Abstract
Objective:
Foetal CT has recently been added to the foetal imaging armamentarium, but this carries with it the risks of ionizing radiation, both to the mother and the foetus. Foetal “black bone” MRI is a new technique that allows assessment of the foetal skeleton without the risk of exposure to ionizing radiation and is a potential new sequence in foetal MRI examination.
Methods:
Retrospective review of all foetal MRI studies over the past 4- to 5-year period identified 36 cases where susceptibility weighted imaging was used. Cases were selected from this group to demonstrate the potential utility of this sequence.
Results:
This sequence is most frequently useful not only in the assessment of spinal abnormalities, most commonly the bony abnormalities in myelomeningocele, but also in cases of scoliosis, segmentation anomalies and sacrococcygeal teratoma.
Conclusion:
Although the utility of this sequence is still being evaluated, it provides excellent contrast between the mineralized skeleton and surrounding soft tissues compared with standard half Fourier acquisition single-shot turbo-spin echo sequences. Further assessment is required to determine whether black bone MRI can more accurately evaluate the level of bony defect in spina bifida aperta, an important prognostic factor. Potential further uses include the assessment of skeletal dysplasias, evaluation of the skull base and craniofacial skeleton in certain congenital anomalies and the post-mortem evaluation of the foetal skeleton potentially obviating the need for necropsy.
Advances in knowledge:
Foetal black bone MRI can be performed using susceptibility weighted imaging and allows better demonstration of the mineralized skeleton compared with standard sequences.
Recent advances in the adult musculoskeletal literature have led to the development of the “black bone” MRI sequence.1,2 This sequence was developed in response to growing concerns regarding the harmful effects of radiation3–5 and was specifically developed for evaluation of the craniofacial skeleton, assessment of which is normally carried out by CT. By methodical evaluation of a gradient echo sequence by stepwise variation of flip angles, the sequence was chosen that gave the highest contrast between bone and soft tissues but the lowest contrast between different soft tissues.
FOETAL SKELETAL IMAGING BY RADIOGRAPHY AND CT
Although foetal plain film radiography and contrast amniography went out of fashion with the advent of ultrasound, foetal CT is now being increasingly used for assessment of foetal skeletal abnormalities.6–8 However, there is still concern regarding harmful effects on the foetus, particularly as depending on the time of examination, the foetus is considered to be more radiosensitive owing to rapid growth, cell division and organogenesis. There is less concern by mid-trimester, and typically these concerns are outweighed by clinical necessity and relatively poor prognostic implications of the pathology being investigated. In the paediatric radiology literature, however, several articles raise concerns regarding the effects of radiation from CT examinations, and a 1:10,000 increased risk of developing either a solid tumour or leukaemia as a result of CT examination has recently been reported.9
FOETAL SKELETAL IMAGING BY MRI
Although the poor detail of bone on MRI has been a significant limitation of MRI in assessment of bony structures, echo-planar imaging has recently been used to assess human long bone development.10 Foetal MRI therefore has the potential to reduce or avoid radiation exposure from foetal CT by replacing CT scanning when imaging the foetal skeleton. This is particularly important if there is uncertainty whether the pregnancy is to be continued.
Susceptibility weighted imaging (SWI) is a technique developed by Siemens.11 Calcification is strongly diamagnetic and therefore decreases the magnetization in bone compared with the applied magnetic field. This reduces the Larmor frequency where there is calcium and causes heterogeneity in the localized magnetic field that leads to intra- and intervoxel dephasing thus causing signal drop-out in the voxel containing calcium as well as adjacent voxels, a phenomenon referred to as “blooming”. The technique gives high contrast between bone and soft tissues, but low contrast between different soft tissues, thus the low signal bone is easily distinguished from the surrounding soft tissues. Thus the SWI sequence can be used for black bone imaging in the foetus.
The scan parameters are provided in Table 1. The sequence is extremely motion sensitive and therefore breath-hold is required. The volume of tissue imaged within one breath-hold (approximately 20 s) is quite shallow, therefore typically three concatenations are performed to cover the required volume, although this can be reduced if only a shallow volume of coverage is needed. The axial plane is best for assessment of the neural arches; the coronal plane gives additional assessment of the ribs and for pedicular widening in cases of spina bifida; and the sagittal plane is best for assessment of the whole spine lengthways. The sequence is high in resolution and therefore the acquired volume can be reformatted into optimal planes, for example, to correct for mild degrees of off-axis imaging. They can also be reformatted into three-dimensional reconstructions, because although the sequence is a two-dimensional sequence, the slice thickness is small and does not cause significant step artefact. The sequence is best run immediately after routine half Fourier acquisition single-shot turbo-spin echo (HASTE) sequences depicting the required anatomy by simply copying the slice parameters thus minimizing the time between sequences and reducing the chance of foetal motion.
Table 1.
MRI scanning parameters on a Magnetom Avanto 1.5 T (Siemens, Erlangen, Germany)
| Parameter | Value |
|---|---|
| Repetition time (ms) | 115 |
| Echo time (ms) | 20.0 |
| Slices | 11 |
| Slice thickness (mm) | 2.0 |
| Slice spacing (mm) | 0 |
| FOV (frequency-encoding direction) (mm) | 300 |
| FOV (phase-encoding direction) | 87.5% |
| Matrix size (frequency-encoding direction) | 256 pixels |
| Matrix size (phase-encoding direction) | 79% (177 pixels) |
| Bandwidth (hertz per pixel) | 80 |
| Interpolation | ON |
| Parallel imaging | iPAT 2/ref. lines PE 24 |
| Concatenations | 3 |
| Number of excitations | 1 |
| Phase oversampling | 75% |
| Flip angle (°) | 30 |
| Pre-scan normalize | ON |
| Distortion correction | ON |
| Flow compensation | ON |
| 2 × 6 channel body array coils | |
| Coils built into table top |
FOV, field of view.
One problem with this sequence in adults was the inability to depict interfaces between bone and air, particularly when evaluating the craniofacial skeleton;2 however, this is not an issue pre-natally because there is no air in the foetus, and those same air-filled structures are filled with fluid.
POTENTIAL USES FOR FOETAL BLACK BONE MRI
We have used the SWI sequence for approximately 4–5 years in our institution to not only assess for the presence of foetal intracranial haemorrhage but also to assess the skeleton, in particular the skull and axial skeleton.
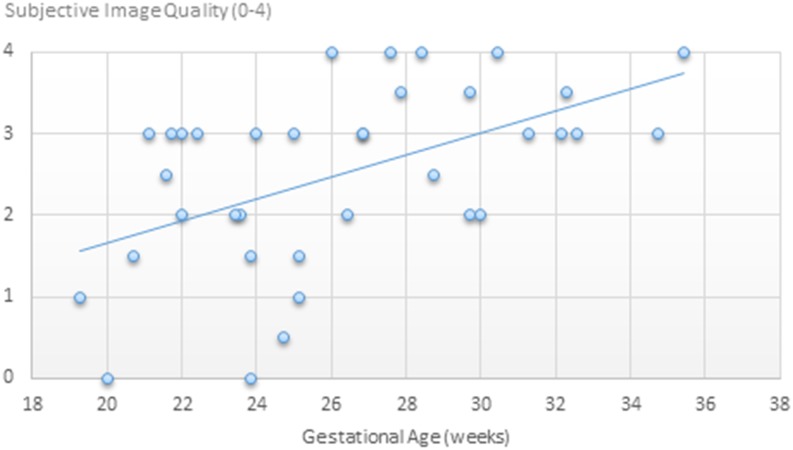
The sequence has so far been used in a total of 36 patients, ranging in gestational age from 19 to 35 weeks, average 26 weeks. The images have been subjectively graded by a single radiologist (SB) on a scale from zero to four (uninterpretable, poor, adequate, good and excellent). Good quality images were obtained after 21 weeks, and excellent quality images were only obtained after 26 weeks (Figure 1). No uninterpretable or poor quality images were seen after 26 weeks. As with foetal MRI in general, motion was the typical cause of degraded image quality, and as expected, this becomes less of an issue later in gestation owing to the growth in size of the foetus and progressively restricted movement.
Figure 1.
Subjective image quality with respect to gestational age.
In our experience, the most frequent utility of this sequence is in the assessment of spinal abnormalities (n = 23) (Figures 2–4), including segmentation anomalies (n = 3) (Figure 5), the bony abnormalities in myelomeningocele (n = 8) (Figure 6), scoliosis and adjoining rib anomalies (n = 8) (Figures 7 and 8) and sacrococcygeal teratoma (n = 2). The utility of this sequence is still being evaluated and needs further assessment to determine whether black bone MRI can more accurately evaluate the level of bony defect in spina bifida aperta, compared with HASTE or ultrasound.
Figure 2.
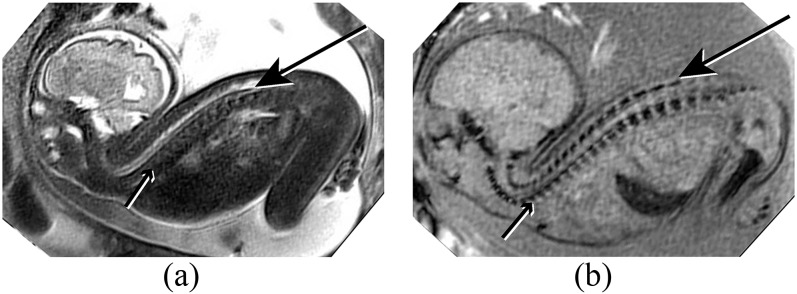
(a) Sagittal half Fourier acquisition single-shot turbo-spin echo (HASTE) image of a foetus with severe cervical retroflexion. The spinal cord (long arrow) is easily seen surrounded by high signal cerebrospinal fluid. The vertebral column is poorly demonstrated on HASTE imaging (short arrow). (b) Similar plane on susceptibility weighted imaging (SWI). The vertebral column and skull base are well demonstrated, including the laminae (long arrow) and vertebral bodies (short arrow). The spinal musculature is higher signal on SWI than on HASTE, providing significantly improved contrast.
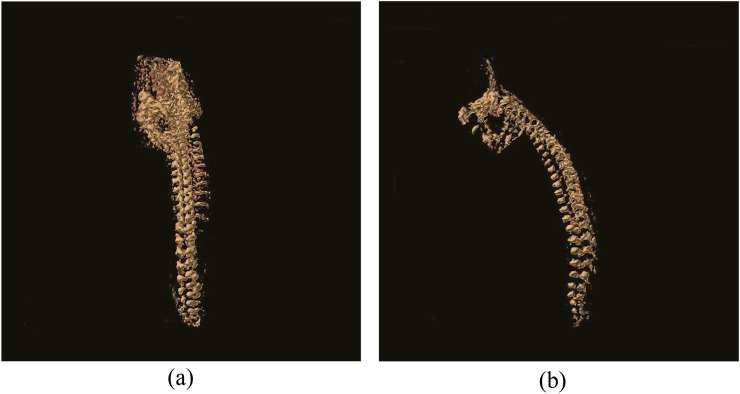
Figure 4.
(a) Coronal view of a three-dimensional (3D) reformatted susceptibility weighted imaging (SWI) sequence of the normal spine. (b) Sagittal view of a 3D reformatted SWI sequence of the normal spine.
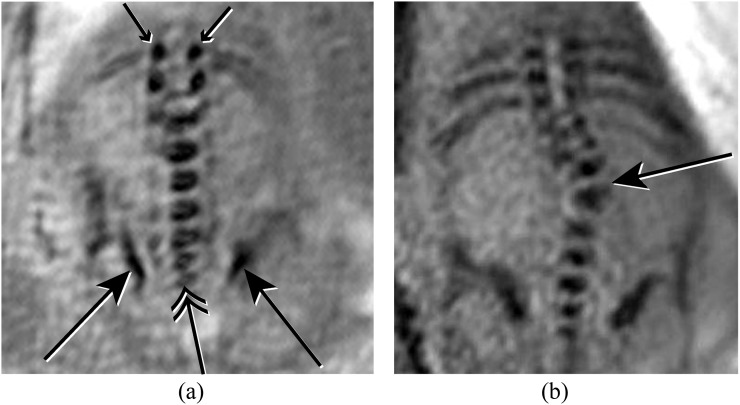
Figure 5.
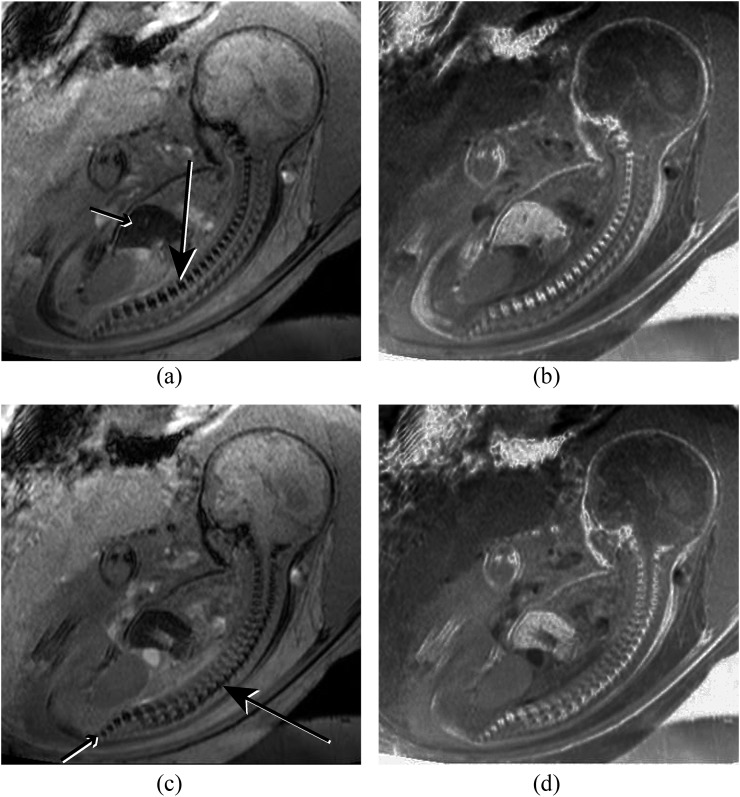
(a) Coronal susceptibility weighted imaging (SWI) image of the lumbosacral spine demonstrating normal iliac bones (long arrows), lumbosacral vertebral bodies (double arrow) and laminae (short arrows). (b) Coronal SWI image of the spine demonstrating a scoliosis secondary to a vertebral segmentation defect (long arrow).
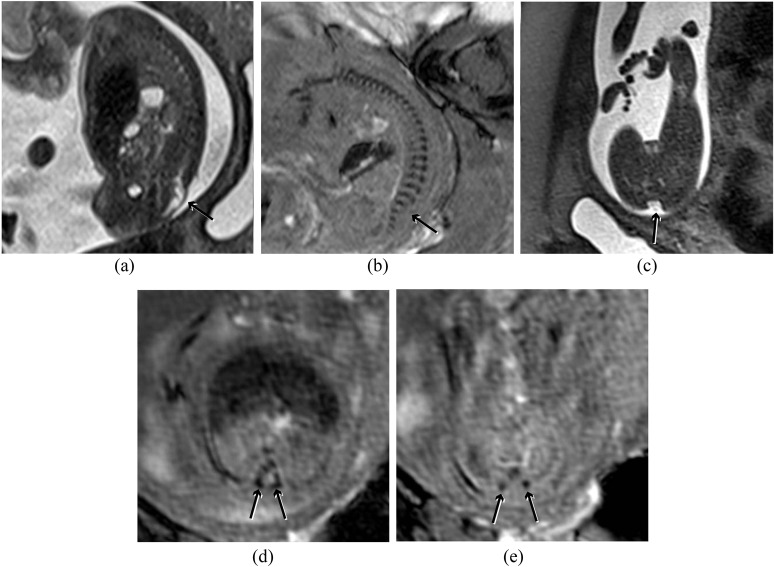
Figure 6.
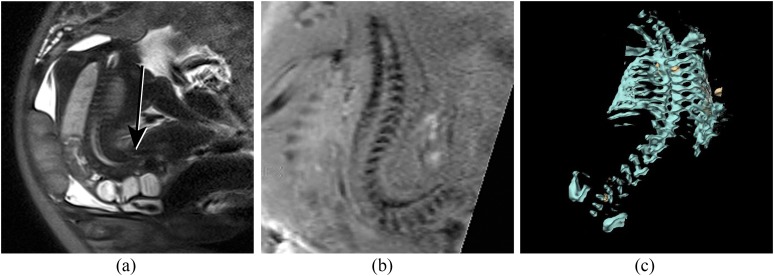
(a) Sagittal half Fourier acquisition single-shot turbo-spin echo (HASTE) image of the spine demonstrating a lumbosacral myelomeningocele (short arrow). (b) Corresponding susceptibility weighted imaging (SWI) image demonstrating the laminae throughout the spine except at the level of the defect (arrow), which can be seen to be at L5. (c) Axial HASTE image demonstrating the dysraphism (arrow), but bony anatomy is poorly demonstrated. (d) Axial SWI image above the lesion demonstrates the laminae pointing towards each other (arrows), which is the normal configuration. (e) Axial SWI image at the level of the lesion demonstrates the laminae to be short (arrows), not pointing to each other and displaced laterally.
Figure 7.
(a) Coronal half Fourier acquisition single-shot turbo-spin echo image through a foetus with limb body wall complex showing spinal curvature. The bony anatomy is poorly demonstrated. (b) Similar plane on susceptibility weighted imaging demonstrates the bony anatomy much more clearly. (c) Three-dimensional reformat on a different foetus with spinal curvature demonstrates the spine, skull base, ribs and iliac bones.
Figure 8.
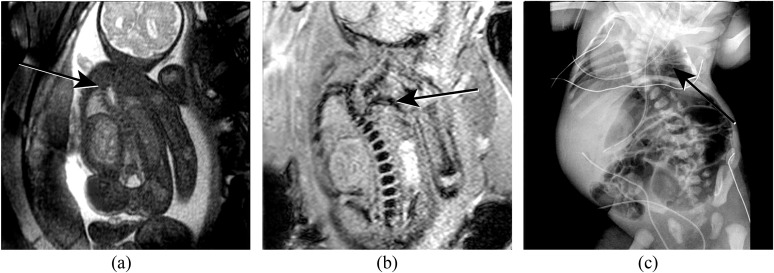
(a) Coronal half Fourier acquisition single-shot turbo-spin echo view through a foetus with vertebral anomalies, anorectal atresia, cardiac anomalies, tracheo-oesophageal fistula, renal and limb anomalies (VACTERL) association. The thoracic spinal curvature is seen (arrow), but the bony anatomy is not well demonstrated. (b) Coronal susceptibility weighted imaging image better demonstrates the bony anatomy and depicts rib non-segmentation (arrow) at the level of the scoliosis. (c) Postnatal radiograph demonstrates the rib non-segmentation (arrow). Additional rib abnormalities and dextrocardia are also seen.
Figure 3.
(a) Sagittal susceptibility weighted imaging (SWI) of the spine nicely demonstrating the vertebral bodies (long arrow). The liver (short arrow) is also low signal owing to the presence of iron. (b) Inverted image of (a) demonstrates an appearance similar to a standard radiograph. (c) Slightly parasagittal SWI image of the spine demonstrating the laminae of the posterior elements (long arrow). The vertebral bodies of the sacrum are also shown (short arrow). (d) Inverted image of (c).
A potential use that we have not yet evaluated would be in the assessment of skeletal dysplasias, which is the most common indication for foetal CT. Owing to the frequent deficient mineralization in these cases, CT can be challenging and the radiation doses necessarily quite high to provide sufficient contrast. SWI is extremely sensitive to calcium, therefore it may prove easier to depict the skeleton in foetuses with poor mineralization than by CT or ultrasound. Further evaluation of this is required.
Another potential main use is in the evaluation of the skull base and craniofacial skeleton in certain congenital anomalies, for example, evaluation of the mandible, hard palate and petrous bones in Goldenhar or Treacher Collins syndromes, pyriform sinus stenosis in single midline median central incisor and for choanal atresia and the petrous bones in coloboma, heart defects, atresia choanae, retardation, genital anomalies, ear anomalies (CHARGE) syndrome. A priori knowledge of bony airway obstruction is useful information in the immediate postnatal period because neonates are obligate nasal breathers.
Although not practiced in some parts of the world, another potential use is in pelvimetry. The sequence has the potential to be used along with biometry of the foetal skull and without the risks of ionizing radiation to both mother and foetus when using the more traditional methods of radiography and CT.
Finally, the technique could also be used in the post-mortem evaluation of the foetal skeleton without the requirement for necropsy. Although evaluation of the skeleton could be carried out by CT, this technique may allow assessment as part of a routine MR necropsy examination12–15 without the need for a separate CT study.
CONCLUSIONS
Foetal black bone MRI is a new technique for evaluation of the foetus, particularly in cases of spinal pathology, and also demonstrates potential use in the evaluation of craniofacial pathologies related to foetal syndromes, for evaluation of foetal skeletal dysplasias, and for foetal MR necropsy.
REFERENCES
- 1.Eley KA, McIntyre AG, Watt-Smith SR, Golding SJ. “Black bone” MRI: a partial flip angle technique for radiation reduction in craniofacial imaging. Br J Radiol 2012; 85: 272–8. doi: 10.1259/bjr/95110289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eley KA, Watt-Smith SR, Golding SJ. “Black bone” MRI: a potential alternative to CT when imaging the head and neck: report of eight clinical cases and review of the Oxford experience. Br J Radiol 2012; 85: 1457–64. doi: 10.1259/bjr/16830245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nievelstein RA, van Dam IM, van der Molen AJ. Multidetector CT in children: current concepts and dose reduction strategies. Pediatr Radiol 2010; 40: 1324–44. doi: 10.1007/s00247-010-1714-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golding SJ. Radiation exposure in CT: what is the professionally responsible approach? Radiology 2010; 255: 683–6. doi: 10.1148/radiol.10100449 [DOI] [PubMed] [Google Scholar]
- 5.Golding SJ. Multi-slice computed tomography (MSCT): the dose challenge of the new revolution. Radiat Prot Dosimetry 2005; 114: 303–7. [DOI] [PubMed] [Google Scholar]
- 6.Ulla M, Aiello H, Cobos MP, Orioli I, García-Mónaco R, Etchegaray A, et al. Prenatal diagnosis of skeletal dysplasias: contribution of three-dimensional computed tomography. Fetal Diagn Ther 2011; 29: 238–47. doi: 10.1159/000322212 [DOI] [PubMed] [Google Scholar]
- 7.Cassart M, Massez A, Cos T, Tecco L, Thomas D, Van Regemorter N, et al. Contribution of three-dimensional computed tomography in the assessment of fetal skeletal dysplasia. Ultrasound Obstet Gynecol 2007; 29: 537–43. [DOI] [PubMed] [Google Scholar]
- 8.Neumann K, Moegelin A, Temminghoff M, Radlanski RJ, Langford A, Unger M, et al. 3D-computed tomography: a new method for the evaluation of fetal cranial morphology. J Craniofac Genet Dev Biol 1997; 17: 9–22. [PubMed] [Google Scholar]
- 9.Miglioretti DL, Johnson E, Williams A, Greenlee RT, Weinmann S, Solberg LI, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr 2013; 167: 700–7. doi: 10.1001/jamapediatrics.2013.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemec U, Nemec SF, Weber M, Brugger PC, Kasprian G, Bettelheim D, et al. Human long bone development in vivo: analysis of the distal femoral epimetaphysis on MR images of fetuses. Radiology 2013; 267: 570–80. doi: 10.1148/radiol.13112441 [DOI] [PubMed] [Google Scholar]
- 11.Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med 2004; 52: 612–18. [DOI] [PubMed] [Google Scholar]
- 12.Widjaja E, Whitby EH, Cohen M, Paley MN, Griffiths PD. Post-mortem MRI of the foetal spine and spinal cord. Clin Radiol 2006; 61: 679–85. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MC, Paley MN, Griffiths PD, Whitby EH. Less invasive autopsy: benefits and limitations of the use of magnetic resonance imaging in the perinatal postmortem. Pediatr Dev Pathol 2008; 11: 1–9. doi: 10.2350/07-01-0213.1 [DOI] [PubMed] [Google Scholar]
- 14.Widjaja E, Whitby EH, Paley MN, Griffiths PD. Normal fetal lumbar spine on postmortem MR imaging. AJNR Am J Neuroradiol 2006; 27: 553–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Whitby EH, Paley MN, Cohen M, Griffiths PD. Post-mortem fetal MRI: what do we learn from it? Eur J Radiol 2006; 57: 250–5. [DOI] [PubMed] [Google Scholar]