Abstract
Objective:
To investigate the accuracy of using CT and MRI to characterize lesions of osteonecrosis of the femoral head (ONFH).
Methods:
Coronal CT and MRI scans were performed on 30 femoral head specimens collected from 23 patients who had undertaken hip arthroplasty owing to ONFH. The results were compared with findings from coronal sectional gross specimens. Two radiologists independently measured the volume of necrotic lesions from CT and MR images using computer software, and the results were averaged. The volume of specimens' necrotic lesion was measured using the water displacement method.
Results:
There was a high degree of consistency between CT, MRI and the coronal sectional gross specimen on the location, shape and spatial structure of lesions. Differences of the lesion volume measured from CT and MR images were not statistically significant between two radiologists. The necrotic lesion volumes measured from CT and MR images and gross specimens were 22.07 ±5.35, 22.21 ± 5.15 and 21.12 ±4.96 cm3, respectively, and the differences were not statistically significant (F = 0.396; p = 0.674).
Conclusion:
For patients with ONFH in Association Research Circulation Osseous stage III or above, CT and MRI can accurately display the characterization of lesion.
Advances in knowledge:
The size and location of necrotic lesions are major factors associated with femoral head collapse. CT is superior to MRI in identifying subchondral fracture. CT can help diagnose and predict the prognosis of ONFH.
Osteonecrosis of the femoral head (ONFH) is a common disease of the hip joint. If untreated, 80% of patients with ONFH will suffer the collapse of femoral head articular surface in a few years of disease progression.1 Once the femoral head collapses, osteoarthritis becomes inevitable and function of the hip joint will be seriously affected, which eventually will lead to artificial joint replacement.2,3 Although hip replacement is an effective approach to relieve pain and improve hip function in the short term, its long-term performance remains unsatisfactory.4,5 Furthermore, non-traumatic ONFH typically occurs in young adults and involves both hips. On the other hand, not all ONFH will progress to femoral head collapse.6 Therefore, being able to accurately predict the risk of femoral head collapse according to the disease severity and to take appropriate therapeutic measures are critical to preserve the hip joint and improve the prognosis of ONFH.7
Studies have shown that the size and location of necrotic lesions are major factors associated with femoral head collapse.6–9 Although various approaches have been reported to illustrate the lesion location and size, all were based on MRI and/or radiographs.6–11 As a main imaging modality for diagnosis of ONFH, CT can clearly display ONFH that is in Association Research Circulation Osseous (ARCO) stage II or above. Multislice CT can achieve isotropic resolution. Volume data acquired from axial scans can be used to reconstruct in any direction to observe lesions. But so far, there has been no study that reports the feasibility and accuracy of using CT to measure the size of ONFH lesions. We hypothesized that CT and MRI could achieve highly consistent results in illustrating the size, shape and location of ONFH lesions in ARCO stage II and above, and both methods can be used to assess the risk of necrotic femoral head collapse.
The objectives of this study are (1) to investigate the accuracy of using CT to capture the size, location, shape and spatial structural relationship of the necrotic lesion by comparing the coronal CT with coronal MR images and findings from coronal sectional gross specimens; and (2) to evaluate the accuracy of using CT to measure necrotic lesion volume, using the measurement from MR images and gross specimen as references. To the best of our knowledge, there has been no study that compared the results of CT images and sectional specimens on the ONFH lesion characteristics, neither has any study reported the difference of necrotic lesion volume measured from CT images and sectional specimens.
METHODS AND MATERIALS
Study population
This was a prospective study. The inclusion criteria for patients were (1) undergone hip arthroplasty owing to late stage ONFH; (2) agreed to participate in the study and provide signed informed consent. Exclusion criteria were (1) the specimens obtained from hip replacement surgery were not subject to CT or MRI examination within 4 h after surgery; (2) the femoral head specimen was fragmented, and the articular surface was incomplete. A total of 23 patients treated at the China–Japan Friendship Hospital, Beijing, China, from January 2006 to December 2012 met the eligibility criteria and were enrolled, including 16 males and 7 females, with a mean age of 36.5 ± 7.2 years (range, 28–52 years). Of them, 16 underwent unilateral hip replacement and 7 received bilateral hip replacement. A total of 30 specimens of necrotic femoral head were obtained. The causes of necrosis were steroid treatment (n = 18), alcohol abuse (n = 4) and idiopathic (n = 8). Based on ARCO staging criteria, 12 were stage III and 18 were stage IV.
Study design
(1) Coronal CT and MRI scans were performed on the necrotic femoral head specimens within 4 h after surgery. (2) Using MR images to determine the anteversion angle of the femoral head, the specimens were then fixed on bench clamp according to the angle. Lines were drawn on the femoral heads along the coronal plane every 5 mm, and then the specimens were cut into 5-mm thick blocks. (3) The findings from the coronal sectional specimens and MR and CT coronal images were compared; the changes in MRI signal intensity and CT imaging density corresponding to the respective areas of gross specimen were observed; and whether CT can clearly display the location, shape and spatial structural relationship of the lesion as shown on the gross specimen and MR images was assessed. (4) The volume of necrotic lesions from gross specimen, CT and MR images was measured separately, and statistical analysis conducted to compare the results. The study was approved by the ethics committee of the hospital prior to initiation, and all patients provided written informed consent.
CT and MRI examination
Toshiba Aquilion™ 16-slice spiral CT (Toshiba Medical Systems Corporation, Tokyo, Japan) was used for coronal scan of femoral head specimens: slice thickness, 1 mm; pitch, 1; 120 kV; 187 mAs; and reconstruction slice thickness, 5 mm. Philips-Intera 1.5 T MR (Philips Medical Systems, Best, Netherlands) was used to obtain coronal fast spin echo T1 weighted (T1W), T2 weighted (T2W), spectral pre-saturation with inversion recovery T1W (SPIR T1W) images. The scanning parameters were slice thickness, 5 mm; gap, 0 mm; field of view (FOV), 150 mm; matrix size, 256 × 256 pixels; T1W and SPIR T1W images repetition time (TR), 617 ms; echo time (TE), 18 ms; T2W images TR, 3248 ms and TE, 100 ms. All CT and MRI data were saved on compact disc in digital imaging and communications in medicine format. Using the smooth femoral neck surgery section as the bottom, the specimen was placed on the scanning bed vertically. Conventional axial scans can get coronal images.
Lesion volume measurement on the gross specimen
ONFH necrotic area and boundary area were identified at each section of specimens. Necrotic tissues were removed with needle-nose pliers and placed into a glass measuring cup filled with 200 ml of water. Once the tissue was completely immersed in water, the total volume of water and of the tissue was recorded. The volume of necrotic tissue (cm3) is equal to the total volume minus the water volume (200 ml)
Lesion volume measurement from CT and MR images
Using Mimics® software (Materialise, Leuven, Belgium), the saved CT and MR T1W or SPIR T1W imaging data were converted into a Mimics project file, then imported as a BMP file. According to the characteristics of ONFH,12 the edge of the necrotic tissue was defined by the high density line or band on CT images and by the inner margin of the low signal intensity band on MR T1W images. If both the necrotic and boundary regions were shown as low-intensity signals and could not be distinguished, SPIR T1W images were used to calculate the volume of necrotic lesions. On SPIR T1W images, the boundary area was shown as a high-intensity ring surrounding the low-intensity necrotic area. The necrotic lesion was sketched on each plane using the software, and the necrotic regions were identified and divided to calculate the volume of the necrotic lesion. Two radiologists with more than 10 years' experience reviewed the imaging data in the same sitting, independently measured the volume of necrotic lesion on CT and MR images, and their results were averaged to represent the final measurements.
Statistical analysis
Paired t-test was used to compare the lesion volumes measured from CT and MR images between the two radiologists. Single-factor analysis of variance was used to compare the lesion volume results from CT and MR images and the gross specimens. All analyses were conducted using SPSS® statistical software v. 17.0 (SPSS Inc., Chicago, IL).
RESULTS
Comparison of the results from CT and MR images and gross specimens of osteonecrosis of the femoral head
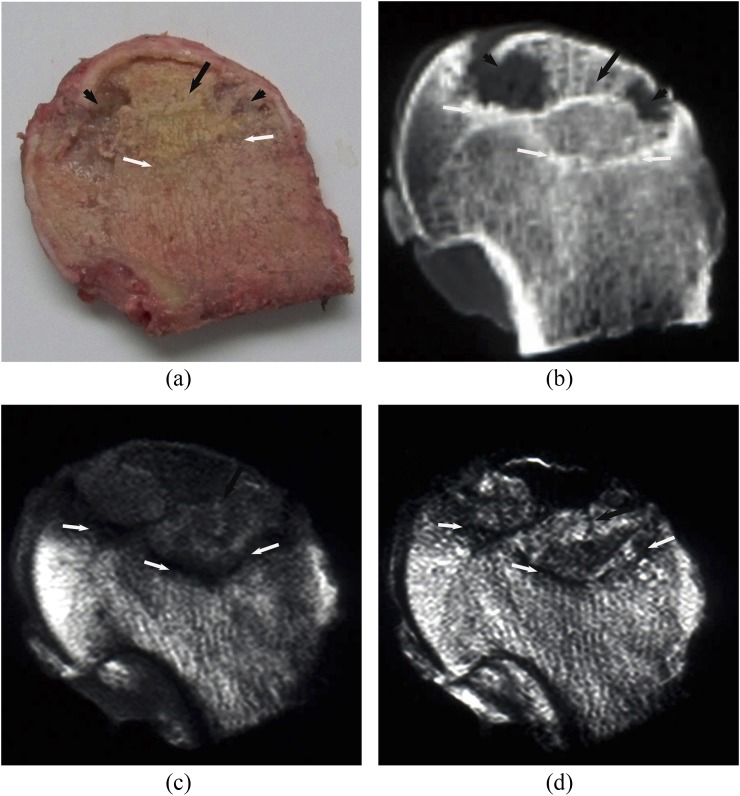
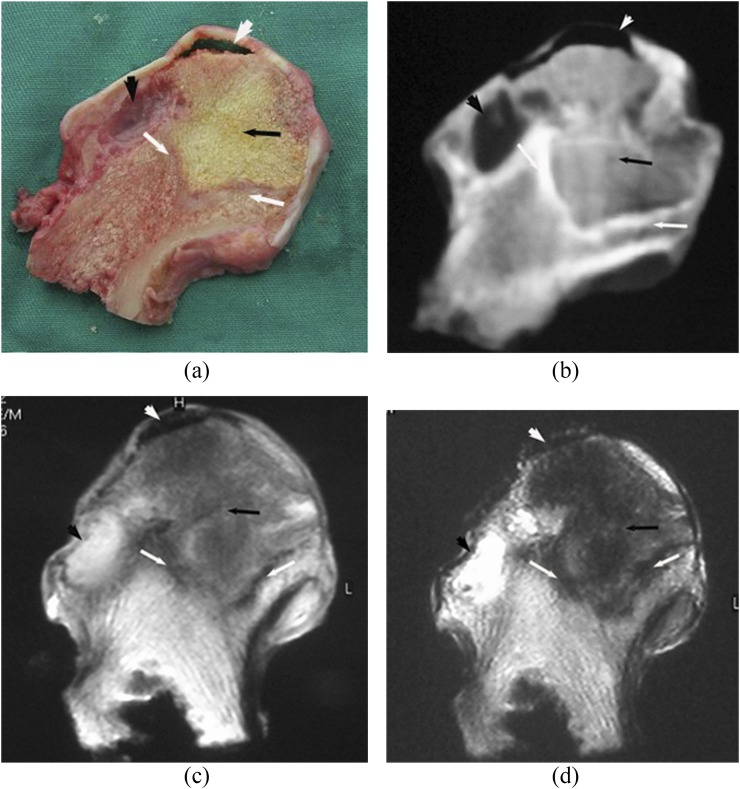
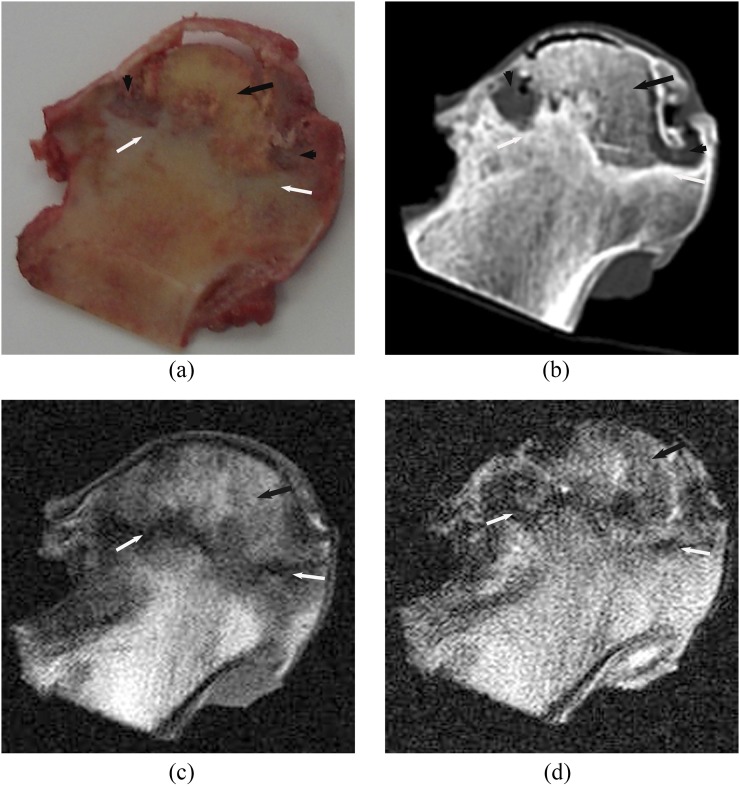
Subchondral area in the anterolateral segment of the femoral head was involved in all necrotic lesions. There were four distinct layers on the coronal sectional specimens of the necrotic femoral head: cartilage, necrotic area, boundary area and extralesional area. The boundary area was brown coloured encompassing the yellow-coloured necrotic area (Figures 1a, 2a, 3a and 4a). Both CT and MRI could clearly and accurately display necrotic, boundary and extralesional areas on the specimens. There was high consistency between CT and MR images and the gross specimens on size, shape and location of lesions (Figure 1b–d). The boundary area on the specimen was shown as clearly marginated high signal density line or band on CT images (Figures 2b, 3b and 4b), as low signal on MR T1W and T2W images (Figures 1c,d an 2c,d) and as high signal on the SPIR T1W images. In four femoral heads, the area was shown as “double-line sign”, that is, a high-intensity rim inside a low-intensity margin on T2W images. Compared with normal tissue, the necrotic area on CT showed isodensity in 19 femoral heads (Figures 1b, 3b), lower density in 10 femoral heads (Figure 4b) and slightly higher density in 1 femoral head. Linear or cystic low density signals were seen at the edge of 18 specimens of the necrotic femoral heads (Figures 2b, 3b and 4b). In the necrotic area, six necrotic femoral head specimens demonstrated fat-type signals on MR images, that is, similar to the normal bone marrow tissue (Figure 1c,d). 2 specimens showed watery signals, that is, low signal on T1W and high signal on T2W images (Figure 2c,d), 17 specimens showed fibre-type signals, that is, low signal on both T1W and T2W images (Figure 3c,d), and mixed signals were found in 5 femoral head specimens (Figure 4c,d). Of those that showed fibre-type signals in the necrotic region, six specimens demonstrated unclear border between the necrotic and boundary areas on T1W and T2W images, but clear edge of necrotic area (low signal) surrounded by boundary area (high signal) on the SPIR T1W image. Normal tissue outside of boundary area showed normal bone density and bone marrow signal. The edge of lesion was clear on both CT and MR images. Thus, it was feasible to import the CT and MRI data into the computer and sketch the border of lesions on each section to calculate the volume. Among 30 femoral heads, 22 subchondral fractures were shown on CT scans, but only 10 were detected on MR images. CT depicted all the subchondral fractures in whom fractures were seen on MR images.
Figure 1.
Steroid osteonecrosis of the femoral head: (a–d) represents the coronal section of a necrotic femoral head specimen (a), the corresponding CT sectional image (b), and MR T1 weighted (T1W) (c) and T2 weighted (T2W) images (d), respectively. There were four distinct layers on the coronal sectional specimen: cartilage (black arrowhead), necrotic area (black arrow), brownish boundary area (white arrows) and extralesional area. CT and MR could clearly show the necrotic, boundary and surrounding normal areas on the specimen. The boundary region was shown as high signal density line on the CT image (white arrows), low signal intensity band on MR T1W and T2W images. Compared with normal tissue, the necrotic region was shown as isodense on CT and isointense on MR images (black arrows).
Figure 2.
Alcoholic osteonecrosis of the femoral head: (a–d) represents the coronal section of a necrotic left femoral head specimen (a), the corresponding CT sectional image (b), and MR T1 weighted (T1W) (c) and T2 weighted (T1W) images (d), respectively. On the coronal sectional specimen, a brown boundary region (white arrows) separates the yellow necrotic region (black arrow) from normal tissue outside of lesions and the edge of the necrotic area showed cystic degeneration (black arrowheads). The boundary region was shown as high signal density band on the CT image (white arrows) and as low signal intensity band on MR T1W and T2W images (white arrows). Most of the necrotic region was shown as isodense on CT (black arrow). The cystic degeneration at the edge of the necrotic region was clearly revealed (black arrowheads). The necrotic region was shown as low signal intensity on the MR T1W image and as mixed high signal intensity on the T2W image (black arrows).
Figure 3.
Steroid osteonecrosis of the femoral head. A high degree of consistency was demonstrated between coronal section of gross specimen (a), corresponding CT sectional image (b), MR T1 weighted (T1W) (c) and T2 weighted (T2W) images (d) on the size, shape and position of necrotic lesion. Boundary region (white arrows) separated and surrounded the necrotic region (black arrows). The necrotic region was shown as isodense on CT and as low signal intensity on MR T1W and T2W images. Cystic degeneration (black arrowheads) and subchondral fracture (white arrowheads) could be seen at the edge of the necrotic region.
Figure 4.
Idiopathic osteonecrosis of the femoral head. The boundary region (white arrow) separated the necrotic region (inside, black arrow) and normal tissue (outside). The region has brownish colour on the gross specimen and was shown as high-density signal line on CT and as low-intensity signal band on MR T1 weighted (T1W) and T2 weighted (T2W) images. The necrotic region was shown as slightly low signal density on CT, and as mixed slightly low signal intensity on MR T1W and T2W images, with cystic change (black arrowhead) at the edge.
Comparison of lesion volumes calculated from CT and MR images and gross specimens of osteonecrosis of the femoral head
Two radiologists measured the volume of necrotic lesions from CT and MR images using computer software (Table 1). There was no statistically significant difference between them on volumes measured from both CT and MR images. Table 2 summarizes the volumes of all 30 necrotic lesions measured from CT and MR images and gross specimens. Single-factor analysis of variance did not find statistically significant difference among them (F = 0.40; p = 0.67).
Table 1.
The comparison of lesion volume measured from CT and MR images between two radiologists
| Specimen number | CT (cm3) |
MRI (cm3) |
||
|---|---|---|---|---|
| Radiologist 1 | Radiologist 2 | Radiologist 1 | Radiologist 2 | |
| 1 | 21.8 | 20.7 | 21.5 | 22.2 |
| 2 | 19.7 | 19.0 | 19.3 | 18.0 |
| 3 | 14.3 | 16.1 | 15.5 | 14.3 |
| 4 | 22.0 | 21.4 | 22.4 | 21.2 |
| 5 | 23.3 | 24.1 | 21.5 | 23.1 |
| 6 | 18.3 | 18.0 | 18.7 | 17.8 |
| 7 | 27.2 | 28.2 | 29.8 | 28.6 |
| 8 | 22.5 | 21.9 | 23.8 | 23.1 |
| 9 | 29.3 | 28.5 | 27.8 | 28.6 |
| 10 | 12.8 | 11.4 | 13.5 | 14.3 |
| 11 | 23.2 | 24.5 | 21.8 | 22.3 |
| 12 | 17.8 | 17.1 | 17.9 | 17.5 |
| 13 | 24.1 | 23.5 | 23.3 | 23.7 |
| 14 | 31.3 | 32.0 | 31.4 | 31.0 |
| 15 | 32.7 | 32.6 | 33.7 | 33.2 |
| 16 | 18.2 | 18.8 | 17.9 | 18.0 |
| 17 | 21.6 | 20.6 | 23.9 | 23.5 |
| 18 | 16.8 | 15.6 | 15.7 | 15.3 |
| 19 | 24.9 | 26.2 | 24.4 | 25.9 |
| 20 | 14.5 | 14.2 | 16.7 | 15.4 |
| 21 | 19.0 | 18.0 | 19.9 | 19.5 |
| 22 | 19.1 | 20.1 | 20.3 | 19.4 |
| 23 | 31.6 | 33.1 | 30.1 | 30.9 |
| 24 | 17.2 | 16.2 | 18.2 | 17.1 |
| 25 | 19.2 | 21.1 | 20.0 | 19.2 |
| 26 | 23.4 | 22.9 | 22.6 | 21.9 |
| 27 | 17.9 | 19.3 | 19.4 | 18.2 |
| 28 | 28.4 | 29.3 | 30.1 | 29.0 |
| 29 | 23.9 | 22.9 | 22.8 | 23.8 |
| 30 | 24.8 | 25.9 | 26.2 | 26.5 |
| Mean | 22.03 ± 5.21 | 22.11 ± 5.54 | 22.34 ± 5.08 | 22.08 ± 5.27 |
| Statistical analysis | t = −0.420; p = 0.677 | t = 1.570; p = 0.127 | ||
Table 2.
The comparison of lesion volume measured from gross specimen, CT and MR images
| Plain radiographs | CT | MRI | Gross specimen |
|---|---|---|---|
| 1 | 21.25 | 21.85 | 20.9 |
| 2 | 19.35 | 18.65 | 18.5 |
| 3 | 15.20 | 14.90 | 15.3 |
| 4 | 21.70 | 21.80 | 21.5 |
| 5 | 23.70 | 22.30 | 23.1 |
| 6 | 18.15 | 18.25 | 18.1 |
| 7 | 27.70 | 29.20 | 26.3 |
| 8 | 22.20 | 23.45 | 21.8 |
| 9 | 28.90 | 28.20 | 27.7 |
| 10 | 12.10 | 13.90 | 10.9 |
| 11 | 23.85 | 22.05 | 22.8 |
| 12 | 17.45 | 17.70 | 16.6 |
| 13 | 23.80 | 23.50 | 22.5 |
| 14 | 31.65 | 31.20 | 29.0 |
| 15 | 32.65 | 33.45 | 31.9 |
| 16 | 18.50 | 17.95 | 19.2 |
| 17 | 21.10 | 23.70 | 21.4 |
| 18 | 16.20 | 15.50 | 15.1 |
| 19 | 25.55 | 25.15 | 24.3 |
| 20 | 14.35 | 16.05 | 13.9 |
| 21 | 18.50 | 19.70 | 17.9 |
| 22 | 19.60 | 19.85 | 18.5 |
| 23 | 32.35 | 30.50 | 29.6 |
| 24 | 16.70 | 17.65 | 16.5 |
| 25 | 20.15 | 19.60 | 18.5 |
| 26 | 23.15 | 22.25 | 21.7 |
| 27 | 18.60 | 18.80 | 17.2 |
| 28 | 28.85 | 29.55 | 27.1 |
| 29 | 23.40 | 23.30 | 21.7 |
| 30 | 25.35 | 26.35 | 24.1 |
| Mean | 22.07 ± 5.35 | 22.21 ± 5.15 | 21.12 ± 4.96 |
| Statistical analysis | F = 0.396; p = 0.674 | ||
DISCUSSION
To evaluate the accuracy of CT in identifying the size, position and shape of ONFH lesions, we performed coronal CT and MRI scans of necrotic femoral head specimens and compared the results with the findings from coronal sectional specimens. Although a previous study had reported the results comparing findings from MRI of necrotic femoral head and coronal sectional pathology of gross specimens,12 our study is the first one that compares the results of CT with the findings from coronal sectional gross specimen. The data indicated that there was a fairly high degree of consistency between CT, MRI and gross specimen in identifying the location, size and morphology of ONFH lesions. Both CT and MRI could display necrotic, boundary and normal regions outside lesions as shown on the gross specimen. The necrotic region was shown as yellow, regular or irregular area on the gross specimen. Compared with normal tissue, density of necrotic tissue lacked specificity. On CT images, it could be isodense, low density or high density. On MR images, it could be fat-type, blood-type, watery or fibre-type signal. The boundary region was shown as a brown band surrounding the yellow-coloured necrotic region on the gross specimen, shown as characteristic high density sclerotic line or band on CT images, and low signal band separating necrotic region and normal tissue on MR T1W and T2W images. When the border between necrotic and boundary regions was unclear, SPIR T1W images could be used to distinguish two regions, which were shown as characteristic high-signal boundary area surrounding the low-signal necrotic area. The lesion border was legible on every section of CT and MR images, both of which could accurately demonstrate the lesion size, location, shape and spatial structure of relationship.
There were several approaches reported to measure the size of lesions, including the curvature of the affected femoral head surface, the percentage of necrotic area and the volume of necrotic lesions. However, all of them were based on MRI of the hip joints and/or plain radiographs.6–11 Owing to the difference in lesion location, heterogeneous morphology and irregular border, we think the percentage or the angle of necrotic areas that were measured from the centre plane of the femoral head or the maximum lesion plane only partially reflected the lesion size. With the development of computer software, we can now distinguish and delineate the lesion border on MR and CT images, calculate the necrotic lesion volume as well as the percentage of the femoral head with necrosis. These measures are generally more objective and accurate in reflecting the size of the lesion. This study compared the results from CT with findings of coronal sectional gross specimens and MRI. The results showed high consistency between these three methods in identifying the location, size and morphology of ONFH lesions. To further demonstrate the accuracy and reliability of CT in determining the lesion size, two radiologists independently measured the lesion volume from CT and MR images, and results from CT were compared with the findings from MR images and gross specimens. The results further confirmed the accuracy and repeatability of CT in measuring ONFH lesion volume.
ONFH is a common disorder of hip joints. Femoral head collapse is the tipping point of disease progression. Once it occurs, patients enter the irreversible late stage. Being able to accurately predict the risk of femoral head collapse is critical to selection of appropriate treatment and improvement of prognosis. Studies have found that lesion size and location are important factors of femoral head collapse.6–9 Although there were several methods introduced to identify the lesion location and measure the lesion size, all of them were based on MR images and/or X-ray plain films.8,9,13,14 So far, no study has reported the feasibility and accuracy of using CT to measure the size of ONFH lesions. This study found that similar to MR image, CT can precisely show the size, shape and location of ONFH lesions. Lesion volume can also be measured from the CT image. Furthermore, the presence of a subchondral fracture is vital for staging osteonecrosis and planning subsequent treatment, the occurrence of which typically indicates the emergence of femoral head collapse. CT is superior to radiography and MRI in identifying subchondral femoral head fracture.15,16 Therefore, CT can play an important role in predicting the prognosis of ONFH.
This study has several limitations: (1) we used the lesion volume measured from coronal sectional gross specimen as the gold standard to evaluate the feasibility and accuracy of CT and MR images in measuring the lesion volume. Since only patients at late stage of ONFH undergo hip replacement surgery, there was no ARCO stage II case in this study. (2) We used water displacement method to measure lesion volume from gross specimen. Underestimation was possible if water permeates into the cancellous bone. (3) This study was conducted in vitro. The signal, density and intensity of CT and MR images of femoral head may be different from that obtained in vivo.
MRI is the most sensitive in the diagnosis of early ONFH. For ONFH lesions in ARCO stage III and above, similar to MRI, CT can objectively demonstrate the location, size and shape of ONFH necrotic lesions; CT images can be used to measure lesion volume; and CT is superior to MRI in identifying subchondral fracture. In summary, CT examination can provide valuable information for the diagnosis and prediction of prognosis, especially among patients with contraindications of MRI examination.
REFERENCES
- 1.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am 1995; 77: 459–74. [DOI] [PubMed] [Google Scholar]
- 2.Jones LC, Hungerford DS. Osteonecrosis: etiology, diagnosis, and treatment. Curr Opin Rheumatol 2004; 16: 443–9. [DOI] [PubMed] [Google Scholar]
- 3.Babis GC, Soucacos PN. Effectiveness of total hip arthroplasty in the management of hip osteonecrosis. Orthop Clin North Am 2004; 35: 359–64. [DOI] [PubMed] [Google Scholar]
- 4.Hungerford DS. Treatment of osteonecrosis of the femoral head: everything's new. J Arthroplasty 2007; 22(Suppl. 1): 91–4. [DOI] [PubMed] [Google Scholar]
- 5.Polkowski GG, Callaghan JJ, Mont MA, Clohisy JC. Total hip arthroplasty very young patients. J Am Acad Orthop Surg 2012; 20: 487–97. doi: 10.5435/JAAOS-20-08-487 [DOI] [PubMed] [Google Scholar]
- 6.Nam KW, Kim YL, Yoo JJ, Koo KH, Yoon KS, Kim HJ. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am 2008; 90: 477–84. doi: 10.2106/JBJS.F.01582 [DOI] [PubMed] [Google Scholar]
- 7.Lieberman JR, Engstrom SM, Meneghini RM, SooHoo NF. Which factors influence preservation of the osteonecrotic femoral head? Clin Orthop Relat Res 2012; 470: 525–34. doi: 10.1007/s11999-011-2050-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishii T, Sugano N, Ohzono K, Sakai T, Sato Y, Yoshikawa H. Significance of lesion size and location in the prediction of collapse of osteonecrosis of the femoral head: a new three-dimensional quantification using magnetic resonance imaging. J Orthop Res 2002; 20: 130–6. [DOI] [PubMed] [Google Scholar]
- 9.Sugano N, Takaoka K, Ohzono K, Matsui M, Masuhara K, Ono K. Prognostication of nontraumatic avascular necrosis of the femoral head. Significant of location and size of the necrotic lesion. Clin Orthop Relat Res 1994; 303: 155–64. [PubMed] [Google Scholar]
- 10.Hernigou P, Lambotte JC. Volumetric analysis of osteonecrosis of the femur. Anatomical correlation using MRI. J Bone Joint Surg Br 2001; 83: 672–5. [DOI] [PubMed] [Google Scholar]
- 11.Kishida Y, Nishii T, Sugano N, Nakanishi K, Sakai T, Miki H, et al. Measurement of lesion area and volume by three-dimensional spoiled gradient-echo MR imaging in osteonecrosis of the femoral head. J Orthop Res 2003; 21: 850–8. [DOI] [PubMed] [Google Scholar]
- 12.Lang P, Jergesen HE, Moseley ME, Block JE, Chafetz NI, Genant HK. Avascular necrosis of the femoral head: high-field-strength MR imaging with histologic correlation. Radiology 1988; 169: 517–24. [DOI] [PubMed] [Google Scholar]
- 13.Ha YC, Jung WH, Kim JR, Seong NH, Kim SY, Koo KH. Prediction of collapse in femoral head osteonecrosis: a modified Kerboul method with use of magnetic resonance images. J Bone Joint Surg Am 2006; 88(Suppl. 3): 35–40. [DOI] [PubMed] [Google Scholar]
- 14.Bassounas AE, Karantanas AH, Fotiadis DI, Malizos KN. Femoral head osteonecrosis: volumetric MRI assessment and outcome. Eur J Radiol 2007; 63: 10–15. [DOI] [PubMed] [Google Scholar]
- 15.Stevens K, Tao C, Lee SU, Salem N, Vandevenne J, Cheng C, et al. Subchondral fractures in osteonecrosis of the femoral head: comparison of radiography, CT, and MR imaging. AJR Am J Roentgenol 2003; 180: 363–8. [DOI] [PubMed] [Google Scholar]
- 16.Yeh LR, Chen CK, Huang YL, Pan HB, Yang CF. Diagnostic performance of MR imaging in the assessment of subchondral fractures in avascular necrosis of the femoral head. Skeletal Radiol 2009; 38: 559–64. doi: 10.1007/s00256-009-0659-0 [DOI] [PubMed] [Google Scholar]