Abstract
Although there is evidence that non-steroidal anti-inflammatory drugs (NSAIDs) might be able to prevent pancreatic cancer, the findings from epidemiological studies have been inconsistent. In this paper, we conducted a meta-analysis of observational studies to examine this possibility. We searched PubMed and Embase for observational (cohort or case-control) studies examining the consumption of aspirin and other NSAIDs and the incidence of or mortality rates associated with pancreatic cancer. Twelve studies including approximately 258,000 participants in total were analysed. The administration of aspirin significantly reduced the incidence of pancreatic cancer (8 studies; odds ratio (OR) = 0.77; 95% confidence interval (CI) = 0.62 to 0.96; I2 = 74.2%) but not the mortality associated with it (2 studies; OR = 0.94; 95% CI = 0.73 to 1.22). Specifically, frequent aspirin use was associated with reduced pancreatic cancer incidence (OR = 0.57; 95% CI = 0.39 to 0.83 for high frequency; OR = 0.57; 95% CI = 0.38 to 0.84 for medium frequency). The summary ORs regarding the incidence of pancreatic cancer and either non-aspirin NSAIDs use (OR = 1.08; 95% CI = 0.90 to 1.31) or overall NSAIDs use (OR = 0.97; 95% CI = 0.86 to 1.10) were not significant. In conclusion, aspirin use might reduce the incidence of pancreatic cancer; however, this finding should be interpreted with caution because of study heterogeneity.
Pancreatic cancer is the fourth leading cause of cancer-related death and one of the ten most common types of malignancies in the world1. In the United States, approximately 45,000 new cases of pancreatic cancer are diagnosed and 37,000 deaths occur each year; the survival rate is less than 1% 5 years after diagnosis1. Only approximately 10% of patients with pancreatic cancer are eligible for surgical resection, and the results of medical therapies remain unsatisfactory2. Therefore, an urgent need exists for a better understanding of the factors that are related to pancreatic cancer development and prognosis. Moreover, the identification of potential chemopreventive strategies for pancreatic cancer is highly desirable.
Cyclooxygenase-2 (COX-2) might play a role in cancer development3,4. Its expression is elevated in pancreatic carcinoma tissue compared with healthy pancreatic tissue5,6. Therefore, non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit the COX-2 pathway, might hold promise for the chemoprevention and treatment of pancreatic cancer. In addition, use of NSAIDs (particularly aspirin) reduces the risk of several cancers, including colorectal7, breast8, gastric and esophageal9,10, lung11, and prostate cancers12. However, the findings from observational epidemiological studies regarding the relationship between aspirin/NSAIDs use and pancreatic cancer risk have been inconsistent13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28. Three meta-analyses have been published on this issue, each with different results. In 2006, Larsson et al.29 carried out a meta-analysis of 11 studies (one randomized controlled trial (RCT), three case-controls, and seven cohorts) and did not find associations between the risk of pancreatic cancer and aspirin, non-aspirin NSAIDs, or NSAIDs use. However, this evidence is not conclusive because the incidence and mortality of cancer were pooled to represent the overall risk of cancer. This strategy might obscure true decreases or increases in the incidence or mortality risk of pancreatic cancer associated with aspirin/NSAIDs use. Another meta-analysis30 that included 8 studies (three case-controls, four cohorts, and one RCT) did not show any association between aspirin/NSAIDs use and pancreatic cancer risk in low, intermediate, or high exposure groups. However, this evidence is limited because the aspirin and NSAIDs groups were pooled for the analysis, which might have masked the individual effects of aspirin versus other NSAIDs on the incidence of pancreatic cancer. More recently, Cui et al.31 conducted a meta-analysis that included 10 studies (four case-controls, five cohorts, and one RCT) and concluded that high-dose aspirin use reduces pancreatic cancer risk. However, this conclusion was not supported because the odds ratio (OR) of the high-dose aspirin group was not significant (odds ratio (OR) = 0.88; 95% confidence intervals (CI) = 0.76 to 1.01; P = 0.069)31.
Thus, these previous meta-analyses must be updated to revise the evidence concerning this issue. Moreover, the frequency and duration risks of aspirin use in real-world practice have not been systematically evaluated. Therefore, based on recent evidence, we performed a systematic review and meta-analysis of 12 observational studies to explore the possibility that NSAIDs use reduces the incidence of pancreatic cancer in real-world settings.
Results
Study selection and characteristics
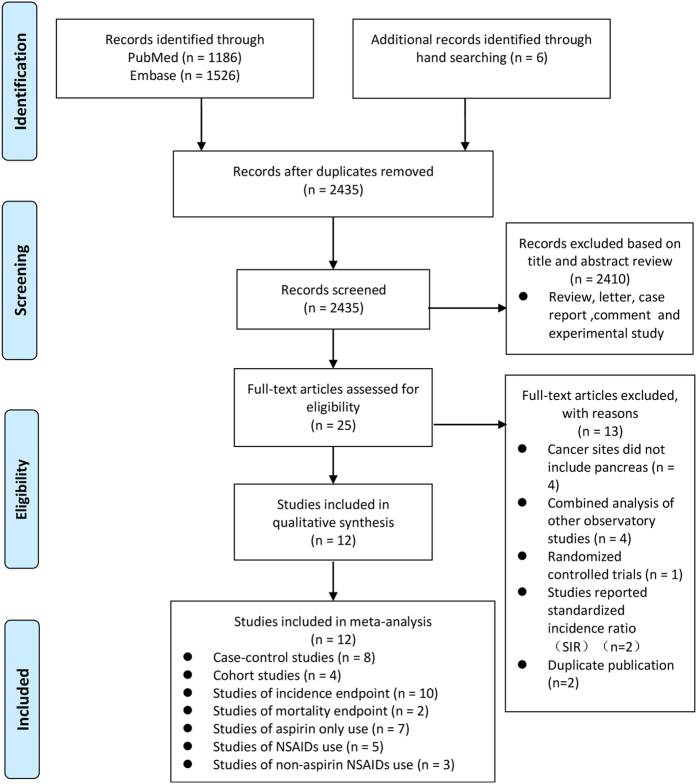
Our initial search yielded 2,718 potentially relevant publications, of which 283 duplicates were excluded. We then excluded 2,410 studies that were deemed irrelevant to the meta-analysis based on their titles and abstracts. After reviewing the full texts of the remaining 25 studies, we identified 12 studies for inclusion in the meta-analysis (for details see Fig. 1)14,16,18,19,20,21,22,23,24,25,26,27. Among reasons for excluding the 13 studies, specifically, we did not include Sørensen et al.28 or Friis et al.13 because these studies reported the standard incidence ratio (SIR) as effect size without adjustment. In addition, we excluded Schreinemachers et al.15 because its cohort was also included in Ratnasinghe et al.16. Moreover, Jacobs and colleagues conducted two cohort studies17,23 based on the same sample to investigate the effects of aspirin use on pancreatic cancer mortality; therefore, we excluded the study that included fewer cases and a briefer follow-up period17.
Figure 1. Article selection flow chart.
The main characteristics of the 12 included studies (8 case-control18,19,20,22,24,25,26,27 and 4 cohort studies14,16,21,23) are shown in Table 1. Approximately 258,000 participants were included in the current meta-analysis. Five studies (4 case-controls20,24,25,26 and 1 cohort21) evaluated overall NSAIDs use, 8 studies (6 case-controls18,19,20,22,25,27 and 2 cohorts14,21) addressed aspirin use, and 3 studies (1 case-control19 and 2 cohorts14,21) measured non-aspirin NSAIDs use with regard to the incidence of pancreatic cancer. Two cohort studies16,23 investigated the association between aspirin use and cancer-specific mortality. The years of publication of the included studies ranged from 2000 to 2014. Of the 12 included studies, 8 were conducted in the United States14,16,19,21,22,23,26,27, and 4 were completed in Europe18,20,24,25. In each study, the participants were either matched or the methodology was adjusted for a wide range of potential confounds. The average Newcastle-Ottawa Scales (NOS) quality score of the observational studies was 7.2 (range = 6 to 9). Greater detail is provided in Supplementary Table S1.
Table 1. Main characteristics included in the meta-analysis.
| First Author [Country] [Ref.]/Year | Design | Study Period | Follow Up (year) | Cases | Controls or Cohort Size | Exposure | Definition of NSAID Use | Strength of Association (95% CI) | Confounders For Adjustment | Outcome | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Streicher et al.[US]/201422 | Case-control | 2005–2009 | NA | 362 | 690 | aspirin | never used | OR, 1 | age, sex, race, smoking status, BMI, diabetes, blood type, education | Incidence | 8a |
| regularly used | OR, 0.52(0.39–0.69) | ||||||||||
| low-dose (75–325 mg per day) aspirin | OR, 0.94(0.91–0.98) | ||||||||||
| regular-dose (325–1200 mg every 4 to 6 hours) aspirin | OR, 0.98(0.96–1.01) | ||||||||||
| aspirin ≤ 6 y | OR, 0.50(0.36–0.70) | ||||||||||
| aspirin >10 y | OR, 0.61(0.37–1.00) | ||||||||||
| Jacobs et al.[US]/201223 | Cohort | 1992–2008 | 17 | 115 | 100139 | aspirin | never used | RR, 1 | age, sex, race, smoking status, BMI, heart disease, stroke, diabetes, hypertension, cholesterol-lowering drug use, aspirin use, NSAID use, history of colorectal endoscopy, physical activity level, education | Mortality | 8a |
| updated analyses for current daily use | RR, 0.95(0.72–1.25) | ||||||||||
| updated analyses for aspirin use <5 y | RR, 0.89(0.64–1.23) | ||||||||||
| updated analyses for aspirin use ≥5 y | RR, 1.03(0.73–1.46) | ||||||||||
| Tan et al.[US]/201119 | Case-control | 2004–2010 | NA | 740 | 1043 | aspirin/non-aspirin NSAIDs | aspirin never used (<1day/month) | OR, 1 | age, sex, smoking status, BMI, diabetes | Incidence | 6a |
| aspirin ever used (≥1day/month) | OR, 0.74(0.60–0.91) | ||||||||||
| aspirin frequency of use 2–5 days/week | OR, 0.61(0.38–0.96) | ||||||||||
| aspirin frequency of use 6+ days/week | OR, 0.63(0.47–0.85) | ||||||||||
| aspirin dosage of 1–2 tablets/day | OR, 0.81(0.63–1.03) | ||||||||||
| aspirin dosage of 3+ tablets/day | OR, 0.72(0.50–1.04) | ||||||||||
| non-aspirin NSAIDs used (≥1 day/month) | OR, 1.01(0.79–1.29) | ||||||||||
| Pugh et al.[UK]/201120 | Case-control | 2004–2007 | NA | 206 | 251 | aspirin/NSAIDs | never used | OR, 1 | age, sex, smoking status, diabetes | Incidence | 6a |
| aspirin use | OR, 0.49(0.29–0.84) | ||||||||||
| NSAIDs use | OR, 0.98(0.50–1.91) | ||||||||||
| Bradley et al.[UK]/201025 | Case-control | 1995–2006 | NA | 1141 | 7954 | aspirin/NSAIDs | never used | OR, 1 | smoking status, BMI, alcohol use, history of chronic pancreatitis, history of rheumatoid arthritis, use of other drugs, diabetes, prior cancer | Incidence | 7a |
| any use of an NSAID until 1 year before diagnosis | OR, 1.03(0.89–1.19) | ||||||||||
| duration of low-dose NSAIDs (<1.0 DDD per day) >5 y | OR, 0.70(0.49–0.99) | ||||||||||
| duration of high-dose NSAIDs (≥1.0 DDD per day) >5 y | OR, 0.85(0.53–1.36) | ||||||||||
| high-dose NSAIDs (1–200 DDDs per day) | OR, 0.99(0.94–1.03) | ||||||||||
| ever used for aspirin and derivatives until 1 year before diagnosis | OR, 0.95(0.81–1.12) | ||||||||||
| high-dose aspirin (≥300 mg a day) | OR, 1.10(0.81–1.50) | ||||||||||
| Bonifazi et al.[Italy]/201018 | Case-control | 1991–2008 | NA | 308 | 477 | aspirin | non-regular used (<1 day/week for more than 6 months) | OR, 1 | age, sex, smoking status, BMI, diabetes, education, study center, year of interview | Incidence | 8a |
| regular used (≥1 day/week for more than 6 months) | OR, 0.87(0.47–1.61) | ||||||||||
| duration of use <5 y | OR, 1.40(0.62–3.17) | ||||||||||
| duration of use ≥5 y | OR, 0.53(0.21–1.33) | ||||||||||
| current users ≥5 y | OR, 0.23(0.06–0.90) | ||||||||||
| Schernhammer et al.[US]/200414 | Cohort | 1980–1998 | 18 | 161 | 88378 | aspirin/non-aspirin NSAIDs | non-regular used (<2 tablets per week) | RR, 1 | age, smoking status, BMI, diabetes, non-vigorous physical activity in metabolic equivalents per week, follow-up cycle | Incidence | 7a |
| use of non-aspirin NSAIDs | RR, 1.20(0.79–1.80) | ||||||||||
| regular use (≥2 tablets per week) | RR, 1.20(0.87–1.65) | ||||||||||
| current aspirin use 1–3 tablets per week | RR, 1.26(0.85–1.85) | ||||||||||
| current aspirin use 4–6 tablets per week | RR, 1.41(0.82–2.40) | ||||||||||
| current aspirin use 7–13 tablets per week | RR, 1.65(1.05–2.59) | ||||||||||
| current aspirin use ≥14 tablets per week | RR, 0.86(0.39–1.89) | ||||||||||
| non-regular used (<5 tablets of aspirin per week) | RR, 1 | ||||||||||
| regular use, 1–5 y | RR, 1.12(0.72–1.74) | ||||||||||
| regular use, 6–10 y | RR, 1.10(0.64–1.89) | ||||||||||
| regular use, >10 y | RR, 1.75(1.18–2.60) | ||||||||||
| Ratnasinghe et al.[US]/200416 | Cohort | 1971–1992 | 21 | 78 | 22756 | aspirin | no aspirin used | RR, 1 | age, sex, race, smoking status, BMI, poverty index, education | Mortality | 9a |
| any aspirin used (≥1 times a week for at least 6 months) | RR, 0.87(0.42–1.77) | ||||||||||
| Anderson et al.[US]/200221 | Cohort | 1992–1999 | 7 | 80 | 28283 | aspirin/non-aspirin NSAIDs/NSAIDs | never used | RR, 1 | age, smoking status, current multivitamin use, diabetes | Incidence | 7a |
| use of only aspirin | RR, 0.56(0.36–0.88) | ||||||||||
| use of NSAIDs | RR, 0.66(0.39–1.11) | ||||||||||
| use of non-aspirin NSAIDs | RR, 1.21(0.77–1.89) | ||||||||||
| ≤1 time/week of aspirin | RR, 0.75(0.45–1.25) | ||||||||||
| 2–5 times/week of aspirin | RR, 0.47(0.22–0.98) | ||||||||||
| ≥6 times/week of aspirin | RR, 0.40(0.20–0.82) | ||||||||||
| Menezes et al.[US]/200227 | Case-control | 1982–1998 | NA | 194 | 582 | aspirin | non-regular used | OR, 1 | age, sex, race, smoking status, BMI, family history of pancreatic cancer, education | Incidence | 6a |
| regular used (at least once a week for six consecutive months) | OR, 1.00(0.72–1.39) | ||||||||||
| Dosage of 1–6 tablets/week | OR, 1.15(0.79–1.67) | ||||||||||
| dosage of ≥7 tablets/week | OR, 0.85(0.49–1.45) | ||||||||||
| duration of use for 0.5–10 years | OR, 0.82(0.54–1.26) | ||||||||||
| duration of use for ≥11 years | OR, 1.21(0.81–1.82) | ||||||||||
| Langman et al.[UK]/200024 | Case-control | 1993–1995 | NA | 367 | 1139 | NSAIDs | no use | OR, 1 | age, smoking status | Incidence | 8a |
| 1 prescription for NSAIDs | OR, 0.94(0.64–1.36) | ||||||||||
| 2–6 prescriptions for NSAIDs | OR, 1.08(0.75–1.54) | ||||||||||
| ≥7 prescriptions for NSAIDs | OR, 1.49(1.02–2.18) | ||||||||||
| Coogan et al.[US]/200026 | Case-control | 1997–1998 | NA | 504 | 5952 | NSAIDs | never used | OR, 1 | age, sex, race, religion, smoking status, alcohol use, family history of digestive cancer, education, interview year, study center | Incidence | 6a |
| continuing regular NSAIDs use (initiated ≥1 y previously) | OR, 0.8(0.5–1.1) | ||||||||||
| duration of NSAIDs use <5 y | OR, 0.8(0.5–1.4) | ||||||||||
| duration of NSAIDs use ≥5 y | OR, 0.6(0.4–1.1) |
a: quality assessment by Newcastle-Ottawa Scales; b: quality assessment by jaded score; NA: not available; y: year; NSAID: non-steroidal anti-inflammatory drug; CI: confidence interval; OR: odds ratio; RR: relative risk; DDD: defined daily dose.
Incidence risk of pancreatic cancer
Aspirin
Six case-control and two cohort studies were included in this combined analysis, and a pooled estimate (OR = 0.77; 95% CI = 0.62 to 0.96) revealed a decrease in the incidence of pancreatic cancer, with moderate heterogeneity amongst studies (P = 0.001; I2 = 74.2%; Fig. 2). The summary OR was 0.75 (95% CI = 0.59 to 0.95; I2 = 73.30%) for the case-control studies and 0.83 (95% CI = 0.40 to 1.76; I2 = 86.50%) for the cohort studies. We conducted a subgroup analyses that considered numerous covariates that might have contributed to the overall heterogeneity (for the results, see Table 2). We pre-specified five subgroups based on geographic region, gender, study quality, pattern of aspirin use, and adjustment for confounds. Notably, most of the studies were adjusted for major confounds such as age, sex, smoking status, history of diabetes and body mass index (BMI). However, not all of the included studies adjusted for important confounds such as alcohol consumption and a family history of pancreatic cancer. Therefore, we pre-specified three strata to explore whether the differences in confounds influenced the results. Significant inverse associations between aspirin use and the incidence of pancreatic cancer were observed across almost all of the strata included in the subgroup analyses. However, considerable heterogeneity was observed amongst the studies within each stratum. A sensitivity analysis revealed that the exclusion of any one study did not substantially alter the overall estimate, with an OR range of 0.72 (95% CI = 0.58 to 0.90) to 0.83 (95% CI = 0.68 to 1.02). No evidence of publication bias was found according to an Egger linear regression test (P = 0.413).
Figure 2. Forest plot showing the association between aspirin use and the incidence of pancreatic cancer.
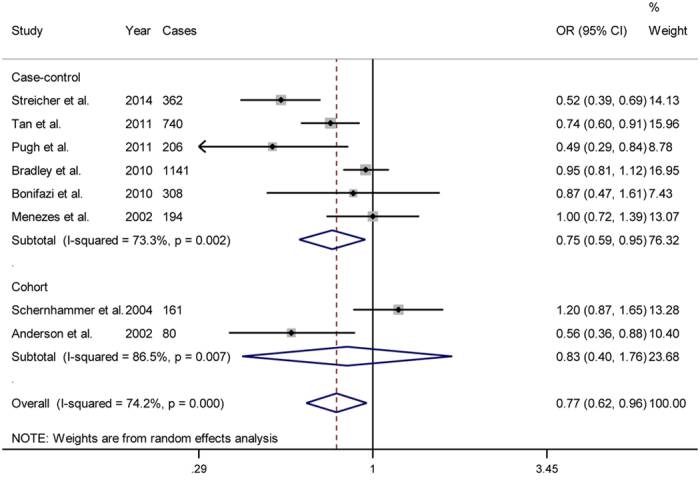
Table 2. Subgroup analysis for aspirin use on pancreatic cancer incidence.
| Study characteristics | Number of Studies | OR (95% CI) | Ρ a | Ρ b | I2 |
|---|---|---|---|---|---|
| Total | 8 | 0.77(0.62–0.96) | 0.000 | 74.2% | |
| Geographic region | 0.97 | ||||
| America14,19,21,22,27 | 5 | 0.77(0.57 to 1.03) | 0.001 | 79.1% | |
| Europe18,20,25 | 3 | 0.77(0.52 to 1.16) | 0.065 | 63.4% | |
| Gender | 0.79 | ||||
| Male and female18,19,20,22,25,27 | 6 | 0.75(0.59 to 0.95) | 0.002 | 73.3% | |
| Female14,21 | 2 | 0.83(0.40 to 1.76) | 0.007 | 86.5% | |
| Study quality | 0.85 | ||||
| Low risk of bias14,18,21,22,25 | 5 | 0.79(0.57 to 1.09) | 0.000 | 81.0% | |
| Medium risk of bias19,20,27 | 3 | 0.75(0.55 to 1.03) | 0.069 | 62.5% | |
| Pattern of aspirin use | 0.50 | ||||
| Ever use19,20,21,25 | 4 | 0.72(0.55 to 0.94) | 0.015 | 71.4% | |
| Regularly use14,18,22,27 | 4 | 0.85(0.56 to 1.30) | 0.001 | 82.0% | |
| Adjustment for confounders | |||||
| BMI | 0.03 | ||||
| Yes14,18,19,22,25,27 | 6 | 0.85(0.67 to 1.06) | 0.001 | 75.4% | |
| No20,21 | 2 | 0.53(0.38 to 0.75) | 0.706 | 0.0% | |
| Family history of pancreatic cancer | 0.15 | ||||
| Yes27 | 1 | 1.00(0.72 to 1.39) | – | – | |
| No14,18,19,20,21,22,25 | 7 | 0.74(0.58 to 0.94) | 0.000 | 76.5% | |
| Alcohol consumption | 0.10 | ||||
| Yes25 | 1 | 0.95(0.81 to 1.12) | – | – | |
| No14,18,19,20,21,22,27 | 7 | 0.74(0.57 to 0.95) | 0.001 | 72.6% | |
a: P value for heterogeneity within each subgroup; b: P value for heterogeneity between subgroups; OR: odds ratio; BMI: body mass index; CI: confidence interval.
Furthermore, we examined the dose, frequency and duration risks of aspirin use; these data are presented in Table 3 and represent a combination of case-control and cohort studies because of the small number of studies within each stratum. Neither high-dose (OR = 0.98; 95% CI = 0.96 to 1.00; P = 0.097; I2 = 0.0%) nor low-dose aspirin use was significantly related to pancreatic cancer prevention (OR = 1.01; 95% CI = 0.82 to 1.24; I2 = 64.4%). Significant declines in the incidence of pancreatic cancer were related to high-frequency (OR = 0.57; 95% CI = 0.39 to 0.83; I2 = 26.1%) and medium-frequency (OR = 0.57; 95% CI = 0.38 to 0.84; I2 = 0.0%) aspirin use. Only one study21 reported low-frequency aspirin use, and its test statistic was not significant (OR = 0.75; 95% CI = 0.45 to 1.25). However, the summary OR associated with the analysis of each aspirin duration risk category was also non-significant (Table 2).
Table 3. Dose-, frequency-, and duration-risk of aspirin use for pancreatic cancer incidence.
| Study characteristics | Number of Studies | Number of Cases | OR (95% CI) | Ρheterogeneity | I2 |
|---|---|---|---|---|---|
| Total | 8 | 4,256 | 0.77(0.62–0.96) | 0.000 | 74.2% |
| Dose | |||||
| Low dose14,19,22,27 | 4 | 1,457 | 1.01(0.82–1.24) | 0.037 | 64.40% |
| High dose14,19,22,25,27 | 5 | 2,926 | 0.98(0.96–1.00) | 0.459 | 0.00% |
| Frequency | |||||
| Low frequency21 | 1 | 80 | 0.75(0.45–1.25) | – | – |
| Medium frequency19,21 | 2 | 820 | 0.57(0.38–0.84) | 0.561 | 0.00% |
| High frequency19,21 | 2 | 820 | 0.57(0.39–0.83) | 0.245 | 26.10% |
| Duration | |||||
| duration less than 5y14,18,22,27 | 4 | 1,025 | 0.84(0.54–1.32) | 0.01 | 73.50% |
| duration more than 5y18 | 1 | 308 | 0.53(0.21–1.33) | – | – |
| duration more than 10y14,22,27 | 3 | 717 | 1.11(0.63–1.96) | 0.005 | 81.10% |
OR: odds ratio; y: year.
Non-aspirin NSAIDs and overall NSAIDs
The analysis of 3 (two cohorts and one case-control) studies suggested that non-aspirin NSAIDs use was not associated with a decrease in the incidence of pancreatic cancer (OR = 1.08; 95% CI = 0.90 to 1.31); these studies showed no heterogeneity (P = 0.676; I2 = 0.0%; Fig. 3a). In addition, 5 studies (four case-controls and one cohorts) revealed that overall NSAIDs use was not associated with a decrease in the incidence of pancreatic cancer (OR = 0.97; 95% CI = 0.86 to 1.10); these studies showed no heterogeneity (P = 0.451; I2 = 0.0%; Fig. 3b).
Figure 3. Forest plot showing the association between other NSAIDs use and the incidence of pancreatic cancer.
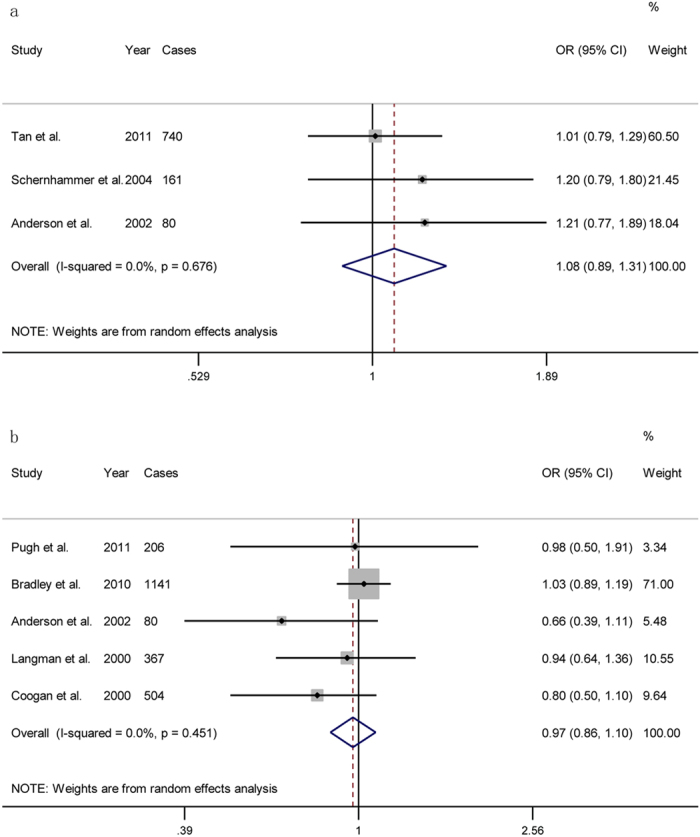
(a): non-aspirin NSAIDs use; (b): all NSAIDs use.
Cancer mortality risk
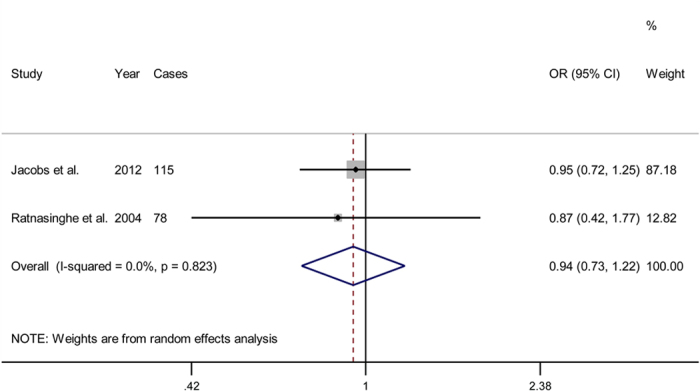
Two cohort studies investigated the association between aspirin use and cancer-specific mortality. Together, these studies revealed that aspirin use was not associated with a decreased mortality risk (OR = 0.94; 95% CI = 0.73 to 1.22). No significant heterogeneity was observed between the 2 studies (P = 0.823; I2 = 0.0%; Fig. 4).
Figure 4. Forest plot showing the association between aspirin use and pancreatic cancer mortality.
Discussion
The current meta-analysis included approximately 258,000 participants from 12 observational studies and investigated the association between NSAID use with the rates of pancreatic cancer and mortality in real-world practice. The major findings of this meta-analysis support the mechanistic hypothesis that aspirin use (specifically, high- and medium-frequency use) is inversely related to the risk of pancreatic cancer. In contrast, neither overall NSAIDs use nor non-aspirin NSAIDs use was associated with a reduced risk of pancreatic cancer.
The main finding of our meta-analysis contradicts a previous meta-analysis (Larsson et al.29) that evaluated the effect of aspirin on pancreatic cancer risk. This previous meta-analysis included 8 studies (one case-control, one RCT and six cohorts) in its analysis of aspirin use; furthermore, it considered incidence and mortality together to represent the overall risk of pancreatic cancer. That analysis revealed that aspirin use is not associated with a reduced risk of pancreatic cancer. However, this evidence was limited because of a methodological weakness. The effect of aspirin on cancer prevention (i.e., a chemoprevention effect) might be masked by its potential therapeutic effect. We only considered observational studies in our meta-analysis to explore the effects of NSAIDs use on the incidence of pancreatic cancer in the real world because they reflect actual practice patterns better than RCTs32, which tend to overstate the effect of a new treatment when it is introduced to an entire target population32. Unlike a previous meta-analysis, however, we did not include a particular study that provided data as SIRs13 without adjustments. Moreover, we excluded Schreinemachers’s study15 from our analysis because its cohort, which is part of the first National Health and Nutrition Examination Survey (NHANES I) study, was included in a duplicate publication by Ratnasinghe et al.16. We believe that both articles were incorrectly included in the meta-analysis by Larsson et al.29. Thus, our meta-analysis, which has a larger sample size, adds to previous findings by demonstrating that non-aspirin NSAIDs and overall NSAIDs use are not associated with the incidence of pancreatic cancer.
We assessed the relationship between aspirin use and pancreatic cancer mortality and did not observe a significant association. However, this finding should be interpreted with caution because of the small number of studies (n = 2) included. In fact, these patients might have died from causes other than pancreatic cancer, thereby creating bias and obscuring the true incidence of pancreatic cancer. Unfortunately, we did not obtain the data needed to evaluate the effect of duration risk on mortality. However, an individual patient data analysis of 8 RCTs suggested that daily aspirin use over at least 5 years significantly reduces mortality due to several common cancers, including pancreatic cancer (hazard ratio (HR) = 0.25; 95% CIs = 0.07 to 0.92)33.
We also investigated the potential implications of aspirin use dose, frequency and duration. A meta-analysis by Cui et al.31 suggested that high-dose aspirin use (OR = 0.88; 95% CI = 0.76 to 1.01; P = 0.069), but not low-dose aspirin use (OR = 0.99; 95% CI = 0.91 to 1.07), reduces the risk of pancreatic cancer; however, the summary OR of the high-dose aspirin group was not significant. Moreover, the incidence and mortality rates associated with pancreatic cancer were pooled together to represent overall risk. Notably, when a study that investigated mortality risk was excluded17 from this meta-analysis, the overall risk estimates associated with the effect of high-dose aspirin use on cancer risk were significant (OR = 0.78; 95% CI = 0.64 to 0.95; P = 0.014)31. We also excluded 3 studies16,18,21 that were incorrectly included in the previous meta-analysis because they did not provide information on high-dose use. Using incidence as an independent endpoint, our analysis did not find a beneficial effect of high-dose aspirin use on the incidence of pancreatic cancer (OR = 0.98; 95% CI = 0.96 to 1.00; P = 0.097; I2 = 0.0%). In addition, we did not include 5 of the previously included studies in our analysis of the effect of low-dose aspirin use on the incidence of cancer. Of these 5 studies, one did not meet our inclusion criteria13, one investigated mortality risk17, one used a RCT design34, and the other 2 did not provide data regarding low-dose aspirin use21,25. Even after excluding these studies, we did not find an association between low-dose aspirin use and the incidence of pancreatic cancer. Furthermore, another meta-analysis by Capurso et al.30 did not identify chemopreventive effects associated with the low use, intermediate use, or high use of NSAID based on a combination of dose and duration. However, these authors combined aspirin and NSAIDs use in each exposure category; this strategy might have masked the effect of aspirin use alone. In addition, duration of use was not assessed separately from dose in this meta-analysis. Our meta-analysis, however, considered aspirin use individually and found that high- and medium-frequent use led to a significant decrease in the incidence of pancreatic cancer. We did not find a significant duration risk of aspirin use on the incidence of pancreatic cancer. Thus, our meta-analysis provides a clearer and more comprehensive understanding of the potential benefits of NSAID use because of its strict inclusion criteria and accumulation of evidence; moreover, it has higher statistical power than previous meta-analyses.
Our analysis identified a significant negative relationship between aspirin use and the incidence of pancreatic cancer when the case-control studies were pooled together; however, this finding was not true for the two cohort studies. However, the exposure assessments of these cohort studies were based on self-reports. Moreover, these studies were both based on women, whereas the case-control samples included both men and women. Therefore, their results might be biased, and the limited data from the 2 cohort studies might limit the interpretation. In addition, our stratified analysis based on geographic region did not reveal significant results in America or Europe, which raises interesting questions. In fact, all of the included American studies reported baseline aspirin use values exceeding 40%, which might have reduced the chance of observing small relative differences in aspirin use as a risk factor30. Moreover, because of the limited number of studies included in the stratified analysis of European studies, the results might be biased. Thus, additional investigations are needed.
Several mechanisms might underpin the associations observed in our study. Our meta-analysis did not find that the negative association between aspirin use and the incidence of pancreatic cancer depended on dosage. Individuals consuming high doses of aspirin primarily did so for pain control or anti-inflammation purposes35; we hypothesise that these reasons are associated with other serious symptoms such as the abdominal discomfort that radiates to the back that occurs during the early stages of pancreatic cancer36. This factor might have partially influenced the observed association. In contrast, low-dose aspirin use is commonly used for the primary or secondary prevention of cardiovascular disease37, and lipid-lowering drugs are often consumed in combination with aspirin to prevent coronary artery disease. This reason might have masked the effect of the reduction in the rate of pancreatic cancer mortality38 and explain the lack of the chemopreventive effect typically associated with low-dose aspirin use. Aspirin use over fewer than 10 years since the onset of cancer might slow carcinogenesis rather than prevent initial tumour development because of the average 10-year latency of this disease39. In addition, one RCT by Cook et al.34 investigated this issue and suggested that low doses of aspirin (100 mg) over a 10-year treatment did not reduce the risk of pancreatic cancer, partially supporting our findings. Our meta-analysis did not identify an association between non-aspirin NSAID use and the incidence of pancreatic cancer, perhaps because only aspirin irreversibly inactivates COX-2 enzymes40.
Given the poor prognosis of pancreatic cancer and the widespread use of aspirin in the general population, successful prevention might have a significant public health effect. Our meta-analysis addresses the beneficial effects of aspirin use; however, physicians should be aware of the optimal dosage and frequency of aspirin use as well as its side effects.
Several limitations should be acknowledged. First, significant heterogeneity was observed across the studies included in the current analysis. Although we stratified the data into subgroups based on geographic region, gender, study quality, pattern of aspirin use, and adjusted confounds, considerable heterogeneity remained. This result is not surprising given the discrepancies of each study with regard to their designs; the differing race, age and lifestyle factors of their participants; sample sizes; definitions of drug exposure; follow-up evaluation lengths; and, most importantly, the type and dose of aspirin used as well as its exact administration schedule.
Second, most studies adjust for major confounds such as age, sex, BMI, smoking status, and diabetes history using multivariate statistical models. However, the adjusted confounds differed across the studies, which might have affected the overall results. In addition, few studies have adjusted for a family history of pancreatic cancer, which is a significant risk factor for the disease41. Researchers do not always make the same decisions concerning confounds. To explore whether the differences across the adjustments for confounds influenced our results, we performed stratified analyses according to the adjustments for BMI, alcohol consumption, and pancreatic cancer history. We found that these variables did not influence the overall results.
A third limitation was the potential misclassification of aspirin use. The consumption levels of the low- and high-dose categories as well as the frequency categories varied across studies; we attempted to minimize this imprecision by pooling the most similar data across the analyses. Nevertheless, this imprecision might have created study heterogeneity in the relevant pooled analysis. Therefore, this result should be considered with caution.
Another limitation is that the quantity of studies included in the analysis was not sufficient to evaluate publication bias, although the P-value associated with the Egger’s test was non-significant (P = 0.413). Moreover, we did not find unpublished data to avoid publication bias.
Finally, our findings concerning the risks associated with the dose, frequency and duration of aspirin use should be interpreted with caution because few studies were included in the stratified analyses, and most studies lacked information regarding these variables. Of the studies that provided this information, these parameters varied across each trial and therefore would have resulted in invalid statistical analyses for these groups.
In summary, the results from the current meta-analysis suggest that aspirin use reduces the incidence of pancreatic cancer but not cancer-specific mortality in the real world. However, these findings should be interpreted with caution because of the considerable heterogeneity amongst the included studies regarding incidence risk and the limited number of included studies concerning mortality risk. Specifically, the negative association between aspirin use and the incidence of pancreatic cancer likely depends on the frequency of this use (i.e., high- and medium- vs. low-frequency doses). Moreover, our meta-analysis did not find any associations between the incidence of pancreatic cancer and either non-aspirin NSAIDs or overall NSAIDs use; the studies included in this analysis had no heterogeneity. However, the small number of studies included in the analyses might limit our interpretations. This study might provide insights that enable a better understanding of the relationship between pancreatic cancer risk and aspirin/NSAIDs use. Future research, especially prospective studies, should be conducted to validate our findings and investigate whether the relationship between aspirin use and the incidence of pancreatic cancer depends on dose or duration of use.
Methods
This study was conducted using the Cochrane methodology and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines42 (Supplementary Table S2).
Search strategy
We searched PubMed and Embase for studies of pancreatic cancer risk and aspirin/other NSAID use published in any language from the inception dates of these databases to June 2015. We searched ClinicalTrials.gov for unpublished studies. The search terms included the generic names of individual drugs, their therapeutic classes, and pancreatic cancer outcome terms (Supplementary Table S3). No restrictions were applied. Furthermore, we reviewed the reference lists of the retrieved articles and recent reviews to identify additional potentially relevant studies.
Study selection
Studies were included when they met the following criteria: 1) a cohort or case-control design was used; 2) the exposure of interest was any NSAID use, including aspirin, non-aspirin NSAIDs and overall NSAIDs; 3) the incidence rate, mortality rate, or both of pancreatic cancer was assessed; and 4) an adjusted OR or relative risk (RR) with 95% CIs were provided. Regarding duplicate publications15,16,17,23, only the most informative and complete studies were included16,23.
Data extraction
The following information was abstracted from all of the included studies using a standardized data collection form: study name (together with the first author’s name and year of publication), study design, study period, study follow-up, study sample size (including both the numbers of cases and controls or the cohort size), study outcomes, the quality score of each study, the types of NSAIDs and consumption categories employed, the ORs and RRs with corresponding 95% CIs for each category, and the adjusted confounds. We also reviewed the supplementary files of each study and contacted the authors for more detailed information when necessary.
Assessments of quality and risk of bias across studies
We assessed the authenticity and quality of the included studies using the NOS43. The NOS evaluates a study from 3 broad perspectives and awards a maximum score of 9 points. We assigned the following risk of bias categories based on the NOS score of each study: low risk of bias (7–9 points), medium risk of bias (4–6 points), and high risk of bias (less than 4 points).
Two investigators (YP Zhang and YD Wan) independently conducted the literature search, study selection, and data extraction as well as the assessments of quality and risk of bias across studies. Any discrepancies were resolved via consensus.
Statistical analyses
The measure of an effect was its associated OR and 95% CIs. Because the absolute risk of pancreatic cancer is low, we generally ignored the distinctions amongst the various measures of relative risk (ORs in case-control studies and RRs in cohort studies); therefore, we reported all of the results as ORs for simplicity44. Multivariate adjusted ORs were pooled in the analysis to minimise potential confounding bias. Between-study heterogeneity was assessed using the Cochran Q statistic (significance level at P < 0.1) and by estimating I2 45. Studies with an I2 statistic of 25% to 50%, 50% to 75%, and >75% were regarded as low heterogeneity, moderate heterogeneity, and high heterogeneity, respectively45. The DerSimonian and Laird46 random-effects model, which incorporates both within- and between-study variability, was used regardless of heterogeneity. The inverse-variance method was used to pool the adjusted ORs. We also conducted a sensitivity analysis using the one-study-out method to test the robustness of the pooled estimate. We intended to assess the publication bias across studies using Egger’s linear regression test47 at the 90% level; however, no testing for funnel plot asymmetry was conducted because of the small number of studies included in all analyses (n < 10)48.
To achieve a clear understanding of the relationship between NSAID use and the incidence of pancreatic cancer, we stratified the data based on drug type (aspirin, non-aspirin NSAIDs, overall NSAIDs) and included two outcomes (the incidence and mortality rates of pancreatic cancer). The effect estimates were pooled both overall and by study design in the aspirin use analysis. Overall NSAIDs use in this report refers to the studies that reported NSAIDs use but did not provide ratios for the use of aspirin or non-aspirin NSAIDs alone. To analyse the dose, frequency, and duration risks associated with aspirin use, we pooled similar data in each category to conduct the analysis. We defined low-dose aspirin use as 100 mg per day and high-dose aspirin use as 300 mg per day. Low-, medium-, and high-frequency aspirin use were defined as ≤1 day per week, 2–5 days per week, and ≥6 days per week, respectively. A two-tailed P-value of <0.05 was considered significant. All statistical analyses were performed using STATA 12.0 (StataCorp, College Station, TX).
Additional Information
How to cite this article: Zhang, Y.-P. et al. Aspirin might reduce the incidence of pancreatic cancer: a meta-analysis of observational studies. Sci. Rep. 5, 15460; doi: 10.1038/srep15460 (2015).
Supplementary Material
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81100304) and the Science and Technology Bureau of Zhengzhou (No. 131PLJRC658), China.
Footnotes
Author Contributions Y.P.Z. conceived the study, participated in the design, collected the data, and drafted the manuscript. Y.D.W. collected the data and performed the statistical analyses. R.T.Z. assisted with data collection, and J.L. conceived the study, participated in the design, and helped draft the manuscript. Y.L.S. edited the manuscript. All of the authors have read and approved the final manuscript.
References
- Jemal A., Siegel R., Xu J. & Ward E. Cancer statistics, 2010. CA Cancer J Clin 60, 277 (2010). [DOI] [PubMed] [Google Scholar]
- Neoptolemos J. P. et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350, 1200 (2004). [DOI] [PubMed] [Google Scholar]
- de Groot D. J., de Vries E. G., Groen H. J. & de Jong S., Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic. Crit Rev Oncol Hematol 61, 52 (2007). [DOI] [PubMed] [Google Scholar]
- Flower R. J. The development of COX2 inhibitors. Nat Rev Drug Discov 2, 179 (2003). [DOI] [PubMed] [Google Scholar]
- Ali S. et al. Concurrent inhibition of NF-kappaB, cyclooxygenase-2, and epidermal growth factor receptor leads to greater anti-tumor activity in pancreatic cancer. J Cell Biochem 110, 171 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tucker O. N. et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res 59, 987 (1999). [PubMed] [Google Scholar]
- Cole B. F. et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst 101, 256 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuder S. A. & Mutgi A. B. Breast cancer and NSAID use: a meta-analysis. Br J Cancer 84, 1188 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abnet C. C. et al. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer 100, 551 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez A., Garcia R. L. & Lopez-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. Bmc Cancer 3, 28 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. et al. Meta-analysis on the association between nonsteroidal anti-inflammatory drug use and lung cancer risk. Clin Lung Cancer 13, 44 (2012). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Effect of aspirin and other non-steroidal anti-inflammatory drugs on prostate cancer incidence and mortality: a systematic review and meta-analysis. Bmc Med 12, 55 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis S. et al. A population-based cohort study of the risk of colorectal and other cancers among users of low-dose aspirin. Br J Cancer 88, 684 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernhammer E. S. et al. A prospective study of aspirin use and the risk of pancreatic cancer in women. J Natl Cancer Inst 96, 22 (2004). [DOI] [PubMed] [Google Scholar]
- Schreinemachers D. M. & Everson R. B. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology 5, 138 (1994). [DOI] [PubMed] [Google Scholar]
- Ratnasinghe L. D. et al. Aspirin use and mortality from cancer in a prospective cohort study. Anticancer Res 24, 3177 (2004). [PubMed] [Google Scholar]
- Jacobs E. J. et al. Aspirin use and pancreatic cancer mortality in a large United States cohort. J Natl Cancer Inst 96, 524 (2004). [DOI] [PubMed] [Google Scholar]
- Bonifazi M. et al. Aspirin use and pancreatic cancer risk. Eur J Cancer Prev 19, 352 (2010). [DOI] [PubMed] [Google Scholar]
- Tan X. L. et al. Aspirin, nonsteroidal anti-inflammatory drugs, acetaminophen, and pancreatic cancer risk: a clinic-based case-control study. Cancer Prev Res (Phila) 4, 1835 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh T. F. G. et al. Aspirin, NSAIDS, Calcium-channel blockers and statins in the aetiology of pancreatic cancer: preliminary results from a case-control study in two centres in the UK. Gut 60 A81 (2011). [Google Scholar]
- Anderson K. E., Johnson T. W., Lazovich D. & Folsom A. R. Association between nonsteroidal anti-inflammatory drug use and the incidence of pancreatic cancer. J Natl Cancer Inst 94, 1168 (2002). [DOI] [PubMed] [Google Scholar]
- Streicher S. A., Yu H., Lu L., Kidd M. S. & Risch H. A. Case-control study of aspirin use and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 23, 1254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E. J., Newton C. C., Gapstur S. M. & Thun M. J. Daily aspirin use and cancer mortality in a large US cohort. J Natl Cancer Inst 104, 1208 (2012). [DOI] [PubMed] [Google Scholar]
- Langman M. J., Cheng K. K., Gilman E. A. & Lancashire R. J. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ 320, 1642 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. C., Hughes C. M., Cantwell M. M., Napolitano G. & Murray L. J., Non-steroidal anti-inflammatory drugs and pancreatic cancer risk: a nested case-control study. Br J Cancer 102, 1415 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan P. F. et al. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomarkers Prev 9, 119 (2000). [PubMed] [Google Scholar]
- Menezes R. J., Huber K. R., Mahoney M. C. & Moysich K. B. Regular use of aspirin and pancreatic cancer risk. Bmc Public Health 2, 18 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen H. T. et al. Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer 88, 1687 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S. C., Giovannucci E., Bergkvist L. & Wolk A. Aspirin and nonsteroidal anti-inflammatory drug use and risk of pancreatic cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15, 2561 (2006). [DOI] [PubMed] [Google Scholar]
- Capurso G. et al. Meta-analysis: the use of non-steroidal anti-inflammatory drugs and pancreatic cancer risk for different exposure categories. Aliment Pharmacol Ther 26, 1089 (2007). [DOI] [PubMed] [Google Scholar]
- Cui X. J. et al. High-dose aspirin consumption contributes to decreased risk for pancreatic cancer in a systematic review and meta-analysis. Pancreas 43, 135 (2014). [DOI] [PubMed] [Google Scholar]
- Hannan E. L. Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC Cardiovasc Interv 1, 211 (2008). [DOI] [PubMed] [Google Scholar]
- Rothwell P. M. et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377, 31 (2011). [DOI] [PubMed] [Google Scholar]
- Cook N. R. et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA 294, 47 (2005). [DOI] [PubMed] [Google Scholar]
- Dovizio M., Bruno A., Tacconelli S. & Patrignani P. Mode of action of aspirin as a chemopreventive agent. Recent Results Cancer Res 191, 39 (2013). [DOI] [PubMed] [Google Scholar]
- Gobbi P. G. et al. The prognostic role of time to diagnosis and presenting symptoms in patients with pancreatic cancer. Cancer Epidemiol 37, 186 (2013). [DOI] [PubMed] [Google Scholar]
- Wolff T., Miller T. & Ko S., Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 150, 405 (2009). [DOI] [PubMed] [Google Scholar]
- Newby L. K. et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation 113, 203 (2006). [DOI] [PubMed] [Google Scholar]
- Yachida S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrono C., Garcia R. L., Landolfi R. & Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 353, 2373 (2005). [DOI] [PubMed] [Google Scholar]
- Permuth-Wey J. & Egan K. M. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer 8, 109 (2009). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G., Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med 6, e1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603 (2010). [DOI] [PubMed] [Google Scholar]
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9, 1 (1987). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539 (2002). [DOI] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177 (1986). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey S. G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J. A. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.