Abstract
Objective:
To study the appearance of primary and metastatic extremity synovial sarcoma (SS) on cross-sectional imaging.
Methods:
In this institutional review board-approved, Health Insurance Portability and Accountability Act-compliant retrospective study, the imaging features of 78 patients (42 males and 36 females; mean age, 40 years) with primary and metastatic extremity SS on MRI and multidetector CT were reviewed, with baseline MRI of the primary available in 31 patients.
Results:
Primary SSs were predominantly well-circumscribed (27/31) and heterogeneously enhancing solid (18/31) or solid-cystic (13/31) tumours. Imaging features visualized included the presence of perilesional oedema (14/31), interfascial (15/31) and intercompartmental extension (7/31), triple sign (11/31), intratumoral haemorrhage (10/31), calcification (6/31), bowl of grapes appearance (5/31) and bone involvement (3/31). Smaller T1 stage tumours (8/31) appeared as heterogeneously enhancing lesions, with some lesions demonstrating interfascial and intercompartmental extension and perilesional oedema. Recurrent/metastatic disease developed in 49/78 (63%) patients. Of these, 20/78 (26%) had metastasis at presentation, while the remaining developed metastatic disease at a median interval of 27 months (range, 3–161 months). Pleuropulmonary metastases (46/78) were the most common sites, with most of the metastases being pleural based. On univariate analysis, larger tumour size, the presence of perilesional oedema, intercompartmental extension, the presence of intralesional haemorrhage and bowl of grapes appearance on MRI were associated with a significantly higher incidence of metastatic disease.
Conclusion:
Certain imaging features of primary SS predict the risk of development of metastatic disease. Imaging features of T1 stage tumours included heterogeneous enhancement, interfascial extension and perilesional oedema. Pleural-based metastases are commonly seen in SSs.
Advances in knowledge:
Imaging features of primary SS correlate with metastatic disease. Pleural-based metastases are often present in SSs.
Synovial sarcoma (SS) is a relatively common high-grade sarcoma, accounting for 5–10% of soft-tissue sarcomas.1,2 The extremities are involved in about 80% of cases, and SS is among the most common malignancies to affect the extremities in young adults.1,3 The majority of patients present with a long history of a slowly growing lump, with average duration of 2–4 years before presentation. Furthermore, smaller tumours often lack aggressive features on imaging, leading to a mistaken presumption of benign aetiology.4,5
Few prior series have described the imaging features of primary SS, including certain typical MRI features. T2 hyperintense, intermediate and hypointense components may be seen in the tumour (triple sign) owing to necrotic, cystic or haemorrhagic areas (hyperintense), cellular elements (intermediate), and calcified or fibrotic regions or areas of old bleed (hypointense). Fluid–fluid levels may be visualized owing to the presence of sedimented blood products. A “bowl of grapes” appearance may be seen owing to the presence of T2 hyperintense areas with intervening T2 hypointense septa.3,5–7 However, most of these studies evaluated SSs occurring throughout the body, including thoracic, abdominopelvic and head and neck SSs, which are all quite variable in presentation and imaging.3,5–7 Besides, none of these studies focused on the imaging features of small SSs, which are often non-aggressive in appearance, or on the pattern of metastatic disease.5,8
In this series, we evaluated the CT and MRI features of 78 patients with primary and metastatic extremity SSs and correlated the imaging features with the metastatic pattern.
METHODS AND MATERIALS
Subjects
This was an institutional review board-approved, Health Insurance Portability and Accountability Act-compliant retrospective study with waiver of informed consent. We identified 373 consecutive patients with histopathologically confirmed SSs treated at our institute between 2002 and 2013 from the pathology database. Patients without cross-sectional imaging available or with SSs involving other parts of the body were excluded from the study. Imaging studies were available for review in 156 patients, of which 78 had extremity SS and were included in our study.
Clinical and histopathological data
Clinical features and histopathological findings were extracted from the electronic medical records, including patient demographics, date of diagnosis, clinical features at presentation, date of diagnosis of metastatic disease, type of treatment, duration of follow-up and final outcome.
Imaging and image analysis
Baseline imaging of the treatment-naïve primary tumour was available in 31 patients (MRI in all patients and CT in 9), and follow-up imaging was available in all 78 patients. All pre-treatment and follow-up imaging studies were reviewed in consensus by two oncoradiology fellowship-trained radiologists (ADB and SHT) with 5 and 8 years' experience. A total of 563 CTs of the chest, abdomen, pelvis and extremities (and brain for 19 patients for a clinical suspicion of metastases) (mean number of studies reviewed per patient, 7; range, 1–37) and 191 MRIs of the extremities (and brain in 21 patients) were reviewed.
The MRI examinations were performed on 1.5-T MRI (GE Healthcare, Milwaukee, WI) or 3-T MRI (Siemens Medical Solutions, Forchheim, Germany) scanners with gadolinium administration at doses of 0.1 mmol kg−1 body weight up to a maximum dose of 20 ml. Sequences acquired included axial and coronal T2 weighted [repetition time (TR)/echo time (TE), 1869/78 ms] sequences, coronal T2 weighted fat-suppressed (TR/TE 2020/95 ms), T1 weighted and diffusion-weighted sequences (b = 0, 500 and 800 s mm−2), and non-enhanced and dynamic contrast-enhanced fat-suppressed T1 weighted gradient-recalled echo (TR/TE, 5.4/2.3 ms). Contrast-enhanced multidetector CT (MDCT) images were acquired on 4-slice (GE Healthcare, Barrington, IL), 16-slice (Siemens Medical Solutions) and 64-slice MDCT (Aquilion 64; Toshiba America Medical Systems, Tustin, CA) scanners, with 5-mm thickness axial images and 4-mm coronal reconstructions. Iopromide (300 mg I ml−1; Ultravist 300; Bayer HealthCare Pharmaceuticals, San Francisco, CA) was administered as intravenous contrast using an automated injector (Stellant®; Medrad, Warrendale, PA) at a rate of 2–3 ml s−1, with a scan delay of 60 s. The images were reviewed on Centricity picture archiving and communication system RA1000 (GE Healthcare) workstation.
Imaging features of the primary tumour that were recorded included the site of origin, largest dimension, margin (well defined or ill defined), outline (smooth, lobulated or irregular), tumour heterogeneity, enhancement, perilesional oedema, interfascial or intercompartmental extension, triple sign, bowl of grapes appearance and the presence of calcification, haemorrhage or fluid–fluid levels. Interfascial extension was defined as post-contrast enhancement, which extended beyond the tumour along the fascial planes. Intercompartmental extension was defined as tumour extending beyond the same compartment in the extremity; for example, from the muscle (deep compartment) into the subcutaneous tissue (superficial compartment), or a posterior compartment tumour extending into the anterior compartment. Smaller tumours (T1 stage, ≤5 cm) were also analysed separately. The presence of metastases at presentation and of development of local or distant recurrent disease during follow-up, their site, number (whether single or multiple) and imaging features were also noted. Intrapulmonary or pleural-based metastases were included under pleuropulmonary metastases, and whether the largest metastasis was pleural-based or pulmonary was separately noted. Metastatic lesions were confirmed either at histopathology (14 patients) or by the presence of unequivocal progression or response to treatment on serial follow-up imaging (34 patients).
Statistical analysis
We studied the effect of patient and imaging characteristics on the pre-treatment MRI on the risk of metastatic spread. A univariate analysis of the imaging features of the primary was performed to correlate them with the development of metastatic disease and survival. This was carried out using Fisher's exact test for categorical variables, Student's t-test for continuous variables with normal distribution and Wilcoxon test for continuous variables with non-normal distribution. Multivariate analysis for this purpose was not possible because of the small number of patients with imaging of primary tumour available for review. We further evaluated the effect on survival of the imaging features that were associated with a higher incidence of metastatic disease using the log-rank test and construction of Kaplan–Meier curves. A two-sided p-value <0.05 was considered statistically significant. All analyses were performed using JMP Pro v. 11.0.0 (SAS Institute Inc., Cary, NC.)
RESULTS
The study consisted of 42 males and 36 females with a mean age of 40 years (range, 9–79 years; standard deviation, 15.8 years). The lower extremity was involved in 66 (85%) cases and the upper extremity in 12 (15%) cases, with the majority of the tumours occurring in the thighs (n = 26) and around the knees (n = 12).
Clinical presentation
The most common presenting symptom was a slowly growing lump in 52/78 (67%), followed by pain or discomfort in 37/78 (47%) patients. One patient presented with back pain owing to metastatic disease.
Imaging of the primary tumour (n = 31)
The mean size of the primary tumour on imaging was 8.5 cm (range, 1.4–22.6 cm; standard deviation, 4.3 cm). On MRI, the tumours were hyperintense compared with skeletal muscles on T2 weighted images (T2WI) and demonstrated intermediate signal intensity on T1 weighted images (T1WI) with T1 hyperintense haemorrhagic foci in 10 patients. Tumour margins were well circumscribed in 27/31 patients and ill defined in 4/31. The tumours were smooth or lobulated in 15/31 patients and irregular in 16/31. Heterogeneously enhancing solid tumours were seen in 18/31 patients, with hypointense necrotic/cystic components (solid-cystic tumours) seen in 13/31. No tumour demonstrated homogeneous enhancement. 8/31 tumours showed extreme T1 and T2 hypointense areas consistent with calcifications on MRI, all of which were confirmed on corresponding radiographs (2 patients) or on CT (6 patients) (Table 1).
Table 1.
Imaging features of the primary tumour
| Characteristic | Number (n = 31) | p-value |
|---|---|---|
| Mean size | 8.5 cm (range, 1.4–22.6 cm) | 0.04a |
| Well-circumscribed vs ill-defined margins | 27 vs 4 | 0.1 |
| Smooth/lobulated vs irregular | 15 vs 16 | 0.11 |
| Solid vs solid-cystic tumours | 18 vs 13 | 0.13 |
| Perilesional oedema | 14 | 0.03a |
| Interfascial extension | 15 | 0.07 |
| Intercompartmental extension | 7 | 0.03a |
| Triple sign | 11 | 0.13 |
| Intratumoral haemorrhage | 10 | 0.02a |
| Bowl of grapes | 5 | 0.04a |
| Calcification | 8 | 0.68 |
| Bone involvement | 3 | 0.3 |
Statistically significant correlation.
Perilesional oedema was present in 14/31 tumours (45%), and 15/31 (48%) demonstrated interfascial extension, while 7/31 (23%) had intercompartmental extension. Intratumoral haemorrhage was seen in 10/31 (32%) patients (with 3 of them showing fluid–fluid levels), 5/31 (16%) demonstrated bowl of grapes sign and 11/31 (35%) exhibited the triple sign (Figure 1). Neurovascular encasement was demonstrated in 6/31 (19%) patients, with associated venous thrombosis in 1 of them. Bony involvement was present in three tumours; two of them were predominantly soft-tissue masses with invasion of the adjacent bones, involving the cortex and extending into the medullary cavity, while the bone was the epicentre of the tumour in the third patient. SS appeared hypodense compared with muscle on CT and demonstrated heterogeneous enhancement, and calcifications were better appreciable.
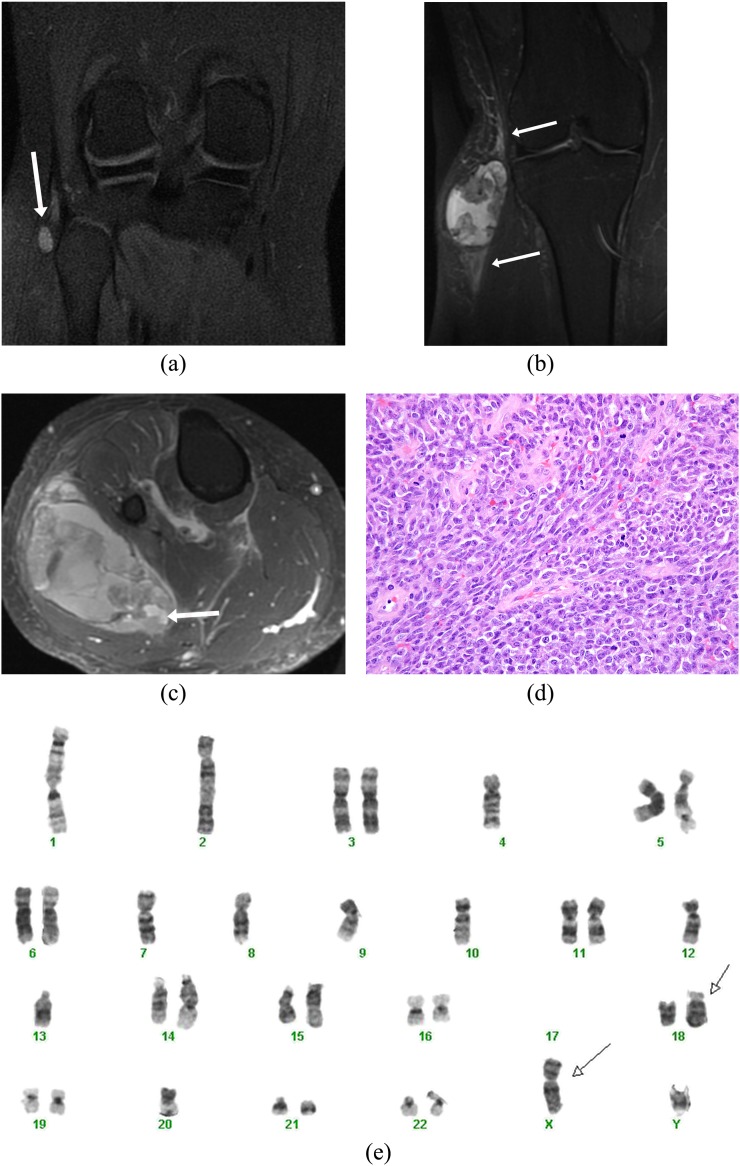
Figure 1.
A 21-year-old male presented with a painless “pea-sized” lump on the right knee. (a) Coronal non-contrast inversion recovery sequence image shows an incidental small well-defined hyperintense lesion on the lateral aspect of the knee detected during an MRI knee study (arrow). A contrast-enhanced study was not performed, and the lesion was presumed to possibly represent a ganglion cyst/collection, and follow-up imaging was suggested. (b) The patient was unfortunately lost to follow up and presented 3 years later with a large swelling involving the right knee. Coronal inversion recovery sequence image reveals dramatic increase in the heterogeneous lesion showing hyper-, iso- and hypointense components (triple sign) along with perilesional oedema (arrows). (c) Axial post-contrast fat-saturated image demonstrates heterogeneous enhancement with presence of fluid–fluid levels (arrow) consistent with blood products. The patient unfortunately also had pleuropulmonary metastases and is currently on chemotherapy. (d) Histology showed a poorly differentiated synovial sarcoma composed of spindled to round cells with a high mitotic rate (original magnification, ×400). (e) Cytogenetic analysis showed a complex karyotype (following neoadjuvant therapy), which included the pathognomonic translocation t(X;18)(p11;q11) (arrows).
Imaging of T1 tumours
8/31 patients had tumours ≤5 cm in size. Contrast enhancement was visualized in all except one patient who had a non-contrast study. Perilesional oedema and interfascial extension were each present in two tumours, while intercompartmental extension was present in one (Figure 2). One patient had a non-contrast MRI of the knee (for evaluating knee pain) with an incidental 1.4-cm homogeneous T2 hyperintense lesion adjacent to the tibial tuberosity, which was considered non-aggressive. 4 years later, the patient presented symptomatically with a large heterogeneously enhancing mass on MRI (Figure 1).
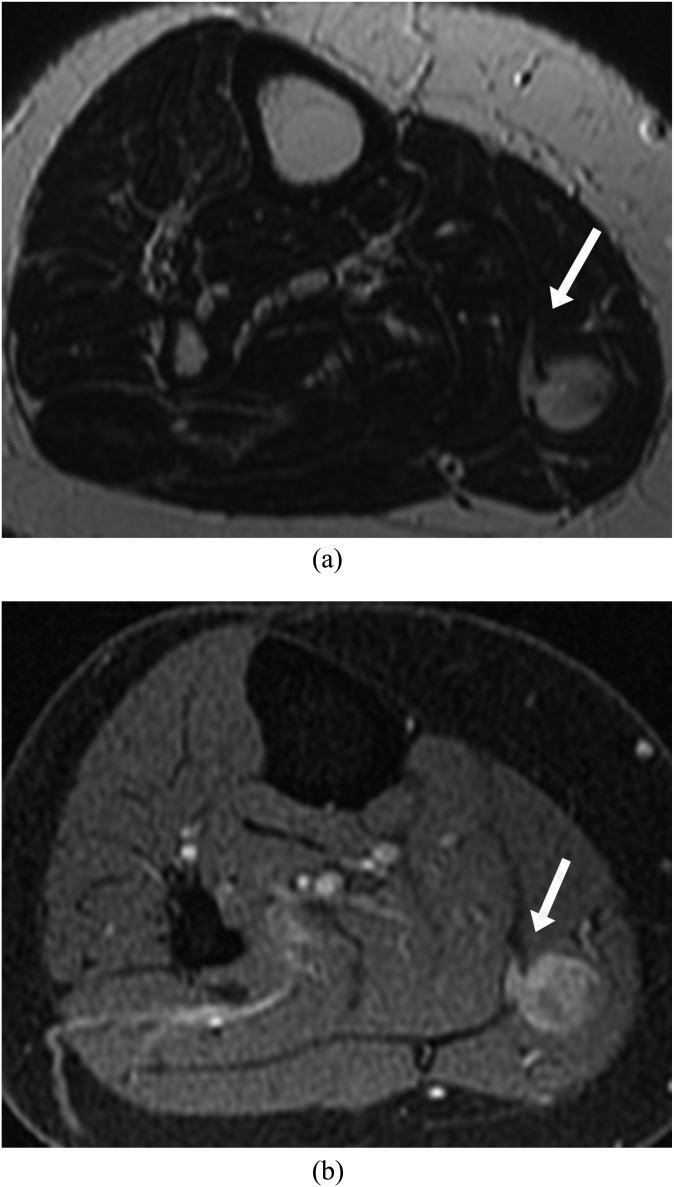
Figure 2.
A 62-year-old female presenting with right mid-calf pain. (a) Axial T2 weighted image shows a well-defined intramuscular lesion with interfascial extension appearing like a tail (arrow). (b) Axial post-contrast fat-saturated T1 weighted image demonstrated enhancement of the lesion as well as the interfascial extension (arrow), thus differentiating it from oedema. The tumour was completely resected, and the patient is disease free for 4 years at the time of last follow-up.
Imaging of recurrent/metastatic disease
Recurrent/metastatic disease developed in 49/78 (63%) patients. Of these, 20/78 (26%) had metastasis at presentation, while the remaining patients developed metastatic disease at a median interval of 27 months (range, 3–161 months).
Local recurrence was seen in 4/78 patients (5%) on follow-up imaging, all of whom also had distant metastatic disease. Recurrent tumours were similar to the primary tumour on MRI, being heterogeneously hyperintense on T2WI and intermediate on T1WI with nodular contrast enhancement.
The most common sites of metastases were pleuropulmonary, bones and lymph nodes (Table 2). 19/78 patients had pleural effusion, all with associated pleuropulmonary metastases, while 2 had ascites, both with associated peritoneal metastases.
Table 2.
Sites of metastatic disease
| Site of metastasis | Number of patients |
|---|---|
| Pleuropulmonary | 46 |
| Bones | 9 |
| Lymph nodes | 8 |
| Liver | 4 |
| Peritoneum | 4 |
| Pancreas, subcutaneous tissue and intramuscular | 2 each |
| Epidural and renal | 1 each |
The pleuropulmonary metastases were predominantly pleural based in the majority of these cases, with the largest deposit being pleural based in 32 of the 46 cases (Figure 3). The mean size of the largest metastatic lesion was 7.6 cm (range, 0.7–20 cm). 6/46 patients had unilateral metastases, not related to the side of the primary tumour, while the rest had bilateral metastases. Cavitation was not present in any of the nodules. All lesions were discrete, seen as nodules or masses. Other forms of pulmonary involvement, such as lymphangitic spread and consolidation, were not visualized.
Figure 3.
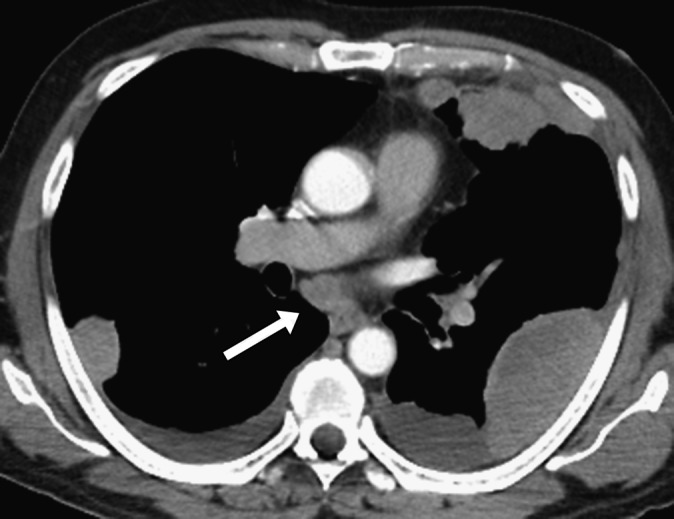
A 56-year-old male with a history of resected right-thigh synovial sarcoma presented with chest pain and mild breathlessness 1 year after surgery. Axial CT image of the chest shows multiple pleural-based metastases and bilateral pleural effusions along with subcarinal adenopathy (arrow).
Bone (n = 9) was the second most common site of involvement, with lytic metastases in 8/9 patients and mixed lytic–sclerotic metastases in 1/9 patients. 3/9 patients had solitary bony involvement, while the rest had multiple metastases. Nodal metastases were seen in eight patients. Seven of these were intrathoracic, all associated with pleuropulmonary metastases. One patient had right pelvic sidewall adenopathy (not present at the time of presentation) with a right calf primary. Brain metastases (n = 3) were supratentorial in all patients, with associated perilesional oedema in all three and haemorrhagic changes in two patients.
Correlation of imaging features with metastatic disease
Statistical analysis of various imaging features of the primary correlating them with the development of metastatic disease revealed that tumour size (p = 0.04), the presence of perilesional oedema (p = 0.03), intercompartmental extension (p = 0.03), the presence of intralesional haemorrhage (p = 0.02) and bowl of grapes appearance (p = 0.04) had statistically significant predictive value (Table 1). Metastases developed in 73% (11/15) patients with perilesional oedema, 88% (7/8) patients with intercompartmental extension, 82% (9/11) patients with intralesional haemorrhage and 100% (5/5) patients with bowl of grapes appearance, whereas 29% (5/17), 38% (9/24), 33% (7/21) and 41% (11/27) patients without the above features, respectively, developed metastatic disease. Other imaging features, including shape, margins, interfascial extension, triple sign, the presence of calcifications and bone involvement, did not significantly correlate with metastases.
Pathology, management and final outcome
42 patients had monophasic tumours, 22 had biphasic tumours and 14 had poorly differentiated tumours. All except five patients were positive for t(X;18) translocation. We found no significant correlation between the imaging findings and pathology.
The primary tumour was resected in 65 patients. 35 patients received neoadjuvant therapy (chemotherapy or radiation or both) before undergoing surgical resection. 50 patients received adjuvant therapy, while 14 did not receive any treatment. Medical management was based on standard ifosfamide- and doxorubicin-based regimens, second-line chemotherapy agents and clinical trials. At the time of last follow-up, 30 patients were alive, 30 were deceased while the remaining 18 were lost to follow up. Out of the 30 patients who died, 27 had metastases, 11 at presentation. The median follow-up for all the patients was 45 months (range, 1–206 months). The median survival for all patients was 43.5 months (range, 7–206 months). Among the 20 patients with metastasis at presentation, 11 expired at a median interval of 24 months (range, 7–61 months), with 5 alive and 4 lost to follow up, while for patients without metastases at presentation, the median survival was 78.5 months. No imaging findings were found to significantly correlate with overall survival on statistical analysis.
DISCUSSION
SS belongs to the group of translocation-associated malignancies and is characterized by the presence of t(X;18) translocation in about 95% of tumours.1,9,10 Pathologically, it is classified into monophasic (consisting of spindle cells), biphasic (consisting of spindle cells and glands) and poorly differentiated types. Despite its name, SS does not arise from the synovium and is rarely intra-articular in location, being most commonly periarticular or close to a bursa or tendon sheath.3,4 SS mainly affects adolescents and young adults and involves both sexes equally.5
MRI is the imaging modality of choice of soft-tissue tumours, as it best characterizes intratumoral architecture along with other features such as perilesional oedema, neurovascular encasement and involvement of adjacent structures.9 A previous study of 30 patients by Tateishi et al7 (including 22 patients of extremity SS) revealed large tumour size, the presence of haemorrhage or triple sign and the absence of calcifications to significantly correlate with high tumour grade and disease-free survival on univariate analysis, whereas tumour size >10 cm had significant correlation with the same on multivariate analysis. In our study, tumour size, intralesional haemorrhage and bowl of grapes appearance correlated significantly with development of metastatic disease but not with disease-free or overall survival.
SSs are among the most common sarcomas to demonstrate calcifications, seen in 28–30% of cases.7 Calcifications were present in 19% tumours in our series. Previous series have described calcifications to be a good prognostic factor.7 None of the six patients with calcifications in our series has expired, but we could not calculate the statistical significance of the prognostic value of calcifications owing to the small number of patients. We found perilesional oedema in 45% of treatment-naïve tumours. This is in contrast with a previous study that reported perilesional oedema to be uncommon in de novo SS and to frequently develop after neoadjuvant therapy.5 We found the presence of perilesional oedema as also intercompartmental extension of the tumour to correlate significantly with metastatic disease. A previous study of 15 cases of soft-tissue sarcoma with peritumoral oedema (including 2 cases of SS) revealed 10/15 patients to have sarcoma cells beyond the tumour on histopathology, and the region of peritumoral oedema is indeed covered by radiation oncologists for treatment.11,12 This indicates that tumours with peritumoral oedema are possibly locally infiltrative and more likely to have a higher incidence of metastases, as demonstrated in our study. Similarly, tumours with demonstrable intercompartmental extension on imaging may be considered possibly infiltrative and accordingly also have a higher incidence of metastases.
A recent study of 95 patients with different soft-tissue sarcomas (including 9 patients with SSs) correlated MRI features with the histological grade and concluded that the presence of peritumoral contrast enhancement is the strongest predictor of tumour grade.13 Other predictors of high grade in the study included larger tumour size, poorly defined margins, heterogeneity on T2WI and high peritumoral T2 signal. However, the study included a heterogeneous group of >15 sarcomas. Furthermore, the tumours were classified as high- or low-grade sarcomas in the study, whereas SS includes monophasic, biphasic and poorly differentiated subtypes. In our study, peritumoral contrast enhancement along the adjacent fascia (interfascial extension) was not found to correlate significantly with metastatic disease. We suggest that this may be owing to confounding factors such as peritumoral hyperaemia, which may also look similar on imaging.
Smaller SSs may masquerade as benign lesions, with well-circumscribed homogeneous or even cystic appearance on the pre-contrast MRI. Particularly, when in a periarticular location, they may be misdiagnosed as ganglion cysts or aggressive fibromatosis.5,8 In our series, we found three tumours that may be confused with complicated cysts on the pre-contrast study. However, we also noted that small SS may demonstrate other suspicious features, such as perilesional oedema or interfascial or intercompartmental extension, and all demonstrated heterogeneous contrast enhancement. The latter is consistent with the findings of Murphey et al,5 who stated that SS does not simulate a cyst (with thin peripheral or septal enhancement alone) on post-contrast imaging. This emphasizes the need for a high index of suspicion and importance of post-contrast studies in all cases of indeterminate soft-tissue lesions.
SSs have a high incidence of metastatic disease, with distant metastases developing in 48–68% of patients as per various studies, 6–14% at presentation.10,14,15 Importantly, while metastases usually develop within the first 5 years, late metastases with indolent behaviour, have been well described in literature, with a significant difference between the 5- and 10-year survival rates, thus emphasizing the importance of long-term radiological follow up.1,16
We found the majority of metastatic SSs present with pleuropulmonary metastases, with only 6% (3/49) patients having extrapulmonary metastases without pleuropulmonary involvement. An important observation in our study was that the intrathoracic metastatic disease was often pleural based, with the largest metastatic lesion being pleural based in 70% cases. This finding has not been described for other sarcomas to the best of our knowledge.
Nodal metastases are uncommon in sarcomas, as they typically have a haematogenous route of dissemination. SS is however one of the few sarcomas to have nodal metastases occurring in up to 8–27% patients. These are nearly always a late manifestation of distant metastatic disease in the thorax (associated with pleuropulmonary metastases), with regional lymph node metastases being exceptionally rare.17–20 The findings in our study are similar, with 10% patients developing nodal metastases. Interestingly, the majority of the involved lymph nodes were intrathoracic in location, associated with pleuropulmonary metastases, rather than related to primary tumour. Other sarcomas that have a relative predilection for nodal metastases include clear-cell sarcoma, angiosarcoma, epithelioid sarcoma, alveolar soft part sarcoma and rhabdomyosarcoma. These tumours may spread to the regional lymph nodes as well.21–23
The 5-year survival rate for SS is 66–71%.10,14 A recent study of 33 patients with metastatic SS concluded that patients with extrapulmonary metastases or with nodal metastases have decreased overall survival on multivariate analysis.20 In our study, 50% (4/8) of patients with metastatic adenopathy and 64% (18/28) of patients with extrapulmonary metastases expired, consistent with these findings.
The differential diagnosis for SS predominantly includes other soft-tissue sarcomas (most common being leiomyosarcoma, liposarcoma and undifferentiated pleomorphic sarcoma, formerly malignant fibrous histiocytoma), all of which are more common in the fifth to sixth decades of life.24–27 The imaging findings frequently overlap, including typical imaging features such as the triple sign, which may also be seen in undifferentiated pleomorphic sarcoma.5 SS may rarely appear almost cystic on pre-contrast MRI (as seen in one of our patients) and may then mimic other cystic lesions such as a poplitaeal cyst (if around the knee joint) or a haematoma, and heterogeneous contrast enhancement helps differentiate between the two in these cases.5 The limitations of the study include the retrospective study design, lack of availability of imaging of the primary tumour in all cases and a selection bias owing to ours being a tertiary cancer institute.
In conclusion, larger tumour size, the presence of perilesional oedema, intercompartmental extension, the presence of intralesional haemorrhage and bowl of grapes appearance were associated with a higher incidence of metastatic disease in our study. T1 stage tumours demonstrated suspicious features, such as heterogeneous enhancement, interfascial extension and perilesional oedema. However, they may appear non-aggressive on pre-contrast imaging, and a high index of suspicion must be maintained. The presence of predominantly pleural-based metastases is characteristic of SS.
REFERENCES
- 1.Eilber FC, Dry SM. Diagnosis and management of synovial sarcoma. J Surg Oncol 2008; 97: 314–20. doi: 10.1002/jso.20974 [DOI] [PubMed] [Google Scholar]
- 2.Spurrell EL, Fisher C, Thomas JM, Judson IR. Prognostic factors in advanced synovial sarcoma: an analysis of 104 patients treated at the Royal Marsden Hospital. Ann Oncol 2005; 16: 437–44. [DOI] [PubMed] [Google Scholar]
- 3.O'Sullivan PJ, Harris AC, Munk PL. Radiological features of synovial cell sarcoma. Br J Radiol 2008; 81: 346–56. doi: 10.1259/bjr/28335824 [DOI] [PubMed] [Google Scholar]
- 4.Bixby SD, Hettmer S, Taylor GA, Voss SD. Synovial sarcoma in children: imaging features and common benign mimics. AJR Am J Roentgenol 2010; 195: 1026–32. doi: 10.2214/AJR.10.4348 [DOI] [PubMed] [Google Scholar]
- 5.Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. From the archives of the AFIP: imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics 2006; 26: 1543–65. [DOI] [PubMed] [Google Scholar]
- 6.Jones BC, Sundaram M, Kransdorf MJ. Synovial sarcoma: MR imaging findings in 34 patients. AJR Am J Roentgenol 1993; 161: 827–30. [DOI] [PubMed] [Google Scholar]
- 7.Tateishi U, Hasegawa T, Beppu Y, Satake M, Moriyama N. Synovial sarcoma of the soft tissues: prognostic significance of imaging features. J Comput Assist Tomogr 2004; 28: 140–8. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima H, Matsushita K, Shimizu H, Isomi T, Nakano Y, Saito M, et al. Synovial sarcoma of the hand. Skeletal Radiol 1997; 26: 674–6. [DOI] [PubMed] [Google Scholar]
- 9.Bakri A, Shinagare AB, Krajewski KM, Howard SA, Jagannathan JP, Hornick JL, et al. Synovial sarcoma: imaging features of common and uncommon primary sites, metastatic patterns, and treatment response. AJR Am J Roentgenol 2012; 199: W208–15. doi: 10.2214/AJR.11.8039 [DOI] [PubMed] [Google Scholar]
- 10.Guillou L, Benhattar J, Bonichon F, Gallagher G, Terrier P, Stauffer E, et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol 2004; 22: 4040–50. [DOI] [PubMed] [Google Scholar]
- 11.White LM, Wunder JS, Bell RS, O'Sullivan B, Catton C, Ferguson P, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61: 1439–45. [DOI] [PubMed] [Google Scholar]
- 12.Bahig H, Roberge D, Bosch W, Levin W, Petersen I, Haddock M, et al. Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 2013; 86: 298–303. doi: 10.1016/j.ijrobp.2013.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao F, Ahlawat S, Farahani SJ, Weber KL, Montgomery EA, Carrino JA, et al. Can MR imaging be used to predict tumor grade in soft-tissue sarcoma? Radiology 2014; 272: 192–201. doi: 10.1148/radiol.14131871 [DOI] [PubMed] [Google Scholar]
- 14.Ferrari A, Gronchi A, Casanova M, Meazza C, Gandola L, Collini P, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer 2004; 101: 627–34. [DOI] [PubMed] [Google Scholar]
- 15.Trassard M, Le Doussal V, Hacène K, Terrier P, Ranchère D, Guillou L, et al. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol 2001; 19: 525–34. [DOI] [PubMed] [Google Scholar]
- 16.Singer S, Baldini EH, Demetri GD, Fletcher JA, Corson JM. Synovial sarcoma: prognostic significance of tumor size, margin of resection, and mitotic activity for survival. J Clin Oncol 1996; 14: 1201–8. [DOI] [PubMed] [Google Scholar]
- 17.Buck P, Mickelson MR, Bonfiglio M. Synovial sarcoma: a review of 33 cases. Clin Orthop Relat Res 1981: 211–15. [PubMed] [Google Scholar]
- 18.Ferrari A, De Salvo GL, Dall'Igna P, Meazza C, De Leonardis F, Manzitti C, et al. Salvage rates and prognostic factors after relapse in children and adolescents with initially localised synovial sarcoma. Eur J Cancer 2012; 48: 3448–55. doi: 10.1016/j.ejca.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 19.Ryan JR, Baker LH, Benjamin RS. The natural history of metastatic synovial sarcoma: experience of the Southwest Oncology group. Clin Orthop Relat Res 1982; 257–60. [PubMed] [Google Scholar]
- 20.Salah S, Salem A. Primary synovial sarcomas of the mediastinum: a systematic review and pooled analysis of the published literature. ISRN Oncol 2014; 2014: 412527. doi: 10.1155/2014/412527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg 1993; 217: 72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 1987; 60: 1800–8. [DOI] [PubMed] [Google Scholar]
- 23.Sood S, Baheti AD, Shinagare AB, Jagannathan JP, Hornick JL, Ramaiya NH, et al. Imaging features of primary and metastatic alveolar soft part sarcoma: single institute experience in 25 patients. Br J Radiol 2014; 87: 20130719. doi: 10.1259/bjr.20130719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belal A, Kandil A, Allam A, Khafaga Y, El-Husseiny G, El-Enbaby A, et al. Malignant fibrous histiocytoma: a retrospective study of 109 cases. Am J Clin Oncol 2002; 25: 16–22. [DOI] [PubMed] [Google Scholar]
- 25.Gordon RW, Tirumani SH, Kurra V, Shinagare AB, Jagannathan JP, Hornick JL, et al. MRI, MDCT features, and clinical outcome of extremity leiomyosarcomas: experience in 47 patients. Skeletal Radiol 2014; 43: 615–22. doi: 10.1007/s00256-014-1823-8 [DOI] [PubMed] [Google Scholar]
- 26.O'Regan KN, Jagannathan J, Krajewski K, Zukotynski K, Souza F, Wagner AJ, et al. Imaging of liposarcoma: classification, patterns of tumor recurrence, and response to treatment. AJR Am J Roentgenol 2011; 197: W37–43. doi: 10.2214/AJR.10.5824 [DOI] [PubMed] [Google Scholar]
- 27.Mastrangelo G, Coindre JM, Ducimetière F, Dei Tos AP, Fadda E, Blay JY, et al. Incidence of soft tissue sarcoma and beyond: a population-based prospective study in 3 European regions. Cancer 2012; 118: 5339–48. doi: 10.1002/cncr.27555 [DOI] [PubMed] [Google Scholar]