Abstract
Topotecan is the most reliable chemotherapy regimen for relapsed small-cell lung carcinoma (SCLC). The efficacy and adverse effects of topotecan as reported by previous studies varied greatly. The inclusion criterion was a prospective study that was able to provide data for 6-month over-all survival (OS) rate, 1-year OS rate, objective responses, and/or adverse effects of single agent topotecan as a second line chemotherapy for SCLC, written in English language as a full article. Any topotecan regimen were allowed. Binary data were meta-analyzed with the random-model generic inverse variance method. We included 14 articles consisted of 1347 patients. Pooled values were estimated as follows. <Refractory relapse> Six-month OS rate: 37% (95% CI: 28–46%). One-year OS rate: 9% (95% CI: 5–13%). Response rate: 5% (95% CI: 1–8%). <Sensitive relapse> Six-month OS rate: 57% (95% CI: 50–64%). One-year OS rate: 27% (95% CI: 22–32%). Response rate: 17% (95% CI: 11–23%). <Adverse effect> Grade III/IV neutropenia 69% (95% CI: 58–80%). Grade III/IV thrombopenia 41% (95% CI: 34–48%). Grade III/IV anemia 24% (95% CI: 17–30%). Non-hematorogical events were rare. Chemotherapy-related death 2% (95% CI: 1–3%). In conclusion, Topotecan provided a possibly promising outcome for sensitive-relapse SCLC and poor outcome for refractory relapse SCLC. Adverse events were mainly hematological.
Lung cancer is the leading cause of cancer-related mortality in the world1. Patients with small-cell lung cancer (SCLC) almost always have a history of smoking and are generally very chemotherapy sensitive. Good sensitivity to chemotherapy and radiotherapy are features of SCLC. However, most patients who have initially responded to chemotherapy and radiotherapy eventually experience recurrence of the cancer in a few months2,3,4,5. Traditionally, the relapse of SCLC from the first line chemotherapy has been divided into two categories: refractory relapse, which occurs within a 60–90-day treatment-free interval (TFI) after the first line chemotherapy, and sensitive relapse, which occurs after at least 60–90 days of TFI6. Although the best management of recurrent SCLC is far from clear, most physicians feel that topoisomerase I inhibitor topotecan (TOP), which is sometimes referred to as nogitecan, is the most reliable chemotherapy regimens at least for sensitive relapse, because these medications have been supported by numerous clinical trials7,8,9,10,11,12,13,14,15,16,17,18,19,20. At present, TOP is the only anti-cancer drug whose efficacy for relapsed SCLC has been proved in a randomized controlled trial (RCT) that used the best supportive care arm as a comparator13. Some RCTs compared the efficacy of TOP for relapsed SCLC and that of other regimens, namely amurubicin11,12,17. However, no medication has been clearly demonstrated to be superior to TOP.
The efficacy and adverse effects (AE) of TOP are of considerable interest for all physicians who take care of patients with SCLC. Nonetheless, the efficacy and AE of TOP as reported by previous studies have seemed to vary greatly. Therefore, we tried to perform a systematic review and a meta-analysis to provide data about survival, objective response, and AEs of TOP when prescribed as the second-line chemotherapy for patients with SCLC.
Methods
Institutional review board approval and patient consent were not required because of the review nature of this study.
Study search
Systematic searching was conducted to find eligible articles. The inclusion criterion for a study to be included in the current meta-analysis was a prospective study that was able to provide data for the 6-month over-all survival (OS) rate, the 1-year OS rate, objective responses, and/or AEs of single agent TOP as second line chemotherapy for SCLC, written in English language as a full article. Any TOP regimen prescribed for both intravenous and oral administration was allowed. Conference abstracts and duplicate use of the same data were excluded. Two investigators independently searched for eligible articles using the PubMed, Web of Science, and Cochrane databases as of February, 2015. The following search formula was used for PubMed: (“small-cell lung cancer” OR “small-cell lung carcinoma” OR “SCLC”) AND (relapsed OR second-line OR 2nd-line OR “second line” OR “previously treated”) AND (nogitecan OR hycamtin OR topotecan OR NGT).
Outcome
Survival was evaluated as 6-month OS rate and 1-year OS rate. If 6-month and/or 1-year survival rate was not directly provided in the article, it was estimated from the survival curve using Parmar’s method21.
For response analysis, response rate (RR), disease control ratio (DCR), complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), not assessable (NA) were evaluated. Both SD and no change were merged as SD. NA, non-evaluable, and early death before response assessment were counted as NA. RR included CR and PR. DCR included CR, PR, and SD. For response assessment, the total number of patients evaluated was equal to the numbers of patients with CR, PR, SD, PD, and NA.
Grade III and IV hematological toxicity including neutropenia, thrombopenia, and anemia; and grade III and IV non-hematological toxicity including fatigue, asthenia, nausea/vomiting, diarrhea, anorexia, dyspnea, and fever were assessed for AE assessment. Febrile neutropenia of any grade was counted. Death that was clearly documented as due to AE was counted. Other minor non-hematological AEs were sometimes mentioned in articles; however they were not assessed for the current systematic review. This was because very rare AEs were usually not mentioned in most articles, thus, including these very rare AEs might have resulted in publication bias. AEs were analyzed based on the number of patients, not on the number of chemotherapy courses.
Survival analysis and response analysis were conducted for patients with sensitive relapse and refractory relapse separately6. AE analysis was conducted for both relapses collectively.
Statistics
Binary data were meta-analyzed with the random-model generic inverse variance method after the standard error was estimated using the Wilson score interval22,23,24. The heterogeneity evaluated with the I2 statistics was interpreted as follows: I2 = 0% indicates no heterogeneity, 0% < I2 < 25% indicates the least heterogeneity, 25% ≤ I2 < 50% indicates mild heterogeneity, 50% ≤ I2 < 75% indicates moderate heterogeneity, and 75% ≤ I2 indicates strong heterogeneity25.
For a subgroup analysis, we divided TOP regimens into three groups: intravenous days 1–5 administration, intravenous weekly administration, and oral administration. Subgroup analyses were not performed for outcomes for refractory relapse because these analyses did not include sufficient numbers of original cohorts.
All analysis was performed in Review Manager ver 5.3 (Cochrane Collaboration, Oxford, UK). Figures illustrated using Review Manager were adjusted as necessary.
Results
Study search
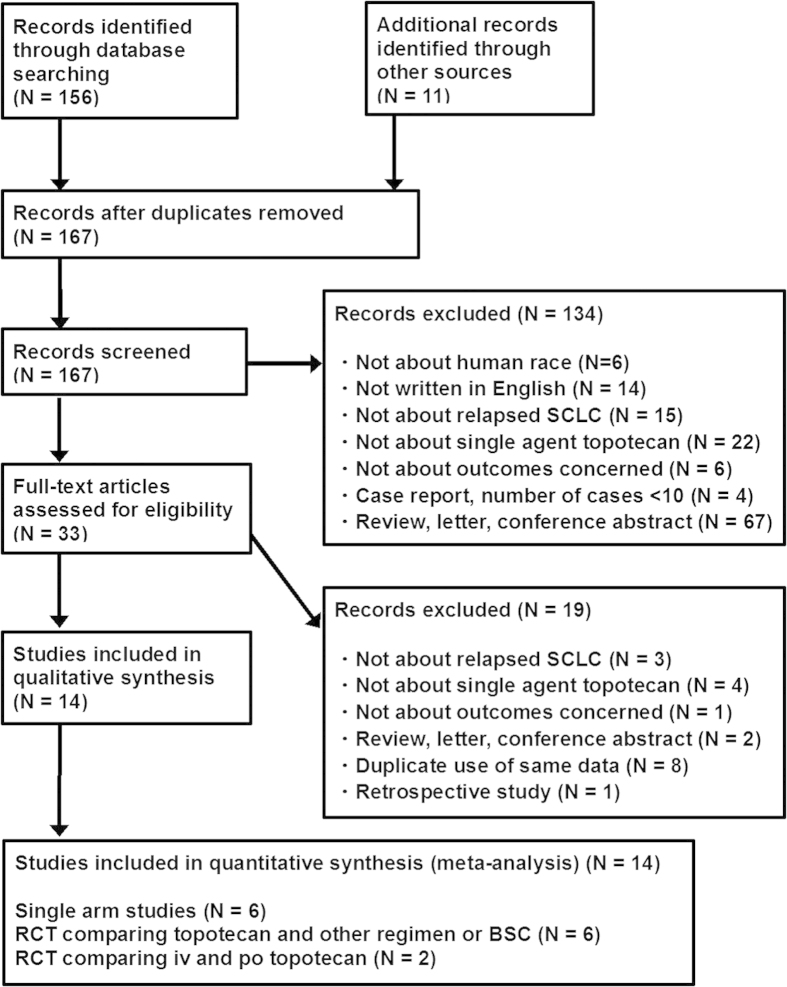
Of 167 articles that met the preliminary criteria, 134 and 19 were excluded through title/abstract screening and full article scrutinizing, respectively. We found 14 eligible articles, which included six single arm studies, two RCTs that compared intravenous and oral TOP, and six RCTs that compared TOP and other regimens (Fig. 1, Table 1)7,8,9,10,11,12,13,14,15,16,17,18,19,20.
Figure 1. Flow chart for study search (PRISMA diagram).
Table 1. Summary of included studies.
| Study | Design | Sub-group | N | PS(%) 0/1/2 | Age | men% | TOP regimen |
|---|---|---|---|---|---|---|---|
| Allen 20147 | RCT, iv-TOP vs iv-TOP+ZA, P2 | S | 41 | 41/59/0 | 60 | 32% | iv 4 mg/m2, d 1/8/15 every 3 w |
| R | 51 | 37/63/0 | 64 | 67% | iv 4 mg/m2, d 1/8/15 every 3 w | ||
| Ardizzoni 19978 | Single arm, iv-TOP | S | 46 | 33/52/15 | 58 | 74% | iv 1.5 mg/m2, d 1–5 every 3 w |
| R | 47 | 17/62/21 | 58 | 64% | iv 1.5 mg/m2, d 1–5 every 3 w | ||
| Eckardt 20079 | RCT, oral-TOP vs iv-TOP, P3 | oral | 155 | 31/56/13 | 63 | 64% | oral 2.3 mg/m2, d 1–5 every 3 w |
| iv | 154 | 23/65/12 | 62 | 64% | iv 1.5 mg/m2, d 1–5 every 3 w | ||
| Huber 200610 | Single arm, iv-TOP | 170 | 21/56/23 | 61 | 78% | iv 1.25 mg/m2, d 1–5 every 3 w | |
| Inoue 200811 | RCT, iv-TOP vs AMR, P2 | 30 | 57/30/13 | 64 | 83% | iv 1.0 mg/m2, d 1–5 every 3 w | |
| Jotte 201112 | RCT, iv-TOP vs AMR, P2 | 26 | 39/54/8 | 68 | 42% | iv 1.5 mg/m2, d 1–5 every 3 w | |
| O’Brien 200613 | RCT, oral-TOP vs BSC, P3 | 71 | 11/62/27 | 60 | 73% | oral 2.3 mg/m2, d 1–5 every 3 w | |
| Park 200814 | Single arm, iv-TOP | 17 | 6/77/18 | 68 | 94% | iv 1.5 mg/m2, d 1–5 every 3 w | |
| Pawel 199915 | RCT, iv-TOP vs CAV | 107 | 17/60/23 | NA | 57% | iv 1.5 mg/m2, d 1–5 every 3 w | |
| Pawel 200116 | RCT, oral-TOP vs iv-TOP, P2 | oral | 52 | 19/65/15 | 60 | 75% | oral 2.3 mg/m2, d 1–5 every 3 w |
| iv | 54 | 33/39/28 | 58 | 80% | iv 1.5 mg/m2, d 1–5 every 3 w | ||
| Pawel 201417 | RCT, iv-TOP vs AMR, P3 | 213 | 34/64/2 | 61 | 60% | iv 1.5 mg/m2, d 1–5 every 3 w | |
| Shah 200718 | Single arm, iv-TOP, P2 | 22 | 18/73/9 | 63 | 55% | iv 4 mg/m2, d 1/8/15 every 4 w | |
| Spigel 201119 | Single arm, iv-TOP, P2 | 38 | 26/74/0 | 64 | 53% | iv 6 mg/m2, d 1/8/15/22/29/36 every 8 w | |
| Takeda 200320 | Single arm, iv-TOP, P2 | 53 | 22/60/18 | 64 | 68% | iv 1.0 mg/m2, d 1–5 every 3 w |
S: sensitive relapse. R: refractory relapse. RCT: randomized controlled trial. TOP: topotecan. iv: intravenous. ZA: ziv-aflibercept. AMR: amurubicin. BSC: best supportive care. P2/3: phase 2/3. N: number of patients. PS: performance status. Age: mean or median age was presented. d: day. w: week.
The number of patients in a study who were treated with TOP ranged from 17 to 309. The total number of patients in all the studies was 1347. In most of the studies, patients with performance status of 1 and men were the majority. Mean or median age presented for each study ranged from 58 to 68 years. Two studies did not mention types of relapse. The cutoff between refractory and sensitive relapse was 90 days except for one study that used 60 days. Intravenous TOP 1.5 mg/m2 on days 1–5 every 3 weeks was the most preferred regimen. Two studies from Japan used intravenous TOP in a low dose of 1.0 mg/m2 on day 1–5 every 3 weeks. Three studies used weekly intravenous TOP in higher doses of 4.0 or 6.0 mg/m2. Three studies used oral TOP 2.3 mg/m2 on days 1–5 every 3 weeks (Table 1).
Refractory relapse
Pooled 6-month and 1-year OS rates estimated from four cohorts were 37% (95% CI 28–46%. I2 = 46%, p for heterogeneity = 0.14) and 9% (95% CI 5–13%. I2 = 0%, p for heterogeneity = 0.94), respectively (Fig. 2).
Figure 2. Meta-analysis for refractory relapse.
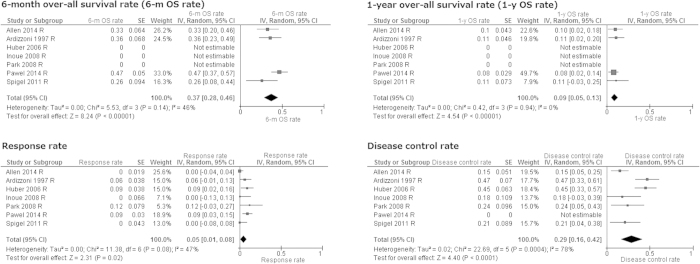
SE: standard error. IV: inverse variance method. 95% CI: 95% confidence interval.
Concerning objective response, random-model meta-analysis using the generic inverse variance method suggested the following pooled values: RR 5% (95% CI 1–8%. I2 = 47%, p for heterogeneity = 0.08), DCR 29% (95% CI 16–42%. I2 = 78%, p for heterogeneity < 0.001), CR 0% (95% CI 0–2%. I2 = 0%, p for heterogeneity = 0.98), PR 3% (95% CI 0–7%. I2 = 40%, p for heterogeneity = 0.16), SD 25% (95% CI 14–36%. I2 = 73%, P for heterogeneity = 0.005%), PD 59% (95% CI 47–70%. I2 = 65%, p for heterogeneity = 0.02), NA 9% (95% CI 0–17%. I2 = 80%, p for heterogeneity < 0.001) (Fig. 2).
Sensitive relapse
The pooled 6-month and 1-year OS rates estimated from 11 cohorts were 57% (95% CI 50–64%. I2 = 77%, p for heterogeneity < 0.001) and 27% (95% CI 22–32%. I2 = 64%, p for heterogeneity = 0.002), respectively (Fig. 3).
Figure 3. Meta-analysis for sensitive relapse.
SE: standard error. IV: inverse variance method. 95% CI: 95% confidence interval.
Random-model meta-analysis using the generic inverse variance method suggested the following pooled values for sensitive relapse: RR 17% (95% CI 11–23%. I2 = 86%, p for heterogeneity < 0.001), DCR 42% (95% CI 35–49%. I2 = 77%, p for heterogeneity < 0.001), CR 1% (95% CI 0–1%. I2 = 1%, p for heterogeneity = 0.43), PR 14% (95% CI 8–21%. I2 = 85%, p for heterogeneity < 0.001), SD 22% (95% CI 19–24%. I2 = 0%, p for heterogeneity = 0.57), PD 48% (95% CI 40–56%. I2 = 82%, p for heterogeneity < 0.001), NA 11% (95% CI 6–16%. I2 = 85%, p for heterogeneity < 0.001) (Fig. 3).
We performed subgroup analysis dividing TOP regimen into three groups. RR of 2% (95% CI: 0–8%), and DCR of 24% (95% CI 11–37%) by intravenous weekly administration were poorer than those by other regimens with significant subgroup differences. Test for subgroup differences were following: RR, I2 = 93.1%, P < 0.001; DCR, I2 = 82.2%, P < 0.001 (Fig. 3). Nonetheless, 6-month OS rate (I2 = 0%, p for subgroup heterogeneity = 0.50), and 1-year OS rate (I2 = 0%, p for subgroup heterogeneity = 0.39) were not largely different among three TOP regimens (Fig. 3).
Adverse effects
Throughout the included articles, hematological AEs were more commonly observed than non-hematological AEs. The pooled grade III/IV incidence of neutropenia, thrombopenia, and anemia were 69% (95% CI 58–80%. I2 = 95%, p for heterogeneity < 0.001), 41% (95% CI 34–48%. I2 = 84%, p for heterogeneity < 0.001), and 24% (95% CI 17–30%. I2 = 88%, p for heterogeneity < 0.001), respectively. Febrile neutropenia was observed with a pooled incidence of 4% (95% CI 2–5%. I2 = 0%, p for heterogeneity = 0.88) (Fig. 4).
Figure 4. Meta-analysis for adverse effects.
SE: standard error. IV: inverse variance method. 95% CI: 95% confidence interval.
Subgroup analysis suggested that weekly regimen had lower frequency for hematological AEs with substantial subgroup heterogeneity. When weekly regimen was selected, pooled incidences were 38% (95% CI 11–66%) for Grade III/IV neutropenia, 21% (95% CI 8–35%) for Grade III/IV thrombopenia, and 6% (95% CI 0–12%) for Grade III/IV anemia (Fig. 4).
The pooled incidences of non-hematological AEs were as follows: fatigue 6% (95% CI 3–9%. I2 = 76%, p for heterogeneity < 0.001), asthenia 3% (95% CI 0–6%. I2 = 66%, p for heterogeneity = 0.02), nausea/vomiting 2% (95% CI 1–3%. I2 = 0%, p for heterogeneity = 0.66), diarrhea 2% (95% CI 0–4%. I2 = 33%, p for heterogeneity = 0.14), anorexia 3% (95% CI 1–5%. I2 = 40%, p for heterogeneity = 0.14), dyspnea 5% (95% CI 2–8%. I2 = 60%, p for heterogeneity = 0.01), fever 2% (95% CI 1–4%. I2 = 33%, p for heterogeneity = 0.16) (Fig. 4).
Chemotherapy-related death was observed with a pooled incidence of 2% (95% CI 1–3%. I2 = 13%, p for heterogeneity = 0.31) (Fig. 4).
Discussion
TOP for patients with sensitive relapse SCLC could provide RR for 17% of cases, and disease control for 42% of cases, which resulted in 57% of 6-month OS rate and 27% of 1-year OS rate (Fig. 3). However, this regimen had poorer outcomes for patients with refractory relapse SCLC (Fig. 2). Hematologic AEs, especially grade III/IV neutropenia were more commonly observed than non-hematological AEs. Approximately 2% of patients died from chemotherapy (Fig. 4).
In addition to the most commonly used regimen of intravenous 1.5 mg/m2 on day 1–5 every three weeks, high dose weekly intravenous TOP regimens and oral TOP 2.3 mg/m2 on day 1–5 every three weeks have been often selected. RCTs by Eckardt et al. in 2007 and by Pawel et al. in 2001 suggested that oral and intravenous TOP on day1–5 every three weeks have similar efficacy and AE profiles9,16. Thus, both regimens could be appropriate choices of treatment for sensitive relapse SCLC (Fig. 3). Oral TOP could provide a convenient administration route with a similar AE profile to intravenous TOP (Fig. 4). Weekly intravenous TOP has been found to be a treatment with good tolerability7,18,19. In our analysis, frequency of hematological AEs by weekly regimen were less than that by other regimens (Fig. 4). Nonetheless, RR of 2% and DCR of 24% for sensitive relapse by intravenous weekly administration may be disappointing (Fig. 3)18,19. Considering 6-month and 1-year OS rates by weekly regimen were not inferior to those by intravenous day 1–5 regimen and oral regimens, weekly regimen could be a choice for patients who have high risk for myelosuppression.
There is still little evidence to guide the third-line chemotherapy. Nonetheless, to improve OS time, a physician provide the third-line chemotherapy for a part of patients whose disease relapsed after the second-line chemotherapy26,27. Here, a clinician faces a challenge how to select a SCLC case who will gain benefit from further treatment. Retrospective chart reviews suggested that patients with normal or low lactase dehydrogenase level, good response to second-line chemotherapy, high body mass index, higher level of hemoglobin, long time to progression after the second-line chemotherapy indicated better response to the third-line chemotherapy26,27.
In 2014, two reports that were potentially conflicting with each other were published. Lara et al. analyzed 329 patients with extensive-stage SCLC who progressed after platinum-based chemotherapy28. This study indicated that platinum-sensitivity status may no longer be strongly associated with progression-free survival and OS. On the other hand, Ardizzoni et al. reported a retrospective study with 631 relapsed SCLC cases6. This concluded that the separation of relapsed SCLC into two types of relapses based on a TFI cut-off of 60 days was valid and could be a standard of care. The current analysis, though mostly using a TFI cut-off of 90 days, also demonstrated that all of 6-month OS rate, 1-year OS rate, RR and DCR were more favorable for patients with sensitive relapse than those with refractory relapse.
Few limitations of the current study should be mentioned. First, the meta-analysis was performed as aggregated data meta-analysis. If we could have obtained individual data from all the original studies, individual patient data meta-analysis would have been preferred. Second, regimen comparison was not done by head-to-head manner. Third, strong heterogeneities for some outcomes made it difficult to interpret results. However, we believe the current meta-analysis provided useful information for clinicians.
In conclusion, TOP provided a possibly promising outcome for patients with sensitive-relapse SCLC and poor outcome for patients with a refractory relapse SCLC. Adverse events were mainly hematological. We believe these data will be informative for physicians who take care of patients with relapsed SCLC.
Additional Information
How to cite this article: Horita, N. et al. Topotecan for Relapsed Small-cell Lung Cancer: Systematic Review and Meta-Analysis of 1347 Patients. Sci. Rep. 5, 15437; doi: 10.1038/srep15437 (2015).
Footnotes
Author Contributions N.H. served for study design, study search, analysis, and drafting as a principal investigator. M.I. worked for study search. M.Y., T.S., T.T., H.N., K.T., Y.S., H.W., K.Nagai, K.Nakashima, R.U., M.S. and M.K. worked for interpretation of data and revising the draft. T.K. contributed for general management. All authors reviewed and finally approved the manuscript.
References
- Alberg A. J. & Nonemaker J. Who is at high risk for lung cancer? Population-level and individual-level perspectives. Semin Respir Crit Care Med. 29, 223–32 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett J. R., Schild S. E., Kesler K. A. & Kalemkerian G. P. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 143, e400S–19S (2013). [DOI] [PubMed] [Google Scholar]
- Asai N., Ohkuni Y., Kaneko N., Yamaguchi E. & Kubo A. Relapsed small cell lung cancer: treatment options and latest developments. Ther. Adv. Med. Oncol. 6, 69–82 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. H. & Mishima M. Second-line chemotherapy for small-cell lung cancer (SCLC). Cancer Treat. Rev. 37, 143–50 (2011). [DOI] [PubMed] [Google Scholar]
- Hartwell D. et al. Topotecan for relapsed small cell lung cancer: a systematic review and economic evaluation. Cancer Treat. Rev. 37, 242–9 (2011). [DOI] [PubMed] [Google Scholar]
- Ardizzoni A., Tiseo M. & Boni L. Validation of standard definition of sensitive versus refractory relapsed small cell lung cancer: a pooled analysis of topotecan second-line trials. Eur. J. Cancer. 50, 2211–8 (2014). [DOI] [PubMed] [Google Scholar]
- Allen J. W. et al. A randomized, phase II trial of weekly topotecan with and without ziv-aflibercept in patients with platinum-treated small-cell lung cancer. J. Clin. Oncol. 32, 2463–70 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardizzoni A. et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J. Clin. Oncol. 15, 2090–6 (1997). [DOI] [PubMed] [Google Scholar]
- Eckardt J. R. et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J. Clin. Oncol. 25, 2086–92 (2007). [DOI] [PubMed] [Google Scholar]
- Huber R. M. et al. Efficacy of a toxicity-adjusted topotecan therapy in recurrent small cell lung cancer. Eur. Respir. J. 27, 1183–9 (2006). [DOI] [PubMed] [Google Scholar]
- Inoue A. et al. Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J. Clin. Oncol. 26, 5401–6 (2008). [DOI] [PubMed] [Google Scholar]
- Jotte R. et al. Randomized phase II trial of single-agent amrubicin or topotecan as second-line treatment in patients with small-cell lung cancer sensitive to first-line platinum-based chemotherapy. J. Clin. Oncol. 29, 287–93 (2011). [DOI] [PubMed] [Google Scholar]
- O’Brien M. E. et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J. Clin. Oncol. 24, 5441–7 (2006). [DOI] [PubMed] [Google Scholar]
- Park S. H. et al. Salvage treatment with topotecan in patients with irinotecan-refractory small cell lung cancer. Cancer Chemother. Pharmacol. 62, 1009–14 (2008). [DOI] [PubMed] [Google Scholar]
- von Pawel J. et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J. Clin. Oncol. 17, 658–67 (1999). [DOI] [PubMed] [Google Scholar]
- von Pawel J. et al. Phase ii comparator study of oral versus intravenous topotecan in patients with chemosensitive small-cell lung cancer. J. Clin. Oncol. 19, 1743–9 (2001). [DOI] [PubMed] [Google Scholar]
- von Pawel J. et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J. Clin. Oncol. 32, 4012–9 (2014). [DOI] [PubMed] [Google Scholar]
- Shah C. et al. A multi-center phase II study of weekly topotecan as second-line therapy for small cell lung cancer. Lung Cancer. 57, 84–8 (2007). [DOI] [PubMed] [Google Scholar]
- Spigel D. R. et al. A phase II study of higher dose weekly topotecan in relapsed small-cell lung cancer. Clin. Lung Cancer. 12, 187–91(2011). [DOI] [PubMed] [Google Scholar]
- Takeda K. et al. A phase II study of topotecan in patients with relapsed small-cell lung cancer. Clin. Lung Cancer. 4, 224–8 (2003). [DOI] [PubMed] [Google Scholar]
- Parmar M. K., Torri V. & Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 17, 2815–2834 (1998). [DOI] [PubMed] [Google Scholar]
- Higgins P. J. & Green S. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). (2011). Available from: http://handbook.cochrane.org/front_page.htm. Accessed on Feburary 1st, 2015.
- Zang J., Xiang C. & He J. Synthesis of median survival time in meta-analysis. Epidemiology. 24, 337–8 (2013). [DOI] [PubMed] [Google Scholar]
- Anonymous Wilson E. B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 22, 209–212 (1927). [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ. 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata M. et al. Outcome and prognostic factors in patients with small cell lung cancer who receive third-line chemotherapy. Tumori. 100, 507–11 (2014). [DOI] [PubMed] [Google Scholar]
- Simons D. et al. Third-line chemotherapy in small-cell lung cancer: an international analysis. Clin Lung Cancer. 15, 110–8 (2014). [DOI] [PubMed] [Google Scholar]
- Lara P. N. Jr. et al. Relevance of platinum (plat) sensitivity status in previously treated extensive-stage small cell lung cancer in the modern era: A patient level analysis of SWOG trials. J. Clin. Oncol. 10, 110–115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]