Abstract
Purpose
There is no standard second-line regimen for malignant melanoma patients with disease progression after first-line chemotherapy, and platinum-alkylating agents combined with paclitaxel have shown modest efficacy.
Materials and Methods
We conducted a phase II, open-label, single-arm study to test the efficacy of docetaxel combined with carboplatin for malignant melanoma patients who failed previous treatment with dacarbazine. Intravenous docetaxel (35 mg/m2 on days 1 and 8 of each cycle) and carboplatin (area under the curve 3 on days 1 and 8 of each cycle) was administered every 21 days. Primary end point was objective response rate (ORR).
Results
Thirty patients were enrolled in the study, and the median follow-up duration was 19.8 months. Among 25 per-protocol patients, there were three responders (1 with complete response and 2 with partial response) and 17 stable disease patients (ORR, 12.0%). Among the per-protocol population, the median progression-free survival (PFS) was 4.3 months and the median overall survival (OS) was 9.6 months. Uveal melanoma patients (n=9) showed the best prognosis compared to other subtypes (median PFS, 7.6 months; OS, 9.9 months). The most common grade 3 or 4 adverse event was neutropenia (n=15, 50.0%).
Conclusion
Docetaxel combined with carboplatin showed association with an acceptable safety profile and overall efficacy for patients with malignant melanoma who had progressed on chemotherapy containing dacarbazine.
Keywords: Melanoma, Docetaxel, Carboplatin, Second-line chemotherapy
Introduction
The incidence of melanoma has shown a rapid increase, with 76,100 estimated new cases and 9,710 estimated deaths per year in the United States in 2014 [1]. Geographic and ethnic differences for malignant melanoma have been reported between Asian and Caucasian patients [2]. The incidence of melanoma in Asia is rare, with an incidence rate of 0.2 to 0.5 per 100,000 patients per year. In Asian populations approximately 65% of all cases are mucosal and acral melanomas, whereas melanoma of the skin on the trunk and legs is the most common in Caucasian populations [3].
New targeted and immunotherapeutic agents have recently been approved for the initial management of patients with stage IV melanoma, including vemurafenib, dabrafenib, and trametinib for patients with the V600 mutated BRAF gene, and immune checkpoint inhibitors such as ipilimumab and nivolumab. In Asia, because acral or mucosal melanoma patients harboring the V600 mutated BRAF gene are rarely seen, many malignant melanoma patients are not eligible for treatment with BRAF targeting agents. In addition, owing to cost and insurance coverage, many patients cannot afford immune checkpoint inhibitors.
Among many immunotherapeutic and cytotoxic chemotherapeutic agents evaluated as systemic treatment for metastatic melanoma, only two were approved for metastatic melanoma before 2011: high-dose interleukin-2 (IL-2) and dacarbazine. High-dose IL-2 has been shown to induce durable remission for some patients, but it is one of the most toxic cancer treatments. Thus, dacarbazine is usually offered as first-line chemotherapy to most malignant melanoma patients, especially in Asia. However, the response rates (RRs) for both therapies are only 10% to 20%, and neither is thought to improve overall survival (OS) [4,5].
There is no standard second-line regimen for malignant melanoma patients with disease progression after first-line chemotherapy, even though progression-free survival (PFS) is short in malignant melanoma. Other cytotoxic agents have been investigated, including the combination of platinumalkylating agents and paclitaxel, which has demonstrated modest antitumor activity against malignant melanoma in various clinical trials with a median PFS of 3 to 4 months and RR of 11% to 26% [6,7]. The toxicity profiles of these agents do not overlap. Docetaxel has shown antitumor efficacy in vitro [8,9], and some studies have reported that it is a more potent antiangiogenesis agent than paclitaxel [10,11]. However, no prospective study to evaluate the efficacy of platinum-alkylating agents combined with docetaxel for malignant melanoma has been reported.
The aim of this study was to assess the efficacy of docetaxel combined with carboplatin as second-line treatment in malignant melanoma patients who failed first-line dacarbazine or temozolomide therapy.
Materials and Methods
1. Patients
Eligible patients were at least 20 years old and had histologically confirmed malignant melanoma (recurred or metastatic) that had progressed during or after at least one cycle of a dacarbazine- or temozolomide-containing regimen in the advanced setting. Additional eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2; at least one measurable lesion by computed tomography (CT) according to Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1; adequate bone marrow, liver, and renal function; and life expectancy of at least 12 weeks. Exclusion criteria were as follows: pregnancy or breast feeding; symptomatic brain metastasis; previous history of treatment with chemotherapy containing taxane or platinum agents; major surgery within 2 weeks before the start of the trial or failure to recover from a surgery; previous history of other malignancies within 5 years except for cured skin basal cell carcinoma or cured in situ cervix cancer; other severe medical conditions including uncontrolled infection, uncontrolled hypertension, heart failure, and myocardial infarction history within 6 months; sensitivity to platinum agents or docetaxel; uncontrolled seizure; and alcohol or drug abuse.
2. Study design and end points
The current phase II, open-label, single-arm study was conducted in a single center, Yonsei Cancer Center, Seoul, Korea. Intravenous docetaxel (35 mg/m2 on days 1 and 8 of each cycle) and carboplatin (area under the curve 3 on days 1 and 8 of each cycle) were administered every 21 days. Dexamethasone (8 mg) was administered orally or intravenously 30 minutes before docetaxel infusion to prevent fluid retention. Study treatment was continued until unacceptable toxicity, tumor progression, patient death, or withdrawal of patient consent. The primary end point of this study was objective response rate (ORR). The secondary end points were disease control rate (DCR), PFS, OS, and safety. Tumor response and disease progression were evaluated according to RECIST ver. 1.1 guideline. Tumors were assessed radiographically using CT every two cycles (every 6 weeks) during the treatment period. Patients who discontinued study treatment were followed every 3 months for 1 year, and every 6 months for the following year, or until death, for collection of data on OS. The National Cancer Institute’s Common Terminology Criteria for Adverse Events ver. 4.0 was used to grade adverse events and to determine the need for dose modifications. The doses of docetaxel and carboplatin were reduced by 20% following the occurrence of grade 4 neutropenia lasting more than 7 days, grade 4 thrombocytopenia, febrile neutropenia, or any grade 3 or 4 nonhematologic toxicity. The protocol was approved by the Institutional Review and Ethics Board of Severance Hospital, Seoul, Korea. The study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines defined by the International Conference on Harmonization. All patients provided written informed consent before enrollment. The trial is registered at ClinicalTrials.gov (NCT02223884).
3. Statistical analyses
The safety population included all patients who received at least one dose of study drug (intent-to-treat population). For efficacy endpoints, primary analyses were performed in the per-protocol patients who at least completed treatment until the first response evaluation. For calculation of sample size, Simon’s two-stage minimax design was applied to test the null hypothesis of 5% as opposed to the alternative hypothesis of 20%. Assuming an α-error of 0.05 and a β-error of 0.2, 13 evaluable patients had to be accrued during stage 1, and if at least one patient responded with complete or partial response, 14 additional patients were to be enrolled in the study during stage 2. A sample size of 30 patients was planned, allowing for a 10% dropout rate. Kaplan-Meier estimates and Cox regression analyses of DFS and OS were calculated. All reported p-values were two-sided. Data were analyzed using SPSS ver. 18.0 statistical software (SPSS Inc., Chicago, IL).
Results
1. Patient characteristics
Between September 2011 and April 2014, 30 malignant melanoma patients who failed chemotherapy containing dacarbazine were enrolled in the study (Fig. 1). Patient demographics and baseline disease characteristics are described in Table 1. Most of the patients had an ECOG performance status of 0 (73.3%), and about half of them had liver (46.7%) or lung metastasis (50.0%). BRAF V600 mutation was found in five patients (16.7%) and c-Kit mutation in two (6.7%). Prior to study enrollment, each patient had received at least one chemotherapy regimen containing dacarbazine. Half of the patients had undergone previous radiotherapy.
Fig. 1.
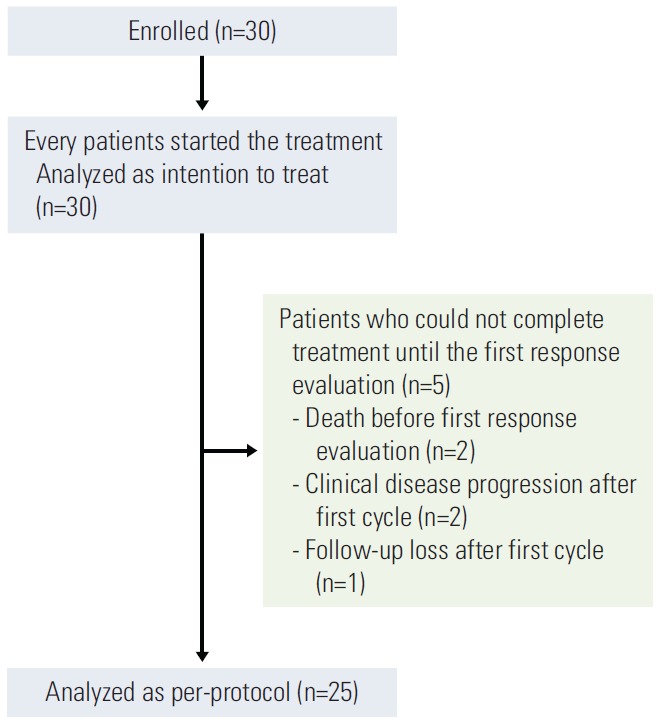
Study flow diagram.
Table 1.
Baseline patient demographic and clinical characteristics (n=30)
| Characteristic | No. (%) |
|---|---|
| Median age (range, yr) | 53 (20-75) |
| Gender | |
| Male | 18 (60.0) |
| Female | 12 (40.0) |
| ECOG performance status | |
| 0 | 22 (73.3) |
| 1 | 8 (26.7) |
| Tumor subtype | |
| Non-CSD | 6 (20.0) |
| Acral | 5 (16.7) |
| Mucosal | 7 (23.3) |
| Uveal | 10 (33.3) |
| Conjunctival | 2 (6.7) |
| Lactate dehydrogenase level at baseline | |
| Normal | 14 (46.7) |
| Above normal | 16 (53.3) |
| Liver metastasis | 14 (46.7) |
| Lung metastasis | 15 (50.0) |
| BRAF mutation | |
| Wild type | 25 (83.3) |
| V600E | 5 (16.7) |
| c-Kit mutation | |
| Wild type | 14 (46.7) |
| Exon 11 mutation | 2 (6.7) |
| Not evaluated | 14 (46.7) |
| Previous chemotherapy regimen | |
| Dacarbazine | 26 (86.7) |
| Dacarbazine and vemurafenib | 3 (10.0) |
| Cisplatin/vinblastine/dacarbazine | 1 (3.3) |
| Previous radiotherapy | 15 (50.0) |
ECOG, Eastern Cooperative Oncology Group; CSD, chronic sun damage.
Patients’ melanomas were categorized according to their locations and degree of exposure to the sun [12] as follows: those arising from the skin with chronic sun-induced (CSD) damage (CSD melanoma), those occurring on the skin without chronic sun-induced damage (non-CSD melanoma), those found on sun-protected skin areas such as the palms, soles, or subungual sites (acral melanomas), and those on mucosal membranes (mucosal melanomas). In addition to these four subtypes, patients with ocular melanomas were also treated in this study. Because there are significant differences in molecular pathogenesis between uveal (choroidal) and conjunctival melanomas [13], the two conditions were analyzed separately. In our study cohort, the most common melanoma subtype was uveal melanoma (n=10, 33.3%), followed by mucosal melanoma (n=7, 23.3%), non-CSD melanoma (n=6, 20.0%), acral melanoma (n=5, 16.7%), and conjunctival melanoma (n=2, 6.7%). Among non-CSD and conjunctival melanoma patients, four out of eight patients (50.0%) had BRAF V600 mutations, which is comparable to the known incidence (approximately 40% to 60%). Among acral and mucosal melanoma patients, one out of 12 patients (8.3%) had BRAF V600 mutation, which is also comparable to the known incidence (less than 10%).
2. Efficacy analysis
The median follow-up duration was 19.8 months. At the cut-off date for data collection on August 20, 2014, one patient with uveal melanoma was still receiving on-going treatment. Of the 30 enrolled patients, 25 (83.3%) could be evaluated for tumor response as five patients did not receive treatment until the first response evaluation. In the intentto-treat population (n=30), the median PFS was 3.7 months, and the median OS was 9.4 months. In the per-protocol population, the median PFS was 4.3 months (22 PFS events among 25 patients), and the median OS was 9.6 months (16 OS events among 25 patients) (Fig. 2). Among 25 per-protocol patients, there were three responders (1 with complete response [CR] and 2 with partial response [PR]) and 17 patients with stable disease (SD). The ORR was 12.0% (n=3; 95% confidence interval [CI], 0% to 24.74%), and DCR was 80.0% (n=20; 95% CI, 64.32% to 95.68%). All deaths were cancer-related. No statistically significant differences were observed among responders, patients with SD, and those with progression regarding ECOG performance status, tumor subtype, lactate dehydrogenase (LDH) level at baseline, liver or lung metastasis, BRAF or c-Kit mutation, previous chemotherapy regimen, and history of previous radiotherapy (Table 2). Responders had significantly longer median PFS (6.0 months vs. 4.9 months vs. 1.3 months, respectively; p < 0.001). ECOG performance status (hazard ratio [HR], 5.399; 95% CI, 1.661 to 17.549; p=0.005) and LDH level at baseline (HR, 4.331; 95% CI, 1.454 to 12.898; p=0.008) were the only two covariates showing association with worse PFS (Table 3). Age, sex, BRAF or c-Kit mutation status, liver or lung metastasis, and previous radiotherapy did not show statistical association with either PFS or OS.
Fig. 2.
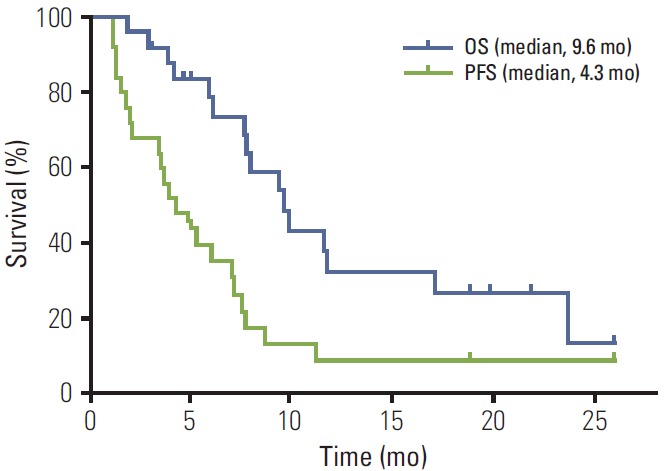
Survival analysis. Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) of per-protocol patients (n=25).
Table 2.
Results of therapy based on response (per-protocol population, n=25 a))
| Variable | All patients (n=25) | Responders (n=3) | SD (n=17) | PD (n=5) | p-value |
|---|---|---|---|---|---|
| ECOG performance status | 0.357 | ||||
| 0 | 20 (80.0) | 3 (100) | 14 (82.4) | 3 (60.0) | |
| 1 | 5 (20.0) | 0 | 3 (17.6) | 2 (40.0) | |
| Tumor subtype | 0.083 | ||||
| Skin | 5 (20.0) | 1 (33.3) | 2 (11.8) | 2 (40.0) | |
| Acral | 5 (20.0) | 2 (66.7) | 2 (11.8) | 1 (20.0) | |
| Mucosal | 6 (24.0) | 0 | 4 (23.5) | 2 (40.0) | |
| Choroidal | 9 (36.0) | 0 | 9 (52.9) | 0 | |
| LDH level at baseline | 0.599 | ||||
| Normal | 13 (52.0) | 1 (33.3) | 10 (58.8) | 2 (40.0) | |
| Above normal | 12 (48.0) | 2 (66.7) | 7 (41.2) | 3 (60.0) | |
| Liver metastasis | 12 (48.0) | 0 | 10 (58.8) | 2 (40.0) | 0.158 |
| Lung metastasis | 13 (52.0) | 2 (66.7) | 9 (52.9) | 2 (40.0) | 0.758 |
| BRAF mutation | 0.620 | ||||
| Wild type | 21 (84.0) | 2 (66.7) | 15 (88.2) | 4 (80.0) | |
| V600E | 4 (16.0) | 1 (33.3) | 2 (5.9) | 1 (20.0) | |
| c-Kit mutation | 0.257 | ||||
| Wild type | 13 (52.0) | 2 (66.7) | 8 (47.1) | 3 (60.0) | |
| Exon 11 mutation | 1 (4.0) | 0 | 0 | 1 (20.0) | |
| Not evaluated | 11 (44.0) | 1 (33.3) | 9 (52.9) | 1 (20.0) | |
| Previous chemotherapy regimen | 0.461 | ||||
| Dacarbazine | 22 (88.0) | 2 (66.7) | 15 (88.2) | 5 (100) | |
| Dacarbazine and vemurafenib | 2 (8.0) | 1 (33.3) | 1 (5.9) | 0 | |
| Cisplatin/vinblastine/dacarbazine | 1 (4.0) | 0 | 1 (5.9) | 0 | |
| Previous radiotherapy | 11 (44.0) | 2 (66.7) | 6 (35.3) | 3 (60.0) | 0.434 |
| Median chemotherapy cycles (range) | 5.5 (2-12) | 8 (8-12) | 6 (2-12) | 2 (2) | |
| Median progression-free survival (mo) | 4.3 | 6.0 | 4.9 | 1.3 | < 0.001 |
| Median overall survival (mo) | 9.6 | 9.6 | 9.9 | 5.9 | 0.194 |
Values are presented as number (%) unless otherwise indicated. SD, stable disease; PD, progressive disease; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase.
Five patients were inevaluable.
Table 3.
Univariate analysis of PFS and OS (per-protocol analysis, n=25)
| Parameter | No (%) | PFS |
OS |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Age (yr) | |||||||
| < 60 | 20 (80.0) | Reference | Reference | ||||
| ≥ 60 | 5 (20.0) | 2.170 | 0.746-6.310 | 0.155 | 0.931 | 0.296-2.929 | 0.903 |
| Gender | |||||||
| Female | 8 (32.0) | Reference | Reference | ||||
| Male | 17 (68.0) | 1.731 | 0.665-4.504 | 0.261 | 3.273 | 0.907-11.819 | 0.070 |
| ECOG | |||||||
| 0 | 20 (80.0) | Reference | Reference | ||||
| 1 | 5 (20.0) | 5.399 | 1.661-17.549 | 0.005 | 1.409 | 0.481-4.130 | 0.532 |
| LDH level at baseline | |||||||
| Normal | 13 (52.0) | Reference | Reference | ||||
| Above normal | 12 (48.0) | 4.331 | 1.454-12.898 | 0.008 | 1.917 | 0.710-5.473 | 0.193 |
| BRAF | |||||||
| Wild type | 21 (84.0) | Reference | Reference | ||||
| V600E | 4 (16.0) | 0.890 | 0.260-3.049 | 0.853 | 0.867 | 0.243-3.094 | 0.825 |
| c-Kit a) | |||||||
| Wild type | 13 (52.0) | Reference | Reference | ||||
| Exon 11 mutation | 1 (4.0) | 3.890 | - | 0.240 | 4.947 | - | 0.195 |
| Liver metastasis | 12 (48.0) | 1.105 | 0.477-2.559 | 0.815 | 1.483 | 0.519-4.237 | 0.462 |
| Lung metastasis | 13 (52.0) | 0.783 | 0.338-1.814 | 0.568 | 1.013 | 0.364-2.815 | 0.980 |
| Previous radiotherapy | 11 (44.0) | 0.866 | 0.371-2.025 | 0.740 | 1.041 | 0.440-2.463 | 0.927 |
PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase.
Only 14 patients underwent c-Kit mutation analysis.
Prognosis and demographics differed according to malignant melanoma subtypes (Table 4). Uveal melanoma patients (n=9) had the best prognosis when treated with docetaxel plus carboplatin compared to those with other subtypes (median PFS, 7.6 months; OS, 9.9 months). All patients with uveal melanoma had the best response as SD. Uveal melanoma patients also experienced a high incidence of liver metastasis (n=8, 88.9%) with no BRAF or c-Kit mutation. Patients with acral melanoma (n=5), another melanoma subtype that is abundant in Asia, had better OS (median OS, 9.6 months) than those with non-CSD or mucosal melanoma.
Table 4.
Results of therapy based on tumor subtype (per-protocol population, n=25)
| Variable | All patients (n=25) | Non-CSD (n=5) | Acral (n=5) | Mucosal (n=4) | Uveal (n=9) | Conjunctival (n=2) | p-value | |
|---|---|---|---|---|---|---|---|---|
| ECOG performance status | 0.014 | |||||||
| 0 | 20 (80.0) | 4 (80.0) | 3 (60.0) | 4 (100) | 9 (100) | 0 | ||
| 1 | 5 (20.0) | 1 (20.0) | 2 (40.0) | 0 | 0 | 2 (100) | ||
| LDH level at baseline | 0.481 | |||||||
| Normal | 13 (52.0) | 3 (60.0) | 2 (40.0) | 3 (75.0) | 5 (55.6) | 0 | ||
| Above normal | 12 (48.0) | 2 (40.0) | 3 (60.0) | 1 (25.0) | 4 (44.4) | 2 (100) | ||
| Liver metastasis | 12 (48.0) | 3 (60.0) | 0 | 1 (16.7) | 8 (88.9) | 0 | 0.009 | |
| Lung metastasis | 13 (52.0) | 3 (60.0) | 3 (60.0) | 3 (75.0) | 3 (33.3) | 1 (50.0) | 0.669 | |
| BRAF mutation | 0.171 | |||||||
| Wild type | 21 (84.0) | 3 (60.0) | 4 (80.0) | 4 (100) | 9 (100.0) | 1 (50.0) | ||
| V600E | 4 (16.0) | 2 (40.0) | 1 (20.0) | 0 | 0 | 1 (50.0) | ||
| c-Kit mutation | 0.563 | |||||||
| Wild type | 13 (52.0) | 1 (20.0) | 4 (80.0) | 2 (50.0) | 5 (55.6) | 1 (50.0) | ||
| Exon 11 mutation | 1 (4.0) | 1 (20.0) | 0 | 0 | 0 | 0 | ||
| Not evaluated | 11 (44.0) | 3 (60.0) | 1 (20.0) | 2 (50.0) | 4 (44.4) | 1 (50.0) | ||
| Previous chemotherapy regimen | 0.160 | |||||||
| Dacarbazine | 22 (88.0) | 3 (60.0) | 5 (100) | 4 (100) | 9 (100) | 1 (50.0) | ||
| Dacarbazine and vemurafenib | 2 (8.0) | 1 (20.0) | 0 | 0 | 0 | 1 (50.0) | ||
| Cisplatin/vinblastine/dacarbazine | 1 (4.0) | 1 (20.0) | 0 | 0 | 0 | 0 | ||
| Previous radiotherapy | 11 (44.0) | 4 (80.0) | 1 (20.0) | 4 (100) | 1 (11.1) | 1 (50.0) | 0.012 | |
| Median chemotherapy cycles (range) | 5.5 (2-12) | 2(2-8) | 6 (2-12) | 6 (2-8) | 7 (4-12) | 2.5 (2-3) | - | |
| Response | 0.164 | |||||||
| CR | 1 (4.0) | 1 (20.0) | 0 | 0 | 0 | 0 | ||
| PR | 2 (8.0) | 0 | 2 (40.0) | 0 | 0 | 0 | ||
| SD | 17 (68.0) | 2 (40.0) | 2 (40.0) | 3 (75.0) | 9 (100) | 1 (50.0) | ||
| PD | 5 (20.0) | 2 (40.0) | 1 (20.0) | 1 (25.0) | 0 | 1 (50.0) | ||
| Median progression-free survival (mo) | 4.3 | 1.8 | 3.9 | 7.1 | 7.6 | 1.2 | 0.061 | |
| Median overall survival (mo) | 9.6 | 5.9 | 9.6 | 7.8 | 9.9 | 7.7 | 0.798 |
Values are presented as number (%) unless otherwise indicated. CSD, chronic sun damage; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
3. Treatment compliance and toxicity
The median number of treatment cycles was 4.5 (range, 1 to 15 cycles). The median relative dose intensity (RDI) was 1 (range, 0.3 to 1) for both docetaxel and carboplatin. Thirteen patients (43.3%) had dose reduction, and treatment initiation was delayed for 16 patients (53.3%). The most common reason for delay and dose reduction was neutropenia.
Most patients (n=29, 96.7%) reported at least one adverse event related to treatment, and the total incidence of grade 3 or 4 adverse events was 66.7% (n=20) (Table 5). The most common adverse event was neutropenia (all grades; n=20, 67.7%), with half of the patients experiencing grade 3 or 4 neutropenia (n=15, 50.0%). The incidence of grade 3 or 4 anemia and thrombocytopenia was 10.0% (n=3) and 13.3% (n=4), respectively. Nonhematologic toxicity was less common and less severe than hematologic toxicity. Observed grade 3 or 4 nonhematologic adverse events included anorexia (n=3, 10.0%), vomiting (n=2, 6.7%), nausea, peripheral neuropathy, epistaxis, skin ulceration, and infection (n=1, 3.3% each). In one patient, peripheral neuropathy led to treatment cessation (Fig. 1).
Table 5.
Incidences of treatment related adverse events (n=30)
| Treatment related adverse event | Grade 3 or 4 | All grades |
|---|---|---|
| Any event | 20 (67.7) | 29 (96.7) |
| Hematologic toxicity | ||
| Febrile neutropenia | 1 (3.3) | 1 (3.3) |
| Neutropenia | 15 (50.0) | 20 (67.7) |
| Anemia | 3 (10.0) | 6 (20.0) |
| Thrombocytopenia | 4 (13.3) | 7 (23.3) |
| Nonhematologic toxicity | ||
| Anorexia | 3 (10.0) | 14 (46.7) |
| Nausea | 1 (3.3) | 13 (43.3) |
| Alopecia | 0 | 13 (43.3) |
| Diarrhea | 0 | 8 (26.7) |
| Myalgia | 0 | 8 (26.7) |
| General weakness | 0 | 7 (23.3) |
| Peripheral neuropathy | 1 (3.3) | 5 (16.7) |
| Vomiting | 2 (6.7) | 4 (13.3) |
| Dyspnea | 0 | 4 (13.3) |
| Weight loss | 0 | 4 (13.3) |
| Mucositis | 0 | 3 (10.0) |
| Abdominal pain | 0 | 2 (6.7) |
| Nail loss | 0 | 2 (6.7) |
| Pleural effusion | 0 | 2 (6.7) |
| Epistaxis | 1 (3.3) | 2 (6.7) |
| Skin ulceration | 1 (3.3) | 2 (6.7) |
| Weight gain | 0 | 2 (6.7) |
| AST elevation | 0 | 2 (6.7) |
| Edema | 0 | 2 (6.7) |
| Infection | 1 (3.3) | 1 (3.3) |
Values are presented as number (%). Incidences of treatment related adverse events with more than 5% or any adverse events in grade 3 or 4 are shown in the table. Neutropenia was the most common adverse event. AST, aspartate aminotransferase.
Discussion
We herein report the results of a phase II study of docetaxel combined with carboplatin as second-line treatment for malignant melanoma patients experiencing disease progression on chemotherapy containing dacarbazine. Our findings suggested that in this patient population, the combination of docetaxel and carboplatin was a reasonable option for second-line treatment.
Several prospective and retrospective studies on second-line treatment for malignant melanoma [6,14-16] have reported a median PFS between 1.3 and 4.1 months and OS between 3.5 and 9.8 months. In these studies, the combination of paclitaxel and carboplatin resulted in the best survival (PFS, 4.1 months; OS, 9.8 months) [16]. Results of our study were comparable with those from previous reports with a median PFS of 4.3 months and a median OS of 9.6 months. ECOG performance status and baseline LDH level were the two covariates with significant effects on PFS. In previous second-line studies, the reported ORR was between 5.3% and 26.0%, whereas DCR between 18.9% and 62% was reported. Our results showed an ORR of 12.0% and a DCR of 80%. The treatment was also tolerable and manageable. The median RDI was 1 for both agents. Major adverse events were hematologic toxicity, and 50.0% of the patients suffered grade 3 or 4 neutropenia. Although dose reduction and delayed treatment were required in about half of the patients, only one patient had to discontinue treatment due to toxicity.
In addition to reporting the overall efficacy of the regimen, the current study successfully demonstrated the profound survival benefit by docetaxel combined with carboplatin in patients with uveal melanoma. Beside non-CSD (20.0%), acral (16.7%), mucosal (23.3%) and conjunctival melanoma (6.7%), uveal melanoma was the most common subtype (n=10, 33.3%). The disease represents only 3% to 5% of all melanoma cases [17], and in the largest reported series of unselected cases to date, patients with metastatic uveal melanoma had a poor prognosis with a median OS of only 3.6 months [18]. In our study, uveal melanoma was the subtype that showed the best survival with a median PFS of 7.6 months and OS of 9.9 months. In previously reported clinical trials of first-line chemotherapy in uveal melanoma [19-21], the median PFS ranged between 1.6 and 4.0 months and OS between 7.7 and 11.8 months. Considering that our study patients had failed at least one prior chemotherapy regimen, the results of this study were very promising despite the small number of patients. On the other hand, a phase II trial of the novel targeted agent selumetinib, a mitogen-activated protein kinase inhibitor, as first-line treatment for uveal melanoma [20] reported a median PFS of 3.7 months and OS of 11.8 months. In this study, the median OS calculated from the starting date of the first-line chemotherapy containing dacarbazine was 13.8 months for uveal melanoma patients. Although none of the uveal melanoma patients in our study had CR or PR, they all had SD. Two previous trials of first-line chemotherapy for uveal melanoma also reported no patients with response. Oncogenic mutations in guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or guanine nucleotide binding protein (G protein), alpha 11 (Gq class) (GNA11) have been reported in more than 80% of uveal melanomas [22,23]; however, in our study, only one uveal melanoma patient had GNA11 Q209L mutation. None of the uveal melanoma patients in our study had BRAF or c-Kit mutation.
Conclusion
In conclusion, our results from this phase II study indicated that weekly docetaxel combined with carboplatin was well tolerated and demonstrated overall efficacy in malignant melanoma patients experiencing disease progression on chemotherapy containing dacarbazine. In particular, the profound survival benefit from this combination chemotherapy among patients with uveal melanoma subtype warrants further clinical investigation.
Acknowledgments
This study was supported supported by a faculty research grant of Yonsei University College of Medicine for 2014 (6-2014-0105).
Footnotes
This study was funded by Boryung Pharmaceutical, Co., Ltd., Seoul, Korea
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Lee HY, Chay WY, Tang MB, Chio MT, Tan SH. Melanoma: differences between Asian and Caucasian patients. Ann Acad Med Singapore. 2012;41:17–20. [PubMed] [Google Scholar]
- 3.Ishihara K, Saida T, Otsuka F, Prognosis and Statistical Investigation Committee of the Japanese Skin Cancer Society Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int J Clin Oncol. 2008;13:33–41. doi: 10.1007/s10147-007-0751-1. [DOI] [PubMed] [Google Scholar]
- 4.Comis RL. DTIC (NSC-45388) in malignant melanoma: a perspective. Cancer Treat Rep. 1976;60:165–76. [PubMed] [Google Scholar]
- 5.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 6.Rao RD, Holtan SG, Ingle JN, Croghan GA, Kottschade LA, Creagan ET, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006;106:375–82. doi: 10.1002/cncr.21611. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty KT, Lee SJ, Zhao F, Schuchter LM, Flaherty L, Kefford R, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol. 2013;31:373–9. doi: 10.1200/JCO.2012.42.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mhaidat NM, Wang Y, Kiejda KA, Zhang XD, Hersey P. Docetaxel-induced apoptosis in melanoma cells is dependent on activation of caspase-2. Mol Cancer Ther. 2007;6:752–61. doi: 10.1158/1535-7163.MCT-06-0564. [DOI] [PubMed] [Google Scholar]
- 9.Mhaidat NM, Zhang XD, Jiang CC, Hersey P. Docetaxel-induced apoptosis of human melanoma is mediated by activation of c-Jun NH2-terminal kinase and inhibited by the mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway. Clin Cancer Res. 2007;13:1308–14. doi: 10.1158/1078-0432.CCR-06-2216. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss KA, Ashton AW, Mahmood R, Russell RG, Sparano JA, Schwartz EL. Inhibition of endothelial cell function in vitro and angiogenesis in vivo by docetaxel (Taxotere): association with impaired repositioning of the microtubule organizing center. Mol Cancer Ther. 2002;1:1191–200. [PubMed] [Google Scholar]
- 11.Grant DS, Williams TL, Zahaczewsky M, Dicker AP. Comparison of antiangiogenic activities using paclitaxel (taxol) and docetaxel (taxotere) Int J Cancer. 2003;104:121–9. doi: 10.1002/ijc.10907. [DOI] [PubMed] [Google Scholar]
- 12.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 13.Griewank KG, Westekemper H, Murali R, Mach M, Schilling B, Wiesner T, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143–52. doi: 10.1158/1078-0432.CCR-13-0163. [DOI] [PubMed] [Google Scholar]
- 14.Perrin C, Pracht M, Talour K, Adamski H, Cumin I, Porneuf M, et al. Metastatic melanoma: results of 'classical' second-line treatment with cytotoxic chemotherapies. J Dermatolog Treat. 2014;25:396–400. doi: 10.3109/09546634.2012.697986. [DOI] [PubMed] [Google Scholar]
- 15.Eisen T, Trefzer U, Hamilton A, Hersey P, Millward M, Knight RD, et al. Results of a multicenter, randomized, double-blind phase 2/3 study of lenalidomide in the treatment of pretreated relapsed or refractory metastatic malignant melanoma. Cancer. 2010;116:146–54. doi: 10.1002/cncr.24686. [DOI] [PubMed] [Google Scholar]
- 16.Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase III, randomized, placebocontrolled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 17.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–5. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123:1639–43. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia S, Moon J, Margolin KA, Weber JS, Lao CD, Othus M, et al. Phase II trial of sorafenib in combination with carboplatin and paclitaxel in patients with metastatic uveal melanoma: SWOG S0512. PLoS One. 2012;7: doi: 10.1371/journal.pone.0048787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvajal RD, Sosman JA, Quevedo JF, Milhem MM, Joshua AM, Kudchadkar RR, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014;311:2397–405. doi: 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmittel A, Scheulen ME, Bechrakis NE, Strumberg D, Baumgart J, Bornfeld N, et al. Phase II trial of cisplatin, gemcitabine and treosulfan in patients with metastatic uveal melanoma. Melanoma Res. 2005;15:205–7. doi: 10.1097/00008390-200506000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–9. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]


