the sum of sensitivity and specificity minus one, for setting an optimal cut-point for use in diagnostic testing [18]. A cut-off APE1/Ref-1 value of 2.83 ng/100 μL resulted in a sensitivity of 93% and a specificity of 59%. In the current study, we showed that increased serum APE1/Ref-1 levels are associated with bladder cancer severity, suggesting that it could act as a potential serologic biomarker for bladder cancer.
In studies of nuclear maxtrix protein 22 (NMP-22) as a urinary marker of bladder cancer, the results vary widely depending on the reporting group: values of 47%-100% for sensitivity and 58%-91% for specificity with this test have been previously reported [22]. In a recent prospective test for NMP-22 test, the outcomes were affected by hematuria, inflammation, and urine creatinine concentration. They concluded that NMP-22 assessment alone cannot be recommended for bladder cancer screening in a high-risk group nor as a reliable alternative to cystoscopy during follow-up [23]. Urine cytology is a more widely accepted noninvasive tumor-specific marker for bladder cancer. However, despite being highly specific for carcinoma in situ and high-grade urothelial carcinoma, it has a relatively low sensitivity for low grade carcinoma [24]. The majority of bladder cancers were diagnosed low grade and stage tumors, the relative value of cytology is limited.
Conclusion
Collectively, we have demonstrated that APE1/Ref-1 protein is detectable in the serum of patients with bladder cancer, and its level is associated with tumor stage and recurrence. Our findings provide a good foundation for development of a new serological biomarker for bladder cancer.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2011-0006231) and the INNOPOLIS Foundation grant funded by the Korean government (No. M2013DD001).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–9. [PubMed] [Google Scholar]
- 4.Vrooman OP, Witjes JA. Molecular markers for detection, surveillance and prognostication of bladder cancer. Int J Urol. 2009;16:234–43. doi: 10.1111/j.1442-2042.2008.02225.x. [DOI] [PubMed] [Google Scholar]
- 5.Raab SS, Grzybicki DM, Vrbin CM, Geisinger KR. Urine cytology discrepancies: frequency, causes, and outcomes. Am J Clin Pathol. 2007;127:946–53. doi: 10.1309/XUVXFXMFPL7TELCE. [DOI] [PubMed] [Google Scholar]
- 6.Brimo F, Vollmer RT, Case B, Aprikian A, Kassouf W, Auger M. Accuracy of urine cytology and the significance of an atypical category. Am J Clin Pathol. 2009;132:785–93. doi: 10.1309/AJCPPRZLG9KT9AXL. [DOI] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep. 2012;32:1–15. doi: 10.1042/BSR20100136. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Wang J, Xiang D, Wang D, Xin X. Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE1/Ref-1) in human ovarian cancer and indentification of the therapeutic potential of APE1/Ref-1 inhibitor. Int J Oncol. 2009;35:1069–79. [PubMed] [Google Scholar]
- 10.Park MS, Lee YR, Choi S, Joo HK, Cho EJ, Kim CS, et al. Identification of plasma APE1/Ref-1 in lipopolysaccharide-induced endotoxemic rats: implication of serological biomarker for an endotoxemia. Biochem Biophys Res Commun. 2013;435:621–6. doi: 10.1016/j.bbrc.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Choi S, Lee YR, Park MS, Joo HK, Cho EJ, Kim HS, et al. Histone deacetylases inhibitor trichostatin A modulates the extracellular release of APE1/Ref-1. Biochem Biophys Res Commun. 2013;435:403–7. doi: 10.1016/j.bbrc.2013.04.101. [DOI] [PubMed] [Google Scholar]
- 12.Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005;7:367–84. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 13.Tell G, Fantini D, Quadrifoglio F. Understanding different functions of mammalian AP endonuclease (APE1) as a promising tool for cancer treatment. Cell Mol Life Sci. 2010;67:3589. doi: 10.1007/s00018-010-0486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puglisi F, Barbone F, Tell G, Aprile G, Pertoldi B, Raiti C, et al. Prognostic role of Ape/Ref-1 subcellular expression in stage I-III breast carcinomas. Oncol Rep. 2002;9:11–7. [PubMed] [Google Scholar]
- 15.Dai N, Cao XJ, Li MX, Qing Y, Liao L, Lu XF, et al. Serum APE1 autoantibodies: a novel potential tumor marker and predictor of chemotherapeutic efficacy in non-small cell lung cancer. PLoS One. 2013;8: doi: 10.1371/journal.pone.0058001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13–5. [PubMed] [Google Scholar]
- 17.Helpap B. New WHO classification of urothelial carcinoma of the urinary bladder. Verh Dtsch Ges Pathol. 2002;86:57–66. [PubMed] [Google Scholar]
- 18.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–72. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 19.German RR, Fink AK, Heron M, Stewart SL, Johnson CJ, Finch JL, et al. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011;35:126–31. doi: 10.1016/j.canep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Offersen BV, Knap MM, Horsman MR, Verheijen J, Hanemaaijer R, Overgaard J. Matrix metalloproteinase-9 measured in urine from bladder cancer patients is an independent prognostic marker of poor survival. Acta Oncol. 2010;49:1283–7. doi: 10.3109/0284186X.2010.509109. [DOI] [PubMed] [Google Scholar]
- 21.Qu J, Liu GH, Huang B, Chen C. Nitric oxide controls nuclear export of APE1/Ref-1 through S-nitrosation of cysteines 93 and 310. Nucleic Acids Res. 2007;35:2522–32. doi: 10.1093/nar/gkl1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossman HB, Messing E, Soloway M, Tomera K, Katz G, Berger Y, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA. 2005;293:810–6. doi: 10.1001/jama.293.7.810. [DOI] [PubMed] [Google Scholar]
- 23.Huber S, Schwentner C, Taeger D, Pesch B, Nasterlack M, Leng G, et al. Nuclear matrix protein-22: a prospective evaluation in a population at risk for bladder cancer. Results from the UroScreen study. BJU Int. 2012;110:699–708. doi: 10.1111/j.1464-410X.2011.10883.x. [DOI] [PubMed] [Google Scholar]
- 24.Lai Y, Ye J, Chen J, Zhang L, Wasi L, He Z, et al. UPK3A: a promising novel urinary marker for the detection of bladder cancer. Urology. 2010;76:514. doi: 10.1016/j.urology.2009.11.045. [DOI] [PubMed] [Google Scholar]


