Abstract
Purpose
Regorafenib, an oral multi-targeted tyrosine kinase inhibitor, is considered the new standard of care in patients with chemotherapy-refractory colorectal cancers (CRCs). However, there are no data on this drug in Korean patients.
Materials and Methods
We evaluated patients who received oral regorafenib 160 mg once daily during the first 3 weeks of each 4-week cycle between August 2013 and September 2013. All patients had previously progressed fluorouracil, irinotecan, and oxaliplatin with or without biologic agents such as cetuximab or bevacizumab.
Results
Thirty-two patients were enrolled (median age, 57 years; male:female ratio, 20:12; Eastern Cooperative Oncology Group performance status [0-1:2], 31:1; colon:rectum, 21:11). The overall response rate was 3.1% and the disease control rate was 50.0% (95% confidence interval [CI]) with one partial response and 15 patients with stable disease. The median progression-free survival was 4.2 months (95% CI, 3.1 to 5.2 months) and the median overall survival has not yet been reached. The most common adverse events of grade two or higher related to regorafenib were hand-foot skin reaction (25%), mucositis (19%), abdominal pain (9%), and liver function test (LFT) abnormalities (9%). Grade 3 or 4 toxicities included LFT abnormalities (9%), abdominal pain (9%), rash (6%), anemia (3%), leukopenia (3%), neutropenic fever (3%), and fatigue (3%). There was no treatment-related death.
Conclusion
Regorafenib appears to have promising activity and tolerable toxicity profiles in Korean patients with refractory CRC, consistent with the CORRECT trial findings.
Keywords: Regorafenib, Korea, Colorectal neoplasms
Introduction
Colorectal cancers (CRCs) are the fourth leading cause of cancer death in Korea, and the number of patients dying of CRC is increasing steadily [1]. One half of all patients develop metastatic disease. With advances in systemic therapy for metastatic CRC, the survival of these patients has been prolonged up to 6 months with fluorouracil (FU), irinotecan and molecular targeted agents, and if untreated up to more than 20 months [2-7]. However, most patients develop resistance to these therapies and experience disease progression. Despite progression in the face of treatment with these currently acceptable cytotoxic chemotherapy and/or biologic agents, a considerable portion of patients still survive with good performance status (PS). Unfortunately, these refractory patients have few additional treatment options.
Regorafenib, an orally administered multikinase inhibitor, is administered on a continuous dosing schedule and exhibits non-specific binding to several intracellular kinases with potent inhibitory activity against vascular endothelial growth factor receptors 1-3 (VEGFR1, VEGFR2, and VEGFR3), PDGFRB, FGFR1, RAF, and TIE2, and the mutant oncogenic kinases KIT, RET, and BRAF [8]. Clinical trials assessing regorafenib in the treatment of CRC, gastrointestinal stromal tumors, lung cancer, renal cell carcinoma, and hepatocellular carcinoma are underway [9-12]. In a phase I extended cohort clinical trial, single-agent regorafenib was associated with stable disease in 19 of 27 assessable patients with heavily pretreated CRC [13]. A recent phase III trial (CORRECT) demonstrated an improvement in overall survival (OS) among regorafenib-treated patients in comparison to those randomized to receive best supportive care after progression on standard therapy (6.4 months vs. 5.9 months, respectively) [14]. However, the CORRECT cohort included only a small portion of Asian patients (15%), and it did not enroll a single Korean patient. There is a known ethnic difference with regard to both drug sensitivity and tumor prognosis. Therefore, though regorafenib showed clinical benefit and tolerable toxicities in a global phase III trial, the effectiveness of regorafenib in Korean patients should be verified in clinical practice in Korea.
The objective of this study was to evaluate the clinical benefit and tolerability of regorafenib monotherapy in Korean patients with metastatic CRC that failed standard treatments, including FU, oxaliplatin, and irinotecan with or without biologic agents.
Materials and Methods
1. Study population
Between August 2013 and September 2013, 32 Korean patients with refractory CRC received regorafenib monotherapy as salvage treatment at three centers in Korea. All patients had previously received progressed FU, irinotecan, and oxaliplatin with or without biologic agents such cetuximab or bevacizumab. The case inclusion criteria were as follows: (1) age over 18; (2) pathologically or cytologically proven metastatic CRC; (3) adequate bone marrow, liver, and renal function; and (4) life expectancy of at least 3 months. All participants provided written informed consent before starting regorafenib.
2. Chemotherapy
Patients were randomized to receive oral regorafenib 160 mg once daily for the first 3 weeks of each 4-week cycle until disease progression, death, unacceptable toxic effects, or withdrawal of consent. Patients were followed up every week during the first two cycles then every 2 weeks from cycle 3. Prespecified dose reductions (to 120 or 80 mg) and delay of the following cycle (up to 28 days) were allowed for management of adverse events. If the patients required an additional dose reduction to 80 mg or a delay of more than 28 days between cycles, regorafenib treatment had to be stopped.
3. Assessments
Tumor response was routinely assessed with computed tomography scan every two cycles. The response was evaluated using a method identical to that used in Response Evaluation Criteria in Solid Tumor (RECIST) ver. 1.1. Toxicities were assessed according to the National Cancer Institute Common Toxicity Criteria ver. 3.0.
4. Statistical analyses
We retrospectively analyzed the treatment outcomes of regorafenib monotherapy in patients with metastatic CRC who had previously received standard chemotherapy with or without biologic agents. Descriptive statistics were calculated as proportions and medians. Treatment outcomes were estimated as the response rate, progression-free survival (PFS), OS, and toxicities. The response rate was determined according to RECIST ver. 1.1. PFS was defined as the time from the first study treatment to the date of disease progression. OS was calculated from the first study treatment until death. The survival data were assessed using the Kaplan-Meier method. We also computed the 95% confidence interval (CI) for the median time to event, and the efficacy analysis was based on the intent to treat group.
Results
1. Patients
Between August 2013 and September 2013, we enrolled 32 patients from three institutions. Patient baseline characteristics are shown in Table 1. The median patient age was 57 years (range, 29 to 79 years), and there were 20 males and 12 females. The median Eastern Cooperative Oncology Group (ECOG) PS was 1 (range, 0 to 2). The primary tumor site for most patients (65.6%) was the colon. There were 18 patients with KRAS wild-type disease and 13 of them had previously received cetuximab-containing therapy. Bevacizumab was used in 18 patients. The time from the initial diagnosis of metastatic or recurrent disease was greater than 25 months in 24 patients (75%).
Table 1.
Baseline patient characteristics (regorafenib, n=32)
| Characteristic | No. (%) |
|---|---|
| Median age (range, yr) | 57 (29-79) |
| Gender | |
| Male | 20 (62.5) |
| Female | 12 (37.5) |
| ECOG performance status | |
| 0-1 | 31 (96.9) |
| 2 | 1 (3.1) |
| Primary site of disease | |
| Colon | 21 (65.6) |
| Rectum | 11 (34.4) |
| Disease status | |
| Recurrent | 15 (46.9) |
| Metastatic | 17 (53.1) |
| KRAS mutation | |
| No | 18 (56.3) |
| Yes | 11 (34.4) |
| Unknown | 3 (9.4) |
| No. of previous systemic anticancer therapiesa) | |
| 1-3 | 20 (62.5) |
| ≥ 4 | 12 (37.5) |
| Previous anti-VEGF treatment (bevacizumab) | 18 (56.3) |
| Previous anti-EGFR treatment (cetuximab) | 13 (40.6) |
| Time from diagnosis of metastasis (mo) | |
| < 25 | 8 (25.0) |
| ≥ 25 | 24 (75.0) |
ECOG, Eastern Cooperative Oncology Group; VEGF, vascular endothelial growth factor; EGFR, epidermal growth factor receptor.
On or after diagnosis of metastatic disease.
2. Response
Response outcomes are listed in Table 2. The response evaluation was performed in the intent-to-treat group. None of the patients showed a complete response and a partial response was noted in only one patient (3.1%). Fifteen patients showed stable disease and 13 patients experienced disease progression. Disease stabilization was achieved in 50% of patients.
Table 2.
Treatment outcomes
| Treatment outcome | No. (%) |
|---|---|
| Response | |
| Complete response | 0 |
| Partial response | 1 (3.1) |
| Stable disease | 15 (46.9) |
| Progressive disease | 13 (40.6) |
| Not available | 3 (9.4) |
| Response rate (%) | 3.1 |
| Disease control rate (%) | 50.0 |
3. Survival
All 32 patients were included in the survival analysis on an intent-to-treat basis. The median PFS was 4.2 months (95% CI, 3.1 to 5.2 months) (Fig. 1). No difference in PFS was observed according to age, sex, primary site of disease, disease status, KRAS mutation status, number of previous palliative chemotherapies, previous treatment with bevacizumab, and time from first diagnosis of metastatic or recurrent disease (Table 3). Median OS has not yet been reached.
Fig. 1.
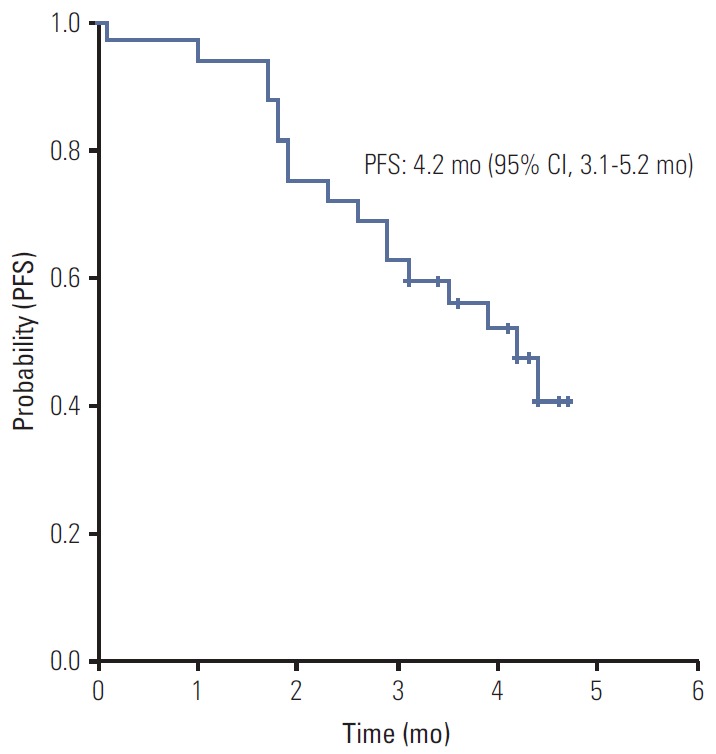
Kaplan-Meier estimate of progression-free survival (PFS) from the start of regorafenib therapy. CI, confidence interval.
Table 3.
Prognostic factors associated with progression-free survival in univariate analysis
| Characteristic | Progression-free survival |
||
|---|---|---|---|
| Median (mo) | Univariate analysis |
||
| HR (95% CI) | p-value | ||
| Age (yr) | 4.2 | 1.08 (0.37-3.08) | 0.88 |
| ≤ 65 | |||
| > 65 | 3.5 | ||
| Gender | 0.72 (0.25-2.05) | 0.54 | |
| Male | 3.5 | ||
| Female | NA | ||
| Primary site of disease | 1.42 (0.52-3.90) | 0.48 | |
| Colon | 4.2 | ||
| Rectum | 2.9 | ||
| Disease status | 0.98 (0.38-2.57) | 0.98 | |
| Recurrent | 4.2 | ||
| Metastatic | 3.9 | ||
| KRAS mutation | 1.12 (0.94-1.34) | 0.18 | |
| No | 4.4 | ||
| Yes | 3.9 | ||
| No. of previous palliative systemic therapies | 1.56 (0.60-4.06) | 0.35 | |
| 1-3 | NA | ||
| ≥ 4 | 3.5 | ||
| Previous anti-VEGF treatment | 0.77 (0.29-2.02) | 0.17 | |
| Yes | 4.2 | ||
| No | 2.9 | ||
| Time from diagnosis of metastasis (mo) | 1.18 (0.41-3.36) | 0.75 | |
| ≤ 25 | 3.9 | ||
| > 25 | 4.4 | ||
HR, hazard ratio; CI, confidence interval; NA, not available; VEGF, vascular endothelial growth factor.
4. Toxicity
Overall, 16 of 32 patients (50%) treated with regorafenib had an adverse event leading to a dose reduction. Toxicityprofiles are shown in Table 4. The most frequent adverse events of grade 2 or more were hand-foot skin reactions (25%) and mucositis (19%). Grade 3 or 4 treatment-related adverse events occurred in 12 patients (37.5%). The most common grade 3 or 4 toxicities included liver function test (LFT) abnormality (9%), abdominal pain (9%), rash (6%), leukopenia (3%), neutropenic fever (3%), anemia (3%), and fatigue (3%). There was no treatment-related death.
Table 4.
Treatment-related adverse events
| Toxicity type | Toxicity |
||
|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | |
| Hematologic toxicity | |||
| Leukopenia | - | - | 1 (3) |
| Neutropenic fever | - | - | 1 (3) |
| Anemia | - | 1 (3) | - |
| LFT abnormality | - | 3 (9) | - |
| Non-hematologic toxicity | |||
| Diarrhea | 1 (3) | - | - |
| Mucositis | 6 (19) | - | - |
| Rash | - | 2 (6) | - |
| Fatigue | - | 1 (3) | - |
| Anorexia | 2 (6) | - | - |
| Abdominal pain | - | 3 (9) | |
| Hand-foot syndrome | 8 (25) | - | - |
Values are presented as number (%). LFT, liver function test.
Discussion
The current study is the first to investigate treatment outcomes and safety of regorafenib in Korean patients with CRC who had received all standard therapies. In this study, regorafenib showed promising activity and tolerable toxicities. Although few patients who received regorafenib achieved an objective tumor response, 16 patients (50%) achieved disease-control. Median PFS in these patients was 4.2 months (95% CI, 3.1 to 5.2 months) and median OS was not reached.
Treatment outcomes of regorafenib in Korean patients appear to be consistent or slightly superior to those in the CORRECT trial [14]. The phase III CORRECT trial enrolled patients with refractory CRC who had received all standard therapies. The CORRECT trial did not include any Korean patients. In the regorafenib-treated group, the objective response rate was 1.0% and the disease control rate was 41%, and the median PFS was 1.9 months (95% CI, 1.6 to 3.9 months) and the median OS was 6.4 months (95% CI, 3.6 to 11.8 months). Results of our analysis for tumor response and disease control were similar to those of the CORRECT trial. However, a difference in PFS was observed between the two studies. Some recent small studies have examined cytotoxic chemotherapies in patients with CRC refractory to standard therapy [15-19]. In this setting, oral FU monotherapy such as capecitabine and S-1 reported was associated with a PFS of 2 months and patients treated with other combinationregimens such as mitomycin plus FU, capecitabine plus gemcitabine, and irinotecan, oxaliplatin plus S-1 showed a PFS of approximately 2 or 3 months. The PFS for regorafenib in the CORRECT trial was similar to those of previous studies. However, the PFS in this analysis is better than that in the CORRECT trial and previous studies. The difference between the PFS in this study and that in other studies might be due to the heterogeneous patient population, small sample size, and retrospective design. In particular, in this study, patient population with previously both bevacizumab and cetuximab (for KRAS wild type) was relatively small as compared to those in the CORRECT trial. The difference in exposure to biologic agents may affect the efficacy and toxicity of regorafenib, multikinase inhibitor.
Palliative chemotherapy in patients with metastatic CRC can improve survival, lessen symptoms, and improve quality of life in patients with unresectable disease. When considering chemotherapy after failure of standard chemotherapy, oncologists must pay attention to the degree and toxicity profile for the intended chemotherapeutic agents. Although there were no treatment-related deaths in this study, grade 3 or 4 treatment-related adverse events occurred in 12 patients (37.5%). LFT abnormality and abdominal pain were observed more frequently in this study as compared to the CORRECT trial. However, rash, hand foot syndrome, and diarrhea were shown with less frequency in this study. A total of 16 among 32 patients (50%) treated with regorafenib experienced an adverse effect leading to dose reduction. In the CORRECT trial, grade 3 or 4 treatment-related adverse events occurred in 207 patients (54%) assigned to the regorafenib group and the dose was reduced in 188 patients (38%) assigned to the regorafenib group [14]. Although there were fewer grade 3 or 4 adverse events among patients treated with regorafenib in our study than in the CORRECT trial, more patients in our study underwent a dose reduction. This finding might be due to our patterns of clinical practice. We obtained significant information regarding the safety of regorafenib from the CORRECT trial before beginning this study. We monitored patients more frequently than indicated in the visiting schedule for the CORRECT trial. Our close and frequent monitoring occurred primarily during the first two cycles of treatment. Early detection and prompt initiation of therapeutic management may enable a reduction in the severity of adverse events. Due to the retrospective nature of this study, we were unable to evaluate quality of life of patients.
Results of this analysis suggest that regorafenib monotherapy could have potential as a new line of therapy in the treatment of refractory CRC in Korean patients. Nevertheless, not all of these patients experienced clinical benefit from regorafenib. To establish the role of regorafenib as a standard palliative treatment, it must be proven that subgroups of patients may experience optimal clinical benefit from treatment with regorafenib. Thus, integrated studies aimed at identification of biomarkers for regorafenib are warranted for personalized medicine, and we plan to conduct a comprehensive genomic analysis to determine the effectiveness of regorafenib using specimens collected for this study.
Conclusion
The current study is the first to investigate treatment outcomes and safety of regorafenib in Korean patients with CRC who had received all standard therapies. In this study, regorafenib showed promising activity and tolerable toxicities.
Footnotes
We acknowledge the Bayer Corp. who kindly donated regorafenib for the study. The MAP was supported by Bayer.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–87. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 3.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 4.Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 5.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 6.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–55. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Tak WY, Gasbarrini A, Santoro A, Colombo M, Lim HY, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412–9. doi: 10.1016/j.ejca.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Schultheis B, Folprecht G, Kuhlmann J, Ehrenberg R, Hacker UT, Kohne CH, et al. Regorafenib in combination with FOLFOX or FOLFIRI as first- or second-line treatment of colorectal cancer: results of a multicenter, phase Ib study. Ann Oncol. 2013;24:1560–7. doi: 10.1093/annonc/mdt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mross K, Frost A, Steinbild S, Hedbom S, Buchert M, Fasol U, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:2658–67. doi: 10.1158/1078-0432.CCR-11-1900. [DOI] [PubMed] [Google Scholar]
- 13.Strumberg D, Scheulen ME, Schultheis B, Richly H, Frost A, Buchert M, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer. 2012;106:1722–7. doi: 10.1038/bjc.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 15.Kim ST, Choi YJ, Park KH, Oh SC, Seo JH, Shin SW, et al. Capecitabine monotherapy as salvage treatment after failure of chemotherapy containing oxaliplatin and irinotecan in patients with metastatic colorectal cancer. Asia Pac J Clin Oncol. 2011;7:82–7. doi: 10.1111/j.1743-7563.2010.01363.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee DJ, Lee J, Lee HY, Lim T, Lee SJ, Yi SY, et al. Salvage S-1 monotherapy in metastatic colorectal cancer patients who failed irinotecan-based or oxaliplatin-based chemotherapy. Med Oncol. 2011;28(Suppl 1):S291–4. doi: 10.1007/s12032-010-9755-1. [DOI] [PubMed] [Google Scholar]
- 17.Kang EJ, Choi YJ, Kim JS, Kim ST, Park KH, Choi IK, et al. Mitomycin-C, 5-fluorouracil, and leucovorin as a salvage therapy in patients with metastatic colorectal adenocarcinoma. Asia Pac J Clin Oncol. 2010;6:286–91. doi: 10.1111/j.1743-7563.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- 18.Salgado M, Reboredo M, Mendez JC, Quintero G, Pellon ML, Romero C, et al. Gemcitabine and capecitabine as third- or later-line therapy for refractory advanced colorectal cancer: a retrospective study. Anticancer Res. 2013;33:4089–96. [PubMed] [Google Scholar]
- 19.Kim SY, Hong YS, Kim BC, Park JW, Choi HS, Jeong SY, et al. A phase II study of S-1 plus irinotecan and oxaliplatin in heavily-treated patients with metastatic colorectal cancer. Invest New Drugs. 2009;27:269–74. doi: 10.1007/s10637-008-9177-5. [DOI] [PubMed] [Google Scholar]


