Abstract
Oxaliplatin is a third-generation platinum derivative used for metastatic or advanced colorectal cancer treatment. Although myelosuppression is the most common cause of oxaliplatin-induced thrombocytopenia, rare cases of oxaliplatin-induced immune-mediated thrombocytopenia are reported. We report a case of a 57-year-old woman with colon cancer who developed gum bleeding and petechiae after oxaliplatin infusion. Laboratory tests revealed grade 4 thrombocytopenia and grade 4 neutropenia. She recovered from the thrombocytopenia and accompanying neutropenia within 4 days with no recurrence following discontinuation of oxaliplatin. Physicians need to be aware of the risk of severe acute thrombocytopenia following oxaliplatin administration.
Keywords: Oxaliplatin, Colorectal neoplasms, Thrombocytopenia, Neutropenia
Introduction
Oxaliplatin is a third generation platinum analog given to patients with metastatic or advanced colorectal cancer. The combination of oxaliplatin, fluorouracil, and leucovorin improves disease-free survival in patients with metastatic and stage II or III colorectal cancer [1-3].
Thrombocytopenia, a common side effect of oxaliplatin, occurs in more than 70% of patients with colorectal cancer receiving oxaliplatin. However, grade 3-4 thrombocytopenia causing life-threatening bleeding presents in only 3%-4% of patients [4]. According to Jardim et al. [5], oxaliplatin-related thrombocytopenia can occur by three mechanisms. The main cause of thrombocytopenia related to oxaliplatin is myelosuppression, which represents asymptomatic thrombocytopenia. In some patients, hepatic sinusoid damage by oxaliplatin leads to portal hypertension and resultant splenic sequestration of platelets. The third mechanism, immune-mediated thrombocytopenia, rarely occurs during oxaliplatin infusion [6]. Although rare, this immune-mediated mechanism can result in grade 3-4 thrombocytopenia with life-threatening bleeding, hypersensitivity reactions, or hemolysis [6]. These symptoms and thrombocytopenia occur suddenly after administration of oxaliplatin and are easily treated by stopping oxaliplatin infusion. However, because of its rarity, physicians are usually not familiar with this oxaliplatin-induced immune-mediated thrombocytopenia. We report a case of immune-mediated thrombocytopenia associated with oxaliplatin.
Case Report
A 56-year-old female without relevant comorbidities was diagnosed with colon adenocarcinoma located in the ascending colon in May 2008. Right hemicolectomy was performed, and the pathologic stage was stage III-C (pT3N2M0). She underwent 12 cycles of adjuvant chemotherapy with mFOLFOX6 (oxaliplatin 85 mg/m2 on day 1, leucovorin 200 mg/m2 on day 1, 5-fluorouracil 400 mg/m2 as an intravenous bolus on day 1 followed by continuous infusion of 1,200 mg/m2/day on days 1 and 2). In December 2009, abdomen-pelvis computed tomography (A-PCT) scan showed recurrent right retrocaval and right renal hilar lymph nodes (LNs). She received three cycles of palliative FOLFIRI (irinotecan 180 mg/m2 on day 1, leucovorin 200 mg/m2 on day 1, 5-fluorouracil 400 mg/m2 as an intravenous bolus on day 1, followed by 1,200 mg/m2 on day 1 and 2). She had palliative right nephrectomy with LNs dissection for a salvage surgery in April 2010. We then gave eight cycles of palliative capecitabine and oxaliplatin because the pathology report mentioned metastasis to two out of 19 LNs around the right kidney.
In October 2011, disease recurrence in a pericaval LN was noted in 18F-fluorodeoxyglucose positron emission tomography–computed tomography (PET-CT). Therefore the patient again received 12 cycles of palliative mFOLFOX6, resulting in stable disease. There was no hypersensitivity reaction associated with oxaliplatin during these chemotherapy cycles.
In January 2013, PET-CT showed progression of metastatic lesions in left hilar and paravertebral area, and palliative mFOLFOX6 was resumed. On the sixth cycle, she felt a squeezing pain along the spine from her head to the lumbar area after oxaliplatin administration. Nine hours after oxaliplatin infusion, she developed mild gum bleeding and petechiae spreading from the needle site over her entire left arm. At this time, complete blood count (CBC) revealed thrombocytopenia and neutropenia: platelet count of 4,000/m3, white blood cell (WBC) count of 210/m3, and absolute neutrophil count (ANC) of 80/m3. The platelet and WBC levels were in the normal range on admission: platelet count of 129,000/m3, WBC count of 3,440/m3, and ANC of 1,850/m3. The bicytopenia resolved in a few days after platelet transfusion and granulocyte-colony stimulating factor (G-CSF) administration (Fig. 1). We thought it better to reduce the dose of oxaliplatin on the next cycle than stop the mFOLFOX6 cycles, because the side effects were not severe and resolved easily.
Fig. 1.
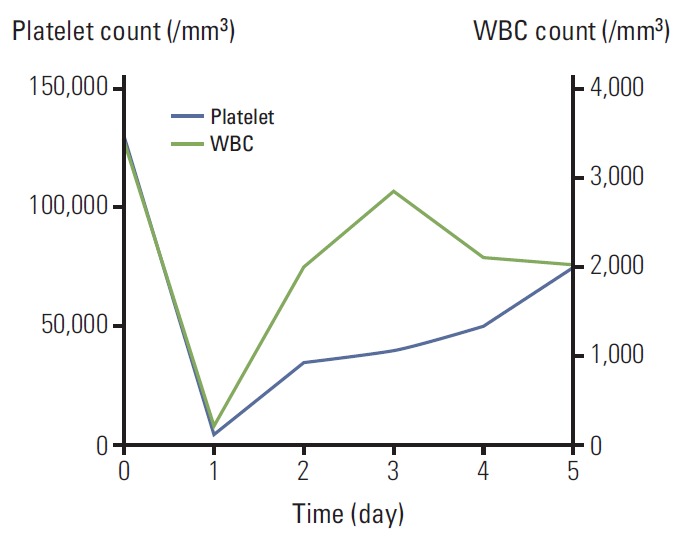
Acute bicytopenia on the sixth cycle of FOLFOX. WBC, white blood cell.
On the seventh cycle of mFOLFOX6, the dose of oxaliplatin was reduced to 70 mg/m2. On admission, the laboratory test showed normal WBC and platelet counts: platelet count of 207,000/m3, WBC count of 3,800/m3, and ANC of 1,599/m3. During oxaliplatin administration, she felt squeezing back pain again. After 5 hours, gum bleeding and petechiae over her entire arm developed. Laboratory test revealed acute-onset thrombocytopenia and neutropenia (platelet 2,000/m3, WBC 670/m3, and ANC 299/m3) (Fig. 2). After three days of supportive care with platelet transfusion and G-CSF injection, the platelet and WBC count recovered to 77,000/m3 and 6,270/m3 respectively, and back pain, gum bleeding and petechiae also improved. She was discharged from the hospital on the fourth day after oxaliplatin administration. We decided to stop the palliative mFOLFOX6 chemotherapy. She receives regular check-ups, and the disease has not progressed further at this time.
Fig. 2.
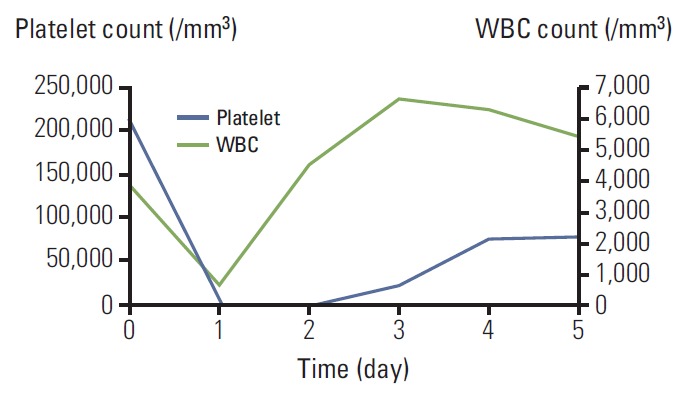
Acute bicytopenia on the seventh cycle of FOLFOX. WBC, white blood cell.
Discussion
Thrombocytopenia of all grades is reported in up to 70% of patients exposed to oxaliplatin. However, only 3%-4% of patients present with grade 3-4 thrombocytopenia with the risk of life-threatening bleeding [1,2,4]. We report a rare case of oxaliplatin-related thrombocytopenia with bleeding.
The most common cause of oxaliplatin-induced thrombocytopenia is bone marrow suppression, which develops in 45%-77% of colorectal cancer patients who receive oxaliplatin [1,2,7]. In these cases, bleeding is not common, and concomitant anemia and neutropenia are frequent. Chemotherapyinduced myelosuppression usually reaches nadir 10-14 days after chemotherapy administration and does not cause extreme thrombocytopenia within several hours of oxaliplatin administration [5]. In the present case, myelosuppression was excluded, as oxaliplatin-dependent thrombocytopenia developed within several hours, accompanied by gum bleeding.
Another mechanism of oxaliplatin-induced thrombocytopenia is splenic sequestration. The hepatic sinusoid can be injured by chemotherapy including oxaliplatin, resulting in portal hypertension leading to splenic sequestration of platelets. This phenomenon is characterized by prolonged thrombocytopenia and splenomegaly [8]. Our patient did not have splenomegaly in A-PCT and recovered from acute thrombocytopenia immediately after platelet transfusion.
Drug-induced immune-mediated thrombocytopenia is also a possible mechanism of oxaliplatin-induced thrombocytopenia. Several studies identified oxaliplatin-dependent platelet glycoprotein IIb/IIIa complex-specific antibodies in patients who developed severe acute thrombocytopenia in only one or two days after oxaliplatin administration [8-10]. A definitive diagnosis of oxaliplatin-induced immune-mediated thrombocytopenia can be established by detection of oxaliplatin-dependent IgG antibodies in patients’ sera [9]. However, it can be diagnosed clinically by acute thrombocytopenia occurring within several hours of oxaliplatin infusion, frequently accompanied by hemolysis or neutropenia. Platelet counts recover rapidly after discontinuation of oxaliplatin and transfusion [5].
These three mechanisms of thrombocytopenia are summarized and compared with the thrombocytopenia in our case (Table 1). Our case was similar to oxaliplatin immune-mediated thrombocytopenia [5]. In our case, the patient presented with symptomatic thrombocytopenia developing several hours after oxaliplatin administration and resolving rapidly after discontinuation of oxaliplatin. Therefore, we diagnosed oxaliplatin-induced immune-mediated thrombocytopenia without a laboratory test for oxaliplatin-dependent IgG antibodies. Accompanying neutropenia in the present case is in line with the previous two case reports of oxaliplatin-related acute pancytopenia [8,11]. In these reports, acute pancytopenia occurred by production of oxaliplatin-dependent antibodies to platelets, red blood cells and neutrophils, respectively.
Table 1.
Three oxaliplatin-induced thrombocytopenia mechanisms compared with our case
| Myelosuppression | Splenic sequestration | Immune-mediated | Present case | |
|---|---|---|---|---|
| Thrombocytopenia characteristics | Subacute, after about 1 week following chemotherapy | Insidious onset | Sudden onset | Sudden onset |
| Usually recovers by the next cycle of chemotherapy | Prolonged thrombocytopenia even after oxaliplatin cessation | Fast and complete recovery after discontinuation of the drug | Complete recovery after discontinuation of the drug | |
| Bleeding | Not common | Not common | Usual Might be life-threatening |
Gum bleeding Petechiae |
| Accompanied anemia and neutropenia | Usual | Not common | Not common | Sudden-onset neutropenia |
| Portal HTN and splenomegaly | No | Yes | No | No |
| Combined hypersensitivity reactions | Not common | Not common | Common (chills, fever, rash, abdominal or back pain, and bronchospasm) | No hypersensitivity reactions |
| Oxaliplatin-dependent IgG Ab | No | No | Yes | Test not done |
| Bone marrow aspirates | Toxicity to megakaryocytic progenitors | - | Increased numbers of megakaryocytes | Test not done |
HTN, hypertension; IgG Ab, immunoglobulin G antibody.
Suzuki et al. [12] reviewed 24 cases of published oxaliplatin-induced acute thrombocytopenia and Ohta et al. [13] reviewed 20 cases. Sixteen of 20 cases in Ohta et al.'s study [13] were also included in Suzuki et al.'s study [12]. These cases suggested that female gender and prolonged exposure to oxaliplatin were risk factors for developing immune-mediated thrombocytopenia. With the exception of symptoms related to thrombocytopenia, patients presented with hypersensitivity reactions such as chills, fever, rash, abdominal or back pain, and bronchospasm. The average number of cycles prior to onset of thrombocytopenia was 16.8 (range, 3 to 25 cycles) in 25 cases in these two studies [12,13]. Most of these oxaliplatin immune-mediated thrombocytopenia cases were induced after 10 cycles of chemotherapy (76% of patients in Suzuki et al. [12] and Ohta et al.’s [13] reports). In the current case, thrombocytopenia occurred in the 40th cycle of oxaliplatin chemotherapy. Our patient had been exposed to oxaliplatin even longer than other cases, although she had not received 40 continuous cycles of oxaliplatin-containing chemotherapy.
We did not realize that the first episode of acute thrombocytopenia in the present case was oxaliplatin-dependent immune-mediated thrombocytopenia, and considered it transient thrombocytopenia of unknown etiology. When the patient received oxaliplatin again, the gum-bleeding and thrombocytopenia developed more rapidly. The possibilities of disseminated intravascular coagulation and idiopathic thrombocytopenia were excluded because prothrombin time and partial thromboplastin time levels were in the normal range and evidence of hemolysis was not found on peripheral blood smear. Myelosuppression was also excluded due to normal range of CBC on admission. Since the platelet and WBC levels decreased suddenly during oxaliplatin administration and recovered following interruption of oxaliplatin, platelet transfusion and G-CSF administration, a diagnosis of oxaliplatin-associated immune-mediated thrombocytopenia was made. Reflecting on our experience, if a physician is not aware of oxaliplatin-induced immune-mediated thrombocytopenia, proper management may be delayed. Currently, there are no specific preemptive measures to prevent or predict oxaliplatin-induced immune-mediated thrombocytopenia. The only method for early detection is a high level of suspicion. There are also no specific management guidelines. Platelet count usually recovers rapidly after discontinuation of oxaliplatin, but platelet transfusion is helpful for increasing platelet levels in case of clinically meaningful bleeding. The value of corticosteroid is not certain yet [5]. Because of the rarity of the condition and lack of uniformity of previous case reports regarding corticosteroid administration, there is currently insufficient evidence for using corticosteroid. The most important countermeasure is not giving oxaliplatin again.
In conclusion, oxaliplatin-induced immune-mediated thrombocytopenia is a rare but important complication of oxaliplatin-containing chemotherapy that presents with acute symptomatic thrombocytopenia accompanied by fever, rash, and abdominal or back pain. A physician who treats patients with oxaliplatin should keep in mind the possibility of oxaliplatin-induced immune thrombocytopenia that may cause life-threatening bleeding, and consider discontinuing oxaliplatin administration and checking complete blood cell counts.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 2.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 3.Kanemitsu Y, Kato T, Shimizu Y, Inaba Y, Shimada Y, Nakamura K, et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone as treatment for liver metastasis from colorectal cancer: Japan Clinical Oncology Group Study JCOG0603. Jpn J Clin Oncol. 2009;39:406–9. doi: 10.1093/jjco/hyp035. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Aravind S, Ranganathan G, Martin A, Nalysnyk L. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large. [DOI] [PubMed]
- 5.Jardim DL, Rodrigues CA, Novis YA, Rocha VG, Hoff PM. Oxaliplatin-related thrombocytopenia. Ann Oncol. 2012;23:1937–42. doi: 10.1093/annonc/mds074. [DOI] [PubMed] [Google Scholar]
- 6.Bautista MA, Stevens WT, Chen CS, Curtis BR, Aster RH, Hsueh CT. Hypersensitivity reaction and acute immune-mediated thrombocytopenia from oxaliplatin: two case reports and a review of the literature. J Hematol Oncol. 2010;3:12. doi: 10.1186/1756-8722-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23:4866–75. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 8.Taleghani BM, Meyer O, Fontana S, Ahrens N, Novak U, Borner MM, et al. Oxaliplatin-induced immune pancytopenia. Transfusion. 2005;45:704–8. doi: 10.1111/j.1537-2995.2005.04373.x. [DOI] [PubMed] [Google Scholar]
- 9.Curtis BR, Kaliszewski J, Marques MB, Saif MW, Nabelle L, Blank J, et al. Immune-mediated thrombocytopenia resulting from sensitivity to oxaliplatin. Am J Hematol. 2006;81:193–8. doi: 10.1002/ajh.20516. [DOI] [PubMed] [Google Scholar]
- 10.Pavic M, Moncharmont P, Seve P, Rigal D, Broussolle C. Oxaliplatin-induced immune thrombocytopenia. Gastroenterol Clin Biol. 2006;30:797–8. doi: 10.1016/s0399-8320(06)73320-6. [DOI] [PubMed] [Google Scholar]
- 11.Fontao-Wendel R, Hoff PM, Lazar A, Freitas D, Novis Y, Patah P, et al. Immune-mediated pancytopenia induced by oxaliplatin: a case report. Transfusion. 2010;50:1453–9. doi: 10.1111/j.1537-2995.2010.02600.x. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Oda H, Sugawara Y, Masuya M, Nakase K, Fujioka M, et al. Oxaliplatin-induced acute thrombocytopenia: a case report and review of the literature. Intern Med. 2013;52:611–5. doi: 10.2169/internalmedicine.52.8933. [DOI] [PubMed] [Google Scholar]
- 13.Ohta S, Cho Y, Oshima S, Hosoya O, Juni K, Kojima H. Oxaliplatin-induced acute-onset thrombocytopenia and hemorrhage: case report and review of the literature. Oncol Lett. 2012;3:1297–300. doi: 10.3892/ol.2012.653. [DOI] [PMC free article] [PubMed] [Google Scholar]


