Abstract
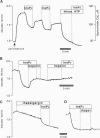
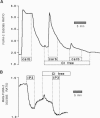
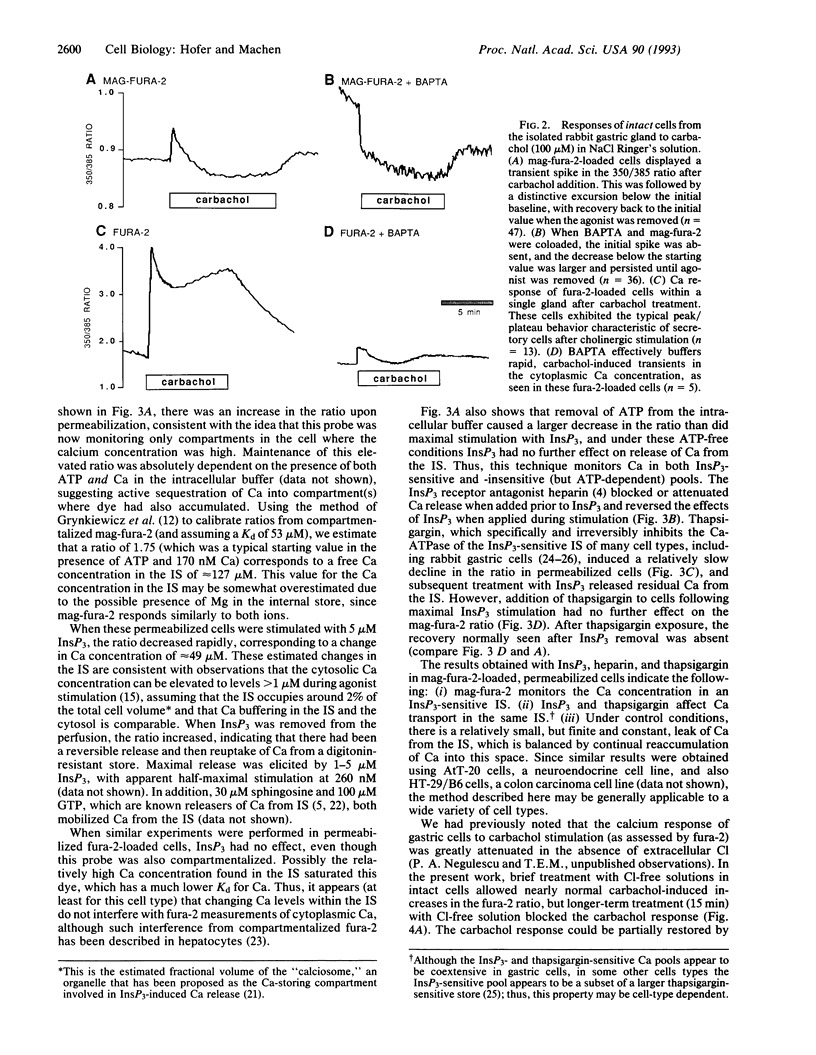
Stimulation of cells with calcium-mobilizing agonists frequently results in inositol 1,4,5-trisphosphate (InsP3)-mediated discharge of Ca from an internal store. We report here a technique for directly monitoring Ca within this and other stores in gastric epithelial cells. This technique takes advantage of the propensity of the acetoxymethyl ester derivative of the fluorescent dye mag-fura-2 (which is sensitive to Ca concentrations above 5 microM) to accumulate in subcellular compartments where it can report changes in the free Ca concentration. Intact dye-loaded cells responded to cholinergic stimulation with a decrease in the 350 nm/385 nm excitation ratio, as measured in individual cells with a digital imaging microscope, consistent with reduced Ca concentration in one or more cellular compartments. When cells were permeabilized with digitonin and incubated in an "intracellular buffer," the cytoplasmic dye was released, leaving the mag-fura-2 in the internal store. InsP3 caused the ratio from the trapped indicator to decrease (i.e., Ca was released) in a dose-dependent manner, and this effect was blocked by the InsP3 receptor antagonist heparin. Ca sequestration into the internal store was ATP-dependent, and reuptake into the InsP3-sensitive pool was blocked by thapsigargin, a specific inhibitor of the Ca-ATPase of the internal store. We used this technique to investigate the role of Cl on the release and reloading of the InsP3-sensitive internal store and found that Ca uptake was reduced in Cl-free solutions, suggesting an important function for Cl in the refilling of this pool.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berglindh T., Obrink K. J. A method for preparing isolated glands from the rabbit gastric mucosa. Acta Physiol Scand. 1976 Feb;96(2):150–159. doi: 10.1111/j.1748-1716.1976.tb10184.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bian J. H., Ghosh T. K., Wang J. C., Gill D. L. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991 May 15;266(14):8801–8806. [PubMed] [Google Scholar]
- Chew C. S., Brown M. R. Release of intracellular Ca2+ and elevation of inositol trisphosphate by secretagogues in parietal and chief cells isolated from rabbit gastric mucosa. Biochim Biophys Acta. 1986 Aug 29;888(1):116–125. doi: 10.1016/0167-4889(86)90077-7. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Petropoulos A. C. Thapsigargin potentiates histamine-stimulated HCl secretion in gastric parietal cells but does not mimic cholinergic responses. Cell Regul. 1991 Jan;2(1):27–39. doi: 10.1091/mbc.2.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh T. K., Bian J. H., Short A. D., Rybak S. L., Gill D. L. Persistent intracellular calcium pool depletion by thapsigargin and its influence on cell growth. J Biol Chem. 1991 Dec 25;266(36):24690–24697. [PubMed] [Google Scholar]
- Ghosh T. K., Bian J., Gill D. L. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990 Jun 29;248(4963):1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Glennon M. C., Bird G. S., Kwan C. Y., Putney J. W., Jr Actions of vasopressin and the Ca(2+)-ATPase inhibitor, thapsigargin, on Ca2+ signaling in hepatocytes. J Biol Chem. 1992 Apr 25;267(12):8230–8233. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hurley T. W., Ryan M. P., Brinck R. W. Changes of cytosolic Ca2+ interfere with measurements of cytosolic Mg2+ using mag-fura-2. Am J Physiol. 1992 Aug;263(2 Pt 1):C300–C307. doi: 10.1152/ajpcell.1992.263.2.C300. [DOI] [PubMed] [Google Scholar]
- Kemmer T. P., Bayerdörffer E., Will H., Schulz I. Anion dependence of Ca2+ transport and (Ca2+ + K+)-stimulated Mg2+-dependent transport ATPase in rat pancreatic endoplasmic reticulum. J Biol Chem. 1987 Oct 5;262(28):13758–13764. [PubMed] [Google Scholar]
- Krause K. H., Pittet D., Volpe P., Pozzan T., Meldolesi J., Lew D. P. Calciosome, a sarcoplasmic reticulum-like organelle involved in intracellular Ca2+-handling by non-muscle cells: studies in human neutrophils and HL-60 cells. Cell Calcium. 1989 Jul;10(5):351–361. doi: 10.1016/0143-4160(89)90061-4. [DOI] [PubMed] [Google Scholar]
- London R. E. Methods for measurement of intracellular magnesium: NMR and fluorescence. Annu Rev Physiol. 1991;53:241–258. doi: 10.1146/annurev.ph.53.030191.001325. [DOI] [PubMed] [Google Scholar]
- Malinowska D. H. Permeabilizing parietal cells. Methods Enzymol. 1990;192:108–124. doi: 10.1016/0076-6879(90)92065-l. [DOI] [PubMed] [Google Scholar]
- Mullaney J. M., Yu M., Ghosh T. K., Gill D. L. Calcium entry into the inositol 1,4,5-trisphosphate-releasable calcium pool is mediated by a GTP-regulatory mechanism. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2499–2503. doi: 10.1073/pnas.85.8.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Freudenrich C. C., Levy L. A., London R. E., Lieberman M. Monitoring cytosolic free magnesium in cultured chicken heart cells by use of the fluorescent indicator Furaptra. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2981–2984. doi: 10.1073/pnas.86.8.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negulescu P. A., Machen T. E. Intracellular Ca regulation during secretagogue stimulation of the parietal cell. Am J Physiol. 1988 Jan;254(1 Pt 1):C130–C140. doi: 10.1152/ajpcell.1988.254.1.C130. [DOI] [PubMed] [Google Scholar]
- Negulescu P. A., Machen T. E. Intracellular ion activities and membrane transport in parietal cells measured with fluorescent dyes. Methods Enzymol. 1990;192:38–81. doi: 10.1016/0076-6879(90)92062-i. [DOI] [PubMed] [Google Scholar]
- Negulescu P. A., Reenstra W. W., Machen T. E. Intracellular Ca requirements for stimulus-secretion coupling in parietal cell. Am J Physiol. 1989 Feb;256(2 Pt 1):C241–C251. doi: 10.1152/ajpcell.1989.256.2.C241. [DOI] [PubMed] [Google Scholar]
- Quamme G. A., Rabkin S. W. Cytosolic free magnesium in cardiac myocytes: identification of a Mg2+ influx pathway. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1406–1412. doi: 10.1016/0006-291x(90)90679-h. [DOI] [PubMed] [Google Scholar]
- Raju B., Murphy E., Levy L. A., Hall R. D., London R. E. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989 Mar;256(3 Pt 1):C540–C548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- Reinlib L., Jefferson D. J., Marini F. C., Donowitz M. Abnormal secretagogue-induced intracellular free Ca2+ regulation in cystic fibrosis nasal epithelial cells. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2955–2959. doi: 10.1073/pnas.89.7.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe M. W., Lemasters J. J., Herman B. Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium. 1990 Feb-Mar;11(2-3):63–73. doi: 10.1016/0143-4160(90)90060-8. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990 Apr 20;61(2):197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Dawson A. P., Scharff O., Foder B., Cullen P. J., Drøbak B. K., Bjerrum P. J., Christensen S. B., Hanley M. R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989 Apr;27(1-2):17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Thomas H. A., Machen T. E. Regulation of Cl/HCO3 exchange in gastric parietal cells. Cell Regul. 1991 Sep;2(9):727–737. doi: 10.1091/mbc.2.9.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent indicators of ion concentrations. Methods Cell Biol. 1989;30:127–156. doi: 10.1016/s0091-679x(08)60978-4. [DOI] [PubMed] [Google Scholar]
- Tsien R., Pozzan T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 1989;172:230–262. doi: 10.1016/s0076-6879(89)72017-6. [DOI] [PubMed] [Google Scholar]
- Webb W. W., Anders M. W. Coupling of ATP synthesis to reversal of rat liver microsomal Ca2+-ATPase. Biochemistry. 1985 Dec 17;24(26):7741–7745. doi: 10.1021/bi00347a036. [DOI] [PubMed] [Google Scholar]