Abstract
Purpose
This study was conducted to validate the survival benefit of metastasectomy plus chemotherapy over chemotherapy alone for treatment of Krukenberg tumors from gastric cancer and to identify prognostic factors for survival.
Materials and Methods
Clinical data from 216 patients with Krukenberg tumors from gastric cancer were collected. Patients were divided into two arms according to treatment modality: arm A, metastasectomy plus chemotherapy and arm B, chemotherapy alone.
Results
Overall survival (OS) was significantly increased in arm A relative to arm B for patients initially diagnosed with stage IV gastric cancer (18.0 months vs. 8.0 months; p < 0.001) and those with recurrent Krukenberg tumors (19.0 months vs. 9.0 months; p=0.002), respectively. Metastasectomy (hazard ratio [HR], 0.458; 95% confidence interval [CI], 0.287 to 0.732; p=0.001), signet-ring cell pathology (HR, 1.583; 95% CI, 1.057 to 2.371; p=0.026), and peritoneal carcinomatosis (HR, 3.081; 95% CI, 1.610 to 5.895; p=0.001) were significant prognostic factors for survival.
Conclusion
Metastasectomy plus chemotherapy offers superior OS when compared to palliative chemotherapy alone in gastric cancer with Krukenberg tumor. Prolonged survival applies to all patients, regardless of gastric cancer stage. Metastasectomy, signet-ring cell pathology, and peritoneal carcinomatosis were prognostic factors for survival. Future prospective randomized trials are needed to confirm the optimal treatment strategy for Krukenberg tumors from gastric cancer.
Keywords: Krukenberg tumor, Metastasectomy, Prognosis, Stomach neoplasms
Introduction
Gastric cancer is the second leading cause of cancer-related death worldwide. In Western countries, the incidence of gastric cancer has been decreasing, whereas it remains a main cause of cancer-related death in Korea. Gastric cancer infrequently metastasizes to the ovary, a hormone-related organ. The incidence of ovarian metastasis or Krukenberg tumor after curative resection of gastric cancer is approximately 0.3%-6.7% [1,2]; however, some autopsy studies have reported incidence rates ranging from 33% to 41% [1,2].
Krukenberg tumor is associated with poor prognosis in gastric cancer [3,4]. In female patients, one of the most important causes of treatment failure for gastric cancer is an ovarian relapse [5,6]. Significant advances have been made in understanding the molecular biology of many cancers. However, the underlying mechanism of the intratumor heterogeneity of gastric cancer has not been clearly established. Furthermore, the prognostic factors and treatment guidelines for patients diagnosed with Krukenberg tumor of gastric origin are insufficient.
Although systemic chemotherapy is the optimal treatment strategy for recurrent or metastatic gastric cancer, it has not provided significant survival benefits. Therefore, several treatment strategies have been investigated to improve overall survival (OS) in metastatic gastric cancer patients with oligometastases or limited metastasis. Several local treatments including metastasectomy, radiofrequency ablation, and stereotactic body radiation therapy have shown impressive results [7,8]. Additionally, resection of metastatic lesions has been shown to increase OS in colorectal cancer (CRC) patients with operable liver and lung metastases [9-12]. Therefore, National Comprehensive Cancer Network guidelines recommend metastasectomy for operable lung and liver lesions in CRC. However, the survival benefit of metastasectomy has not been clearly validated for Krukenberg tumors in gastric cancer. Most Krukenberg tumors are diagnosed metachronously, and only a few patients with Krukenberg tumor are clinically diagnosed synchronously. In most hospitals, patients initially diagnosed with ovarian metastasis in advanced gastric cancer are primarily treated with chemotherapy. However, there is limited clinical data available regarding the survival benefit of ovarian metastasectomy in patients with advanced gastric cancer [13]. Moreover, controversies regarding the best treatment strategy for Krukenberg tumor in gastric cancer have caused confusion among physicians. Therefore, we investigated the survival benefit of ovarian metastasectomy in synchronous or metachronous Krukenberg tumor in gastric cancer.
Materials and Methods
1. Patients
Of 27,103 patients who were diagnosed with gastric cancer between March 2004 and February 2012 at Yonsei University Medical Center, 9,217 (34%) were women. Among female gastric cancer patients, 216 with Krukenberg tumor detected by abdominal-pelvis computed tomography (CT) or gynecologic ultrasonography were included in this study and reviewed retrospectively (Severance Hospital, n=172; Gangnam Severance Hospital, n=44). Patient information was obtained from outpatient clinical or admission records and information regarding patient survival was obtained from the Korean National Statistics Registry Database. The protocols were approved by the Yonsei University Health System Institutional Review Board.
In general, curative surgery plays an important role in gastric cancer without distant metastasis. Therefore, for data analysis, patients were divided into two groups according to initial gastric cancer stage: stage I-III and stage IV. Patients received surgery or palliative chemotherapy according to the initial disease stage. Patients suspected of having Krukenberg tumor underwent imaging studies to confirm disease resectability. However, 87% of patients (93/107) who underwent oophorectomy had disease that already extended beyond the ovary, in which case oophorectomy was performed for palliative symptom control. The residual disease state of each patient was documented as the presence or absence of gross residual disease, which was classified as negative resection margins (R0), microscopic tumor infiltration (R1), and macroscopic residual tumor (R2). R0 resection was achieved in only 38% (41/107) of patients who underwent oophorectomy.
Overall, 125 patients were initially diagnosed with stage IV gastric cancer and 91 with recurrent Krukenberg tumor after they underwent curative resection of gastric cancer. Among the patients initially diagnosed with stage IV gastric cancer, Krukenberg tumors were detected synchronously and metachronously in 84 patients and 41 patients, respectively.
To compare OS, patients with initial stage IV gastric cancer (n=125) were divided into two arms according to treatment modality. Arm A1 comprised 49 patients who received both chemotherapy and metastasectomy for Krukenberg tumor. Arm B1 comprised 76 patients who received chemotherapy alone. Patients with recurrent Krukenberg tumor (n=91) were assigned to arm A2 or arm B2. Arm A2 comprised 58 patients who received chemotherapy and metastasectomy for recurrent Krukenberg tumor, and arm B2 comprised 33 patients who received chemotherapy alone. In arms A1 and B1, OS was defined as the time from the date of pathologic diagnosis of gastric cancer to the date of death or last follow-up. In arms A2 and B2, OS was defined as the time from the date of Krukenberg tumor diagnosis by imaging to the date of death or last follow-up.
2. Statistical analyses
All statistical analyses were performed using IBM SPSS ver. 20.0 (IBM Co., Armonk, NY). For continuous variables, two-tailed Student t tests were used to compare the demographic and clinical characteristics between patient arms. For discrete variables, a chi-square test was used. Survival rates and 95% confidence intervals (CIs) were calculated using the Kaplan-Meier method. The influence of the covariates on survival length between treatment arms was assessed using the log-rank test. A p-value of < 0.05 was considered significant. Significant variables in the univariate analysis were entered into multivariate analysis using the Cox proportional hazards model.
Results
1. Clinical characteristics
The median follow-up duration for all patients was 30.0 months until the OS data cutoff date (June 30, 2013), at which time 90% of the patients had discontinued treatment. The median age of patients at Krukenberg tumor diagnosis was 43.4 years (range, 21 to 78 years) and the average size of metastatic ovarian tumors was 6.8 cm (range, 1.5 to 24 cm).
The clinical characteristics of patients with initial stage IV gastric cancer (n=125) are listed in Table 1. Patients were divided into two arms according to treatment modality: arm A, metastasectomy plus chemotherapy; arm B, chemotherapy alone. Comparison of the patients who received chemotherapy plus metastasectomy revealed they had significantly larger Krukenberg tumors (median size, 7.99 cm vs. 5.76 cm; p=0.004), fewer metastases outside the ovaries (85.7% vs. 97.4%; p=0.028), and a more normal range of serum cancer antigen (CA) 19-9 level (65.3% vs. 39.5%; p=0.009) than patients who received chemotherapy alone.
Table 1.
Clinical characteristics of 125 patients with initial stage IV gastric cancer
| Variable | Arm A1a) (n=58) | Arm B1a) (n=33) | p-valueb) |
|---|---|---|---|
| Median age (yr) | 43.3 (26-69) | 42.1 (27-72) | 0.428 |
| < 50 | 39 (80.0) | 64 (84.2) | 0.508 |
| ≥ 50 | 10 (20.4) | 12 (15.8) | - |
| Laterality | 0.315 | ||
| Bilateral | 37 (75.5) | 51 (67.0) | - |
| Unilateral | 12 (24.5) | 25 (33.0) | - |
| Krukenberg tumor size (cm) | 7.99 (3.4-19) | 5.76 (1.5-24) | 0.004 |
| Pathologic differentiation | 0.236 | ||
| WD-MD | 7 (14.3) | 6 ( 7.9) | - |
| PD-SRC | 42 (85.7) | 69 (90.8) | - |
| Chronology | 0.676 | ||
| Synchronous | 34 (69.3) | 50 (65.8) | - |
| Metachronous | 15 (30.6) | 26 (34.2) | - |
| Metastasis site | |||
| Peritoneum | 38 (77.6) | 66 (86.8) | 0.175 |
| Liver | 6 (12.2) | 10 (13.2) | 0.881 |
| Bone | 5 (10.2) | 11 (14.4) | 0.723 |
| Lung | 2 (4.1) | 5 (6.6) | 0.704 |
| Other | 23 (46.9) | 32 (42.1) | 0.699 |
| Extent of disease | 0.028 | ||
| Limited to the ovary | 7 (14.3) | 2 (2.6) | - |
| Beyond the ovary | 42 (85.7) | 74 (97.4) | - |
| R status | |||
| R0 resection | 14 (28.6) | - | - |
| R2 resection | 35 (71.4) | - | - |
| Serum CEA (ng/mL) | 3.05 (0.01-36.3) | 5.80 (0.01-121) | 0.277 |
| Normal | 41 (83.7) | 56 (73.7) | 0.083 |
| > 5 | 4 (8.2) | 15 (19.7) | - |
| Serum CA 19-9 (U/mL) | 96.64 (0.1-1,850) | 484.5 (0.1-12,100) | 0.067 |
| Normal | 32 (65.3) | 30 (39.5) | 0.009 |
| > 24 | 14 (28.6) | 37 (48.7) | - |
| Serum CA-125 (U/mL) | 74.1 (5.5-244) | 187 (11-1,555) | 0.051 |
| Normal | 14 (28.6) | 11 (14.5) | 0.159 |
| > 35 | 14 (28.6) | 23 (30.3) | - |
Values are presented as median (range) or number (%). WD-MD, well differentiated adenocarcinoma and moderately differentiated adenocarcinoma; PD-SRC, poorly differentiated adenocarcinoma and signet ring cell carcinoma; CEA, carcinoembryonic antigen; CA, cancer antigen.
Patients were divided into two arms according to treatment modality: arm A, metastasectomy plus chemotherapy; arm B, chemotherapy alone,
p-values from chi-square test except for Krukenberg tumor size, and median age at Krukenberg tumor diagnosis, which were determined by a two-tailed Student t test.
The clinical characteristics of patients with recurrent Krukenberg tumor of gastric origin (n=91) are listed in Table 2. Patients who received chemotherapy plus metastasectomy had significantly higher frequency of bilateral tumors (72.4% vs. 48.5%; p=0.022), and a more normal range of serum CA 19-9 level (65.6% vs. 45.5%; p=0.035) than those who received chemotherapy alone.
Table 2.
Clinical characteristics of 91 patients with recurrent Krukenberg tumor
| Variable | Arm A2a) (n=58) | Arm B2a) (n=33) | p-valueb) |
|---|---|---|---|
| Median age (yr) | 43.9 (21-78) | 45.9 (25-75) | 0.372 |
| < 50 | 41 (70.7) | 18 (54.5) | 0.121 |
| ≥ 50 | 17 (29.3) | 15 (45.5) | - |
| Relapse free survival (mo) | 24.3 (3-109) | 27.8 (4-91) | 0.435 |
| Laterality | 0.022 | ||
| Bilateral | 42 (72.4) | 16 (48.5) | - |
| Unilateral | 16 (27.6) | 17 (51.5) | - |
| Krukenberg tumor size (cm) | 7.39 (3-18) | 5.95 (1.9-15) | 0.068 |
| Pathologic differentiation | 0.499 | ||
| WD-MD | 6 (10.3) | 5 (15.2) | - |
| PD-SRC | 52 (89.7) | 28 (84.8) | - |
| AJCC stage | 0.824 | ||
| I, II | 26 (44.8) | 14 (42.4) | - |
| III | 32 (55.2) | 19 (57.6) | - |
| Metastasis site | |||
| Peritoneum | 45 (77.6) | 26 (78.8) | 0.894 |
| Liver | 4 (6.9) | 4 (12.1) | 0.454 |
| Bone | 6 (10.3) | 5 (15.2) | 0.519 |
| Lung | 2 (3.4) | 0 (0) | 0.533 |
| Other | 33 (56.9) | 13 (39.4) | 0.108 |
| Extent of disease | 0.25 | ||
| Limited to the ovary | 7 (12.1) | 1 (3.0) | - |
| Beyond the ovary | 51 (87.9) | 32 (97.0) | - |
| R status | |||
| R0 resection | 27 (46.6) | - | - |
| R2 resection | 31 (53.4) | - | - |
| Serum CEA (ng/mL) | 2.79 (0.13-22.2) | 332 (0.65-10,410) | 0.319 |
| Normal | 44 (75.9) | 24 (72.7) | 0.276 |
| > 5 | 8 (13.8) | 8 (24.2) | - |
| Serum CA 19-9 (U/mL) | 118.73 (0.1-2,270) | 1,702 (0.1-20,000) | 0.097 |
| Normal | 38 (65.6) | 15 (45.5) | 0.035 |
| > 24 | 14 (25.0) | 15 (45.5) | - |
| Serum CA-125 (U/mL) | 36.4 (4-241) | 60.82 (5-227.8) | 0.117 |
| Normal | 33 (56.9) | 10 (30.3) | 0.08 |
| > 35 | 11 (19.0) | 9 (27.3) | - |
Values are presented as median (range) or number (%). WD-MD, well differentiated adenocarcinoma and moderately differentiated adenocarcinoma; PD-SRC, poorly differentiated adenocarcinoma and signet ring cell carcinoma; AJCC, American Joint Committee on Cancer; CEA, carcinoembryonic antigen; CA, cancer antigen.
Patients were divided into two arms according to treatment modality: arm A, metastasectomy plus chemotherapy; arm B, chemotherapy alone,
p-values from chi-square test except for Krukenberg tumor size, median age at Krukenberg tumor diagnosis, and relapse free survival, which were determined by a two-tailed Student t test.
2. Treatment outcome
The median OS of patients with initial stage IV gastric cancer was 12.0 months (95% CI, 9.7 to 14.3 months). The median OS of arm A1 and arm B1 was 18.0 months (95% CI, 15.2 to 20.8 months) and 8.0 months (95% CI, 6.6 to 9.4 months), respectively. Therefore, patients in the chemotherapy plus metastasectomy arm had a significantly better OS than patients in the chemotherapy arm (p < 0.001) (Fig. 1).
Fig. 1.
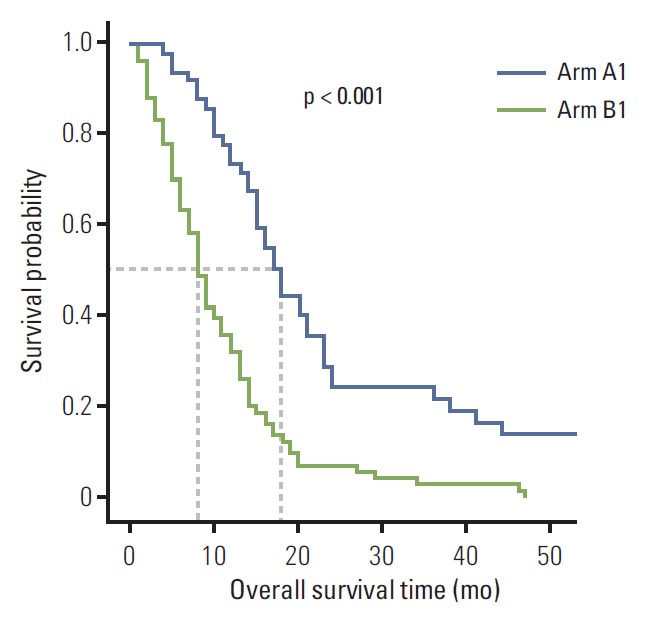
Kaplan-Meier overall survival based on treatment arm in initial stage IV gastric cancer. Patients were divided into two arms according to treatment modality: arm A, metastasectomy plus chemotherapy; arm B, chemotherapy alone.
The median OS of patients with recurrent Krukenberg tumors was 15.0 months (95% CI, 12.7 to 17.3 months). The median OS time of arm A2 and arm B2 was 19.0 months (95% CI, 14.4 to 23.6 months) and 9.0 months (95% CI, 6.2 to 11.8 months), respectively. Patients in the chemotherapy plus metastasectomy arm had a significantly better OS than patients in the chemotherapy alone arm (p=0.002) (Fig. 2).
Fig. 2.
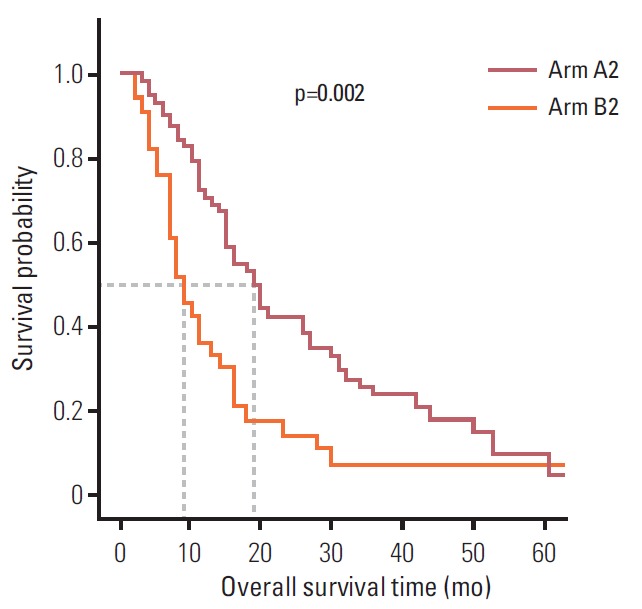
Kaplan-Meier overall survival based on treatment arm with recurred Krukenberg tumor. Patients were divided into two arms according to treatment modality: arm A, metastasectomy plus chemotherapy; arm B, chemotherapy alone.
Upon univariate analysis of all patients, metastasectomy, signet-ring cell pathology, presence of peritoneal carcinomatosis, gastrectomy, and elevated serum levels of carcinoembryonic antigen (CEA; >5 ng/mL), CA 19-9 (>24 U/mL), and CA-125 (>35 U/mL) were prognostic factors associated with survival. After adjusting for covariates in multivariate analysis, metastasectomy (hazard ratio [HR], 0.458; 95% CI, 0.287 to 0.732; p=0.001), signet-ring cell pathology (HR, 1.583; 95% CI, 1.057 to 2.371; p=0.026), and presence of peritoneal carcinomatosis (HR, 3.081; 95% CI, 1.610 to 5.895; p=0.001) were independent predictors of OS (Table 3).
Table 3.
Univariate and multivariate analysis showing factors associated with overall survival in 216 patients
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Metastasectomy | 0.404 (0.302-0.539) | < 0.001 | 0.458 (0.287-0.732) | 0.001 |
| Age (≥ 50 yr) | 1.065 (0.769-1.477) | 0.704 | - | - |
| Metachronous disease | 0.870 (0.587-1.289) | 0.487 | - | - |
| Unilateral ovarian metastases | 1.097 (0.809-1.487) | 0.552 | - | - |
| Size of Krukenberg tumor (< 5 cm) | 0.749 (0.547-1.024) | 0.070 | - | - |
| Signet-ring cells | 0.642 (0.479-0.859) | 0.003 | 1.583 (1.057-2.371) | 0.026 |
| Peritoneal carcinomatosis | 3.034 (1.990-4.625) | < 0.001 | 3.081 (1.610-5.895) | 0.001 |
| Gastrectomy | 2.022 (1.507-2.712) | < 0.001 | 1.293 (0.787-2.124) | 0.311 |
| Relapse free survival (≥ 12 mo) | 1.433 (0.958-2.144) | 0.080 | - | - |
| CEA | 1.434 (1.061-1.938) | 0.052 | - | - |
| CA 19-9 | 1.614 (1.193-2.182) | 0.002 | 0.683 (0.447-1.042) | 0.077 |
| CA-125 | 2.091 (1.420-3.078) | < 0.001 | 0.653 (0.421-1.014) | 0.057 |
HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; CA, cancer antigen.
It was difficult to statistically analyze survival differences between patients in whom metastasis was limited to the ovary and those who have metastasis beyond the ovary because only 8% of patients showed metastasis limited to the ovary. Most of these patients were alive at the time of the study. A few patients who showed metastasis to other sites were subjected to additional surgery with oophorectomy, such as total hysterectomy and bowel resection.
As shown in Fig. 3, the R0 resection group (n=41) had a significantly longer OS (HR, 0.405; 95% CI, 0.254 to 0.646; log-rank p < 0.001) than the R1, R2 resection group (n=66). The median OS was 30.0 months (95% CI, 24.0 to 36.0) in the R0 resection group and 15.0 months (95% CI, 13.6 to 16.4) in the R1, R2 resection group.
Fig. 3.
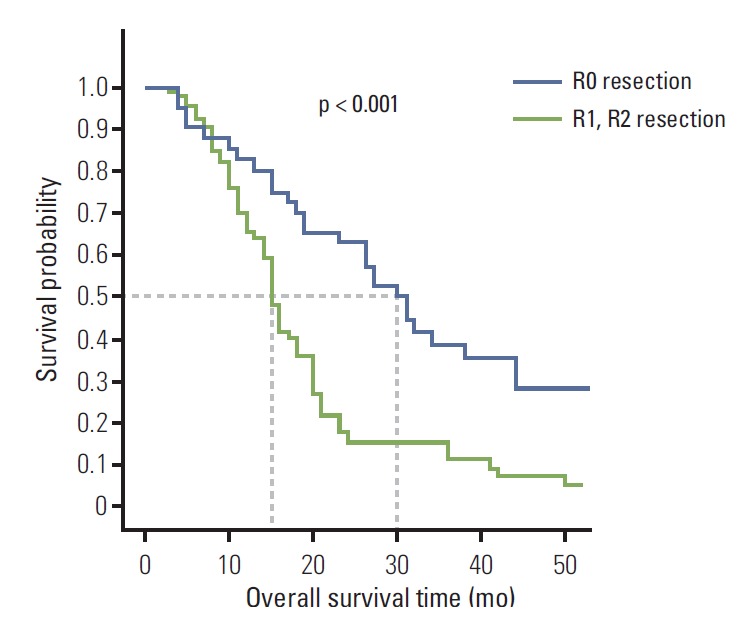
Kaplan-Meier overall survival based on curative resection of Krukenberg tumor in stomach cancer. The residual disease state of each patient was documented as the presence or absence of gross residual disease, which was classified as negative resection margins (R0), microscopic tumor infiltration (R1), and macroscopic residual tumor (R2).
Oophorectomy was found to still be beneficial when other unresectable metastasis were present, for both metastatic and recurrent disease. Analysis of all cases except single ovarian metastasis revealed that the median OS time of arm A1 and arm B1 was 16.0 months (95% CI, 13.7 to 18.3 months) and 8.0 months (95% CI, 6.6 to 9.4 months; p < 0.001), respectively. Additionally, the median OS time of arm A2 and arm B2 was 16.0 months (95% CI, 12.5 to 19.5 months) and 8.0 months (95% CI, 5.8 to 10.2 months; p=0.039), respectively.
The frequencies and response rates of chemotherapy regimens initially used after ovarian metastasis diagnosis were also analyzed (Table 4). Overall, 111 patients were treated with chemotherapy for ovarian metastasis, with platinum (n=43), taxane (n=26), and irinotecan (n=8) chemotherapy regimens being the most frequently used. Chemotherapy regimens did not differ significantly between arms A and B (p=0.535). Patients who received chemotherapy for ovarian metastasis were evaluated using the Response Evaluation Criteria in Solid Tumors ver. 1.1. Tumor assessment included measurable metastatic ovarian lesions and not overall gastric cancer lesions. The response rates for the chemotherapy regimens were as follows: platinum, 26%; irinotecan, 25%; and taxane, 12%.
Table 4.
Frequencies and response rates of chemotherapy regimens initially used after ovarian metastasis diagnosis
| Variable | Arm A1a) |
Arm A2 |
Arm B1 |
Arm B2 |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | RR (%)b) | No. | RR (%) | No. | RR (%) | No. | RR (%) | No. | RR (%) | |
| Platinumc) | 8 | 25 | 1 | 0 | 21 | 14 | 13 | 46 | 43 | 26 |
| Irinotecand) | 0 | 0 | 0 | 0 | 4 | 25 | 4 | 25 | 8 | 25 |
| Taxanee) | 4 | 0 | 2 | 0 | 17 | 18 | 3 | 0 | 26 | 12 |
| Total | 12 | 17 | 3 | 0 | 42 | 17 | 20 | 35 | 77 | 21 |
Patients were divided into two arms according to treatment modality: arm A, metastasectomy plus chemotherapy; arm B, chemotherapy alone,
RR, response rate (comlete response or partial response patients/total patients),
Cisplatin+TS-1, cisplatin+capecitabine (XP), cisplatin+5-fluorouracil (5-FU) (FP), oxaliplatin+capecitabine (XELOX), oxaliplatin+5-FU/leucovorin (LV) (FOLFOX), oxaliplatin+TS-1 (SOX),
Irinotecan mono, irinotecan+TS-1, irinotecan+5-FU/LV (FOLFIRI),
Paclitaxel mono, paclitaxel+5-FU/LV, paclitaxel+TS-1, docetaxel mono, docetaxel+5-FU/LV, docetaxel+TS-1, docetaxel+capecitabine.
Discussion
Most patients diagnosed with Krukenberg tumor of gastric origin have poor prognosis. Many studies have shown that the median survival after Krukenberg tumor diagnosis is 7-11 months [13]. In the past, symptomatic patients received palliative operation for symptom relief. Recently, the development of diagnostic tools has increased early detection of Krukenberg tumors and their curative resectability. Nevertheless, the optimal treatment strategy for Krukenberg tumors has not been established.
Our study showed that patients with Krukenberg tumor of gastric origin who underwent both chemotherapy plus metastasectomy had longer OS than those who underwent chemotherapy alone, regardless of stage. The difference in OS was actually underestimated because the OS in arms A2 and B2 was determined from the date of recurrent Krukenberg tumor diagnosis and not the date of the initial gastric cancer diagnosis. Despite the small proportion of R0 resections, a prolonged OS was observed in the chemotherapy plus metastasectomy arm. In our study, one patient in arm A1 survived more than 9 years after the initial diagnosis of gastric cancer, while one patient in arm A2 survived more than 7.5 years after resection of metachronous Krukenberg tumor.
Some studies have reported the survival benefit of metastasectomy for Krukenberg tumor; however, most of these included a small number of patients (approximately 50 patients) and Krukenberg tumors of different origins, including gastric, colon, and breast cancers [14]. Many reports have suggested that metastasectomy provides a better survival benefit for Krukenberg tumors of CRC origin [15-17]. Among studies of the survival benefit of metastasectomy for Krukenberg tumor, ours is the largest conducted to date and the only one that investigated Krukenberg tumors of gastric cancer origin exclusively. A few studies have demonstrated that metastasectomy of metachronous recurrent Krukenberg tumor of gastric origin provided longer OS [18-20]. Based on our results, metastasectomy should be performed in stage IV gastric cancer patients diagnosed synchronously or metachronously with Krukenberg tumor. Our recommendation is consistent with those of previous reports [21,22].
In the present study, the prognostic factors associated with survival in patients with Krukenberg tumor of gastric origin were analyzed. Metastasectomy, signet-ring cell pathology, and peritoneal carcinomatosis were identified as significant prognostic factors. Several studies have also shown that metastasectomy is a prognostic factor for better OS in patients with Krukenberg tumors [18,19]. In the present study, absence of peritoneal carcinomatosis was associated with better prognosis, which is consistent with the results of a previous study that showed limited disease extent as a prognostic factor [2]. Complete resection is easily achieved when the extent of disease is limited; therefore, active Krukenberg tumor metastasectomy should be conducted in patients who are not expected to have residual disease after operation. Adenocarcinomas composed of signet ring cells tend to metastasize to the ovaries more frequently than adenocarcinomas of other histologic types [23]. Signet ring cell features have not been well established as a prognostic factor for Krukenberg tumors; however, in the present study, they were a poor prognostic factor for Krukenberg tumors of gastric cancer origin.
Published studies of the role of chemotherapy in the treatment of Krukenberg tumors have included only small patient numbers or case reports. In the present study, response rates to chemotherapy regimens were analyzed in 111 patients diagnosed with Krukenberg tumor, and response rates ranged from 12% to 26%. In most of our cases, Krukenberg tumor was diagnosed during later stages of gastric cancer progression. Therefore, at the time of Krukenberg tumor diagnosis, patients have already received standard first-line chemotherapy for advanced or metastatic gastric cancer.
In our experience, ovarian metastases show less chemotherapy responsiveness than other sites of metastasis. Early detection of ovarian metastases is important to successful treatment. In the present study, serum CEA, CA 19-9, and CA-125 level were not useful predictors of Krukenberg tumor. Despite continual efforts to develop a practical biomarker that can predict relapse or metastasis of ovary metastasis with gastric cancer, no clinical tests have been established [24].
Clinical heterogeneity is most likely due to the diverse molecular profile of gastric cancer. Thus, identifying diversity in the molecular profile of gastric cancer that governs the clinical behavior of tumors could lead to new and more effective clinical strategies. Recent studies of gastric cancer have identified genes that differ according to histologic factors and age, as well as those useful for gastric cancer prognosis prediction [24]. We will continue to identify genes and develop a practical biomarker in future studies.
It should be noted that several factors may have affected the decision of surgery for treatment of the patients evaluated in the present study, including the extent of metastasis, possibility of curative surgery, surgeon’s opinion, etc. Additionally, the difference in the chemotherapy regimen between arm A and arm B may have influenced patient survival or toxicity.
CT was used to identify patients who would benefit from the curative resection of Krukenberg tumors. Although imaging modalities, including CT scanning, have been developed to detect intraperitoneal metastasis, CT has been shown to only have a 50% accuracy for detecting intraperitoneal metastatic cancers [25]. Therefore, peritoneal carcinomatosis is difficult to diagnosis by CT scan. Laparoscopic examination has shown better accuracy in detecting peritoneal carcinomatosis; however, this procedure is invasive and can result in complications.
Conclusion
In conclusion, we demonstrated that metastasectomy was associated with longer survival in patients with Krukenberg tumors in gastric cancer. Therefore, metastasectomy should be performed in stage IV gastric cancer patients diagnosed synchronously or metachronously with Krukenberg tumor. Our data also suggest that metastasectomy plus chemotherapy may play a role in the treatment of Krukenberg tumors of gastric origin. Furthermore, we found that metastasectomy, signet ring cells, and peritoneal carcinomatosis were prognostic factors for Krukenberg tumors. Future prospective randomized trials are needed to confirm our findings and will be important in establishing standard treatment guidelines for patients with Krukenberg tumor in metastatic gastric cancer.
Acknowledgments
This research was supported by a CMB-Yuhan research grant from the Yonsei University College of Medicine (6-2013-0065) and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (No. 2010-0024248).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Wang J, Shi YK, Wu LY, Wang JW, Yang S, Yang JL, et al. Prognostic factors for ovarian metastases from primary gastric cancer. Int J Gynecol Cancer. 2008;18:825–32. doi: 10.1111/j.1525-1438.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim HK, Heo DS, Bang YJ, Kim NK. Prognostic factors of Krukenberg's tumor. Gynecol Oncol. 2001;82:105–9. doi: 10.1006/gyno.2001.6210. [DOI] [PubMed] [Google Scholar]
- 3.Petru E, Pickel H, Heydarfadai M, Lahousen M, Haas J, Schaider H, et al. Nongenital cancers metastatic to the ovary. Gynecol Oncol. 1992;44:83–6. doi: 10.1016/0090-8258(92)90017-d. [DOI] [PubMed] [Google Scholar]
- 4.Hale RW. Krukenberg tumor of the ovaries: a review of 81 records. Obstet Gynecol. 1968;32:221–5. [PubMed] [Google Scholar]
- 5.Duarte I, Llanos O. Patterns of metastases in intestinal and diffuse types of carcinoma of the stomach. Hum Pathol. 1981;12:237–42. doi: 10.1016/s0046-8177(81)80124-4. [DOI] [PubMed] [Google Scholar]
- 6.Saphir O. Signet-ring cell carcinoma. Mil Surg. 1951;109:360–9. [PubMed] [Google Scholar]
- 7.Kim KH, Lee KW, Baek SK, Chang HJ, Kim YJ, Park DJ, et al. Survival benefit of gastrectomy ± metastasectomy in patients with metastatic gastric cancer receiving chemotherapy. Gastric Cancer. 2011;14:130–8. doi: 10.1007/s10120-011-0015-7. [DOI] [PubMed] [Google Scholar]
- 8.Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19:1146–53. doi: 10.1093/annonc/mdn026. [DOI] [PubMed] [Google Scholar]
- 9.Elias D, Cavalcanti A, Sabourin JC, Lassau N, Pignon JP, Ducreux M, et al. Resection of liver metastases from colorectal cancer: the real impact of the surgical margin. Eur J Surg Oncol. 1998;24:174–9. doi: 10.1016/s0748-7983(98)92878-5. [DOI] [PubMed] [Google Scholar]
- 10.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62. [PubMed] [Google Scholar]
- 11.Iwatsuki S, Esquivel CO, Gordon RD, Starzl TE. Liver resection for metastatic colorectal cancer. Surgery. 1986;100:804–10. [PMC free article] [PubMed] [Google Scholar]
- 12.Imamura H, Matsuyama Y, Shimada R, Kubota M, Nakayama A, Kobayashi A, et al. A study of factors influencing prognosis after resection of hepatic metastases from colorectal and gastric carcinoma. Am J Gastroenterol. 2001;96:3178–84. doi: 10.1111/j.1572-0241.2001.05278.x. [DOI] [PubMed] [Google Scholar]
- 13.Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. 2006;30:277–99. doi: 10.1097/01.pas.0000190787.85024.cb. [DOI] [PubMed] [Google Scholar]
- 14.Ekbom GA, Gleysteen JJ. Gastric malignancy: resection for palliation. Surgery. 1980;88:476–81. [PubMed] [Google Scholar]
- 15.Ayhan A, Guvenal T, Salman MC, Ozyuncu O, Sakinci M, Basaran M. The role of cytoreductive surgery in nongenital cancers metastatic to the ovaries. Gynecol Oncol. 2005;98:235–41. doi: 10.1016/j.ygyno.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 16.Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol. 1998;69:103–8. doi: 10.1006/gyno.1998.4955. [DOI] [PubMed] [Google Scholar]
- 17.Zang RY, Zhang ZY, Li ZT, Chen J, Tang MQ, Liu Q, et al. Effect of cytoreductive surgery on survival of patients with recurrent epithelial ovarian cancer. J Surg Oncol. 2000;75:24–30. doi: 10.1002/1096-9098(200009)75:1<24::aid-jso5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Kang SB, Park NH, Choi YM, Lee HP. Krukenberg tumors of the ovary: a clinical analysis of 16 cases. J Korean Cancer Assoc. 1990;22:194–201. [Google Scholar]
- 19.Lu LC, Shao YY, Hsu CH, Hsu C, Cheng WF, Lin YL, et al. Metastasectomy of Krukenberg tumors may be associated with survival benefits in patients with metastatic gastric cancer. Anticancer Res. 2012;32:3397–401. [PubMed] [Google Scholar]
- 20.Cheong JH, Hyung WJ, Chen J, Kim J, Choi SH, Noh SH. Survival benefit of metastasectomy for Krukenberg tumors from gastric cancer. Gynecol Oncol. 2004;94:477–82. doi: 10.1016/j.ygyno.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Cheong JH, Hyung WJ, Chen J, Kim J, Choi SH, Noh SH. Surgical management and outcome of metachronous Krukenberg tumors from gastric cancer. J Surg Oncol. 2004;87:39–45. doi: 10.1002/jso.20072. [DOI] [PubMed] [Google Scholar]
- 22.Nio Y, Tsubono M, Kawabata K, Masai Y, Hayashi H, Meyer C, et al. Comparison of survival curves of gastric cancer patients after surgery according to the UICC stage classification and the General Rules for Gastric Cancer Study by the Japanese Research Society for gastric cancer. Ann Surg. 1993;218:47–53. doi: 10.1097/00000658-199307000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor: an overview. Arch Pathol Lab Med. 2006;130:1725–30. doi: 10.5858/2006-130-1725-AILAKT. [DOI] [PubMed] [Google Scholar]
- 24.Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011;17:1850–7. doi: 10.1158/1078-0432.CCR-10-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH. Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer. 1993;72:1631–6. doi: 10.1002/1097-0142(19930901)72:5<1631::aid-cncr2820720523>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]


