Abstract
Hypomorphic mutation of apoptosis-inducing factor (AIF) in the whole body or organ-specific knockout of AIF compromises the activity of respiratory chain complexes I and IV, as it confers resistance to obesity and diabetes induced by high-fat diet. The mitochondrial defect induced by AIF deficiency can be explained by reduced AIF-dependent mitochondrial import of CHCHD4, which in turn is required for optimal import and assembly of respiratory chain complexes. Here we show that, as compared to wild type control littermates, mice with a heterozygous knockout of CHCHD4 exhibit reduced weight gain when fed with a Western style high-fat diet. This finding suggests widespread metabolic epistasis among AIF and CHCHD4. Targeting either of these proteins or their functional interaction might constitute a novel strategy to combat obesity.
Keywords: Apoptosis, diabetes, metabolism, obesity, programmed cell death
Introduction
Apoptosis-inducing factor (AIF, official protein name: AIFM1) is a flavoprotein that is tethered to the outer side of the inner mitochondrial membrane.1 Upon cell death-associated permeabilization of the outer membrane2 and calpain-mediated cleavage of its membrane anchorage,3 AIF is released from mitochondria to the cytosol and translocates to the nucleus. AIF has the property to directly interact with DNA.4,5 However, its contribution to nuclear apoptosis and chromatin degradation requires the interaction with additional protein factors including cyclophilin A.6,7
Beyond its implication in developmental cell death,8,9 AIF also contributes to pathological cell death, in particular in the brain and in the retina, where it may mediate caspase-independent cell loss of neurons and photoreceptors, respectively.10-14 In addition, AIF plays a major role in normal mitochondrial pathogenesis.15 Thus, yeast, mouse or human cells lacking AIF exhibit a reduced abundance of respiratory chain protein complexes (in particular complexes I and IV), which compromises oxidative phosphorylation.16-18 In humans, several mutations of AIF have been described. Such mutants cause X-linked pathologies that resemble mitochondriopathies with regard to their clinical manifestations ranging from deafness and cognitive impairment to severe encephalomyopathy and cardiomyopathy.19-23
The mechanisms accounting for mitochondrial defect induced by deficient AIF expression have recently been elucidated.24 Thus, AIF is required for the translational import of a mitochondrial intermembrane protein called coiled-coil-helix-coiled-coil-helix domain containing 4 (CHCHD4). CHCHD4, which is the human homolog of yeast Mia40,25,26 in turn plays a major role in the import, folding and oxidative maturation (due to the introduction of intramolecular disulfide bonds) of other intermembrane proteins. Knockdown or knockout of CHCHD4 results in a mitochondrial defect that is similar to that observed in AIF-deficient cells.24 Moreover, transfection of cells with a CHCHD4 variant whose mitochondrial import does not rely on AIF can repair the respiratory defect of AIF-deficient cells, demonstrating that AIF and CHCHD4 are epistatic with respect to mitochondrial biogenesis and respiratory function.24 Here, we addressed the question as to whether AIF and CHCHD4 also are epistatic with respect to their broad metabolic effects.
Results and Discussion
Mice bearing a hypomorphic mutation of apoptosis-inducing factor (AIF) exhibit the so-called Harlequin phenotype with cerebellar ataxia as a prominent hallmark.27 Before such mice develop signs of neuropathology, they exhibit a progressive reduction in the abundance of respiratory chain complexes I and IV in multiple organs. At this stage, such mice are resistant against the induction of obesity and type 2 diabetes by high fat diet.28 More convincingly, mice bearing a muscle-specific AIF knockout (which causes an age-dependent dilated cardiomyopathy and skeleton muscle degeneration) or a hepatocyte-specific AIF knockout (which does not cause any detectable phenotype) results in increased glucose tolerance, reduced fat mass, and increased insulin sensitivity. Such mice also resist weight gain and metabolic syndrome induced by a Western style high fat diet.28 Thus, even a partial AIF defect may have positive effects on whole body metabolism.
Driven by these considerations, we decided to investigate the metabolic phenotype of CHCHD4-deficient mice. Homozygous knockout of Chchd4 (Chchd4−/−) results in embryonic lethality around E8, presumably due to a severe mitochondrial deficiency.24 However, mice bearing a heterozygous knockout of Chchd4 (Chchd4+/−) are viable and have a life expectancy of at least 1.5 years (contrasting with mice bearing the Harlequin mutation or a muscle-specific AIF knockout) without any obvious phenotypic or behavioral alterations.
Both male and female Chchd4+/− mice were fertile, and Chchd4+/− pups resulting from the cross of WT and Chchd4+/− parents were born at an expected Mendelian frequency of close-to-50% (Table 1). Heterozygous knockout of Chchd4 resulted in a reduction of CHCHD4 expression by approximately 50% (as compared to wild type mice), yet failed to affect the expression of AIF (Fig. 1) and failed to manifest major respiratory chain defects (not shown). Importantly, when fed with a Western style high-fat diet, female Chchd4+/− mice exhibited a markedly reduced weight gain as compared to their age- and sex-matched WT littermates (Fig. 2).
Table 1.
Genotype frequency of adult Chchd4+/− mice
| Total number | WT genotype | Chchd4+/− |
|---|---|---|
| 845 | 372 (44%) | 473 (56%) |
Mice arising from multiple crosses of female or male Chchd4+/− mice with wild type (WT) control mice were genotyped at weaning.
Figure 1.
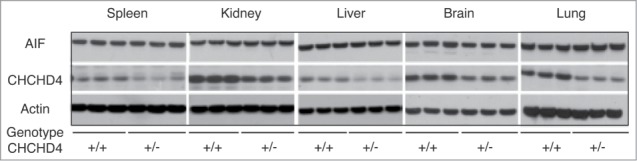
Impact of heterozygous knockout of CHCHD4 on the abundance of CHCHD4 protein and that of AIF. Levels of AIF and CHCHD4 expression were determined in various organs of adult mice. Triplicate samples from wild type (chchd4+/+) and heterozygous (chchd4+/−) were analyzed by immunoblot for the abundance of the indicated proteins. Actin expression was determined as a loading control.
Figure 2.
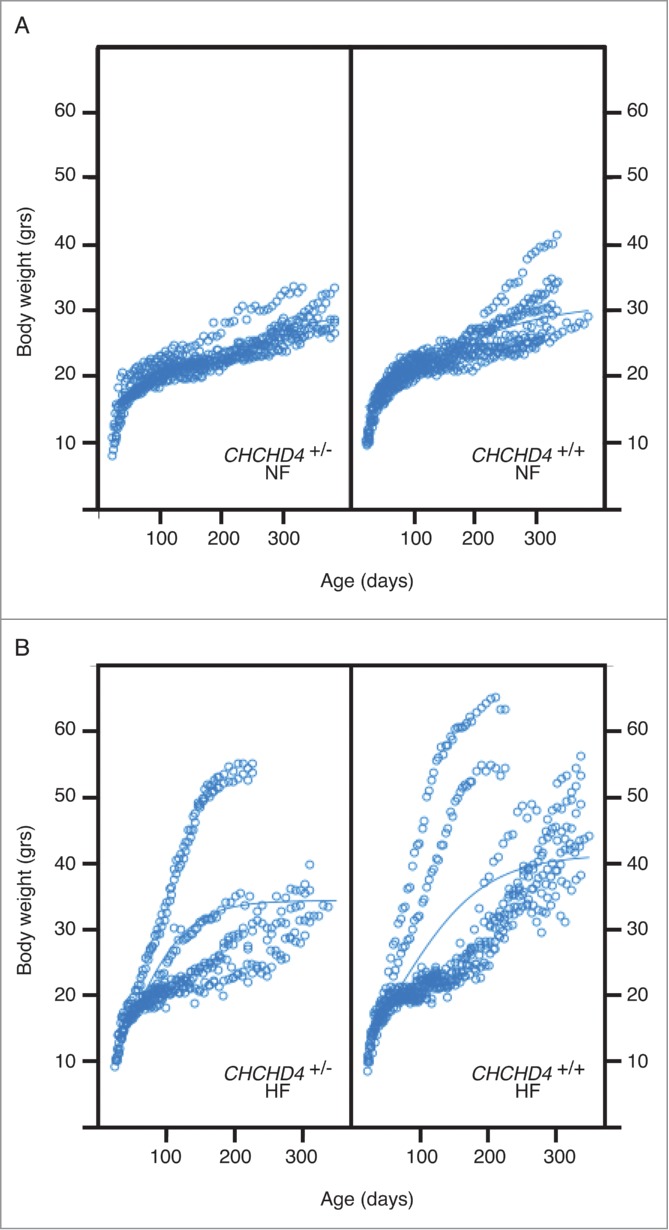
Impact of CHCHD4 on weight gain induced by high-fat diet. Weight gain was measured for wild type (chchd4+/+) and heterozygous (chchd4+/−) mice fed normal chow (A; NF: normal feeding) or high-fat diet (B; HF: high fat feeding). Maximum fitted values at age 300 to 310 days were: 28.2g ± 0.29 for chchd4+/−/NF mice; 30.5g ± 0.68 for chchd4+/+/NF; 33.6g ± 0.84 for chchd4+/−/HF mice; 44.6g ± 1 for chchd4+/+/HF. Values were significantly different for mice fed with high fat (HF) diet (p < 0.00001), while those for animals fed with normal (NF) diet were similar. Values are means ± SEM for animals of more than 300 days age in each category.
The aforementioned results indicate that defects in AIF and CHCHD4 result in similar metabolic phenotypes with respect to the resistance to diet-induced obesity. While AIF deficiency causing such a phenotype is linked to a major respiratory chain defect that is pathogenic (at least in the case of Harlequin mice and the muscle-specific deletion of AIF), the partial CHCHD4 deficiency linked to the Chchd4+/− genotype does not cause any obvious pathology, yet renders mice partially resistant against diet-induced obesity. This suggests that the beneficial effects of the AIF deficiency on whole body metabolism may be uncoupled from the negative effects of the mitochondriopathy.
At this stage, it remains to be determined through which molecular mechanisms the Chchd4+/− genotype confers resistance against obesity. As a possibility, Chchd4+/− mice might manifest a subclinical mitochondrial defect that affects particular cell types, hence explaining its beneficial effects. As an alternative, a partial defect in mitochondrial import might activate subtle homeostatic pathways (such as the mitochondrial unfolded protein response)29 that avoid the manifestation of obesity (or other age-associated changes in metabolism) without any negative impact on mitochondrial respiration.30 Finally, it is possible that Chchd4 deficiency affects non-mitochondrial signaling pathways by virtue of its capacity to regulate the subcellular localization of p53,31 a master transcription factor that controls the differentiation of both white and brown fat cells.32,33 Future work must distinguish between these possibilities.
Irrespective of these possibilities, CHCHD4 emerges as a new putative target for therapeutic interventions on obesity and metabolic syndrome. A peptide derived from the N-terminus of CHCHD4 can competitively disrupt the interaction between AIF and CHCHD4,24 suggesting the possibility of creating small molecules that affect the AIF-CHCHD4 axis. It will be interesting to explore whether such molecules might be used for the avoidance or treatment of obesity.
Materials and Methods
Antibodies
Antibodies against the following proteins were used: actin (mouse mAb; Millipore); AIF (mouse mAb; Santa Cruz and rabbit pAB; Cell Signaling); CHCHD4 (rabbit pAB; Santa Cruz).
Animals
Mutant heterozygous Chchd4 animals were constructed by Texas Institute for Genomic Medicine (TIGM) using a gene-trapping strategy.24 All procedures and animal experimentation protocols were reviewed and deemed acceptable by the registered ethical Committee n°26 and carried out in the animal facility of Gustave Roussy.
Genotyping of Chchd4 heterozygous mice
3 to 4 weeks after weaning, tail snip DNA was extracted using the Maxwell16 mouse tail DNA purification kit (Maxwell). Using the AmpliTaq Gold master Mix (Applied Biosystems), PCR was performed for the detection of the wt or mutant Chchd4 allele interrupted by a gene trap vector. The wt allele was amplified using the Primers IST11943B12-F (TGGGCTGGTTAGTCAGTGATTGG) and IST11943B12-R (GTGCTCCTCATAGGGATCATTGG) and the mutant allele was amplified using IST11943B12-R and LTR2 (AAATGGC GTTACTTAAGCTAGCTTGC).
Tissue extract preparation for immunoblot
Wild type (Chchd4+/+) or heterozygous (Chchd4+/−) adult female mice were anesthetized and killed by decapitation. All the dissected organs were snap-frozen and then homogenized, using Precellys homogenizer (Bertin), in an ice-cold RIPA 1X buffer (Sigma Aldrich), supplemented with protease (EDTA- free protease inhibitor tablet - Roche Applied Science) and phosphatase inhibitors (PhosSTOP phosphatase inhibitor tablet - Roche Applied Science). The proteins present in the extracts were quantified (Bio-Rad DC protein assay) and samples were finally resolved directly by SDS-PAGE (NUPAGE; Invitrogen) after boiling in 1xSB (2% SDS, 10% glycerol, 62.5 mM Tris-HCl, pH 6.8, 100 mM dithiothreitol). After electrophoresis, the gel was subjected to immunoblot analysis to visualize specific protein bands.
Regimens
Normal control Chow diet (A03) and high fat diet (A03 supplemented with 30% porcine fat, and 5% soya oil) were prepared by SAFE (Augy, France). Wild type (chchd4+/+) and heterozygous (chchd4+/−) mice were fed normal chow or high-fat diet starting from 4 weeks of age until the termination of the experiment. Animals were kept under 12h light/dark cycle and weighted every 3 to 4 days.
Statistics
Growth curves were analyzed by means of R statistical software [R Development Core Team (2008). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.] by fitting a nonlinear model (by logistic regression, R package nlme). Data are expressed as mean ± SEM.
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
Funding
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI).
Acknowlegments
We are grateful to Isabelle Godin, Karine Ser- Le Roux and Aurélie Sauvage for their help with animal experimentation.
References
- 1.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, et al.. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999; 397:441-6; PMID:9989411; http://dx.doi.org/ 10.1038/17135 [DOI] [PubMed] [Google Scholar]
- 2.Patterson SD, Spahr CS, Daugas E, Susin SA, Irinopoulou T, Koehler C, Kroemer G. Mass spectrometric identification of proteins released from mitochondria undergoing permeability transition. Cell Death Differ 2000; 7:137-44; PMID:10713728; http://dx.doi.org/ 10.1038/sj.cdd.4400640 [DOI] [PubMed] [Google Scholar]
- 3.Norberg E, Karlsson M, Korenovska O, Szydlowski S, Silberberg G, Uhlen P, Orrenius S, Zhivotovsky B. Critical role for hyperpolarization-activated cyclic nucleotide-gated channel 2 in the AIF-mediated apoptosis. EMBO J 2010; 29:3869-78; PMID:21037554; http://dx.doi.org/ 10.1038/emboj.2010.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye H, Cande C, Stephanou NC, Jiang S, Gurbuxani S, Larochette N, Daugas E, Garrido C, Kroemer G, Wu H. DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat Struct Biol 2002; 9:680-4; PMID:12198487; http://dx.doi.org/ 10.1038/nsb836 [DOI] [PubMed] [Google Scholar]
- 5.Mate MJ, Ortiz-Lombardia M, Boitel B, Haouz A, Tello D, Susin SA, Penninger J, Kroemer G, Alzari PM. The crystal structure of the mouse apoptosis-inducing factor AIF. Nat Struct Biol 2002; 9:442-6; PMID:11967568; http://dx.doi.org/ 10.1038/nsb793 [DOI] [PubMed] [Google Scholar]
- 6.Zhu C, Wang X, Deinum J, Huang Z, Gao J, Modjtahedi N, Neagu MR, Nilsson M, Eriksson PS, Hagberg H, et al.. Cyclophilin A participates in the nuclear translocation of apoptosis-inducing factor in neurons after cerebral hypoxia-ischemia. J Exp Med 2007; 204:1741-8; PMID:17635954; http://dx.doi.org/ 10.1084/jem.20070193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cande C, Vahsen N, Kouranti I, Schmitt E, Daugas E, Spahr C, Luban J, Kroemer RT, Giordanetto F, Garrido C, et al.. AIF and cyclophilin A cooperate in apoptosis-associated chromatinolysis. Oncogene 2004; 23:1514-21; PMID:14716299; http://dx.doi.org/ 10.1038/sj.onc.1207279 [DOI] [PubMed] [Google Scholar]
- 8.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, et al.. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 2001; 410:549-54; PMID:11279485; http://dx.doi.org/ 10.1038/35069004 [DOI] [PubMed] [Google Scholar]
- 9.Joza N, Galindo K, Pospisilik JA, Benit P, Rangachari M, Kanitz EE, Nakashima Y, Neely GG, Rustin P, Abrams JM, et al.. The molecular archaeology of a mitochondrial death effector: AIF in Drosophila. Cell Death Differ 2008; 15:1009-18; PMID:18309327; http://dx.doi.org/ 10.1038/cdd.2008.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hisatomi T, Sakamoto T, Murata T, Yamanaka I, Oshima Y, Hata Y, Ishibashi T, Inomata H, Susin SA, Kroemer G. Relocalization of apoptosis-inducing factor in photoreceptor apoptosis induced by retinal detachment in vivo. Am J Pathol 2001; 158:1271-8; PMID:11290545; http://dx.doi.org/ 10.1016/S0002-9440(10)64078-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penninger JM, Kroemer G. Mitochondria, AIF and caspases–rivaling for cell death execution. Nat Cell Biol 2003; 5:97-9; PMID:12563272; http://dx.doi.org/ 10.1038/ncb0203-97 [DOI] [PubMed] [Google Scholar]
- 12.Cande C, Vahsen N, Garrido C, Kroemer G. Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ 2004; 11:591-5; PMID:15017385 [DOI] [PubMed] [Google Scholar]
- 13.Zhu C, Wang X, Huang Z, Qiu L, Xu F, Vahsen N, Nilsson M, Eriksson PS, Hagberg H, Culmsee C, et al.. Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ 2007; 14:775-84; PMID:17039248; http://dx.doi.org/ 10.1038/sj.cdd.4402053 [DOI] [PubMed] [Google Scholar]
- 14.Osato KY, Sato Y, Ochiishi T, Osato A, C Zhu C, Sato M, Swanpalmer J, Modjtahedi N, Kroemer G, Kuhn HG, et al.. Apoptosis-inducing factor deficiency decreases the proliferation rate and protects the subventricular zone against ionizing radiation. Cell Death and Disease 2010; 1:e84; PMID:21368857; http://dx.doi.org/ 10.1038/cddis.2010.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hangen E, Blomgren K, Benit P, Kroemer G, Modjtahedi N. Life with or without AIF. Trends Biochem Sci 2010; 35:278-87; PMID:20138767; http://dx.doi.org/ 10.1016/j.tibs.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 16.Vahsen N, Cande C, Briere JJ, Benit P, Joza N, Larochette N, Mastroberardino PG, Pequignot MO, Casares N, Lazar V, et al.. AIF deficiency compromises oxidative phosphorylation. EMBO J 2004; 23:4679-89; PMID:15526035; http://dx.doi.org/ 10.1038/sj.emboj.7600461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wissing S, Ludovico P, Herker E, Buttner S, Engelhardt SM, Decker T, Link A, Proksch A, Rodrigues F, Corte-Real M, et al.. An AIF orthologue regulates apoptosis in yeast. J Cell Biol 2004; 166:969-74; PMID:15381687; http://dx.doi.org/ 10.1083/jcb.200404138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joza N, Oudit GY, Brown D, Benit P, Kassiri Z, Vahsen N, Benoit L, Patel MM, Nowikovsky K, Vassault A, et al.. Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy. Mol Cell Biol 2005; 25:10261-72; PMID:16287843; http://dx.doi.org/ 10.1128/MCB.25.23.10261-10272.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghezzi D, Sevrioukova IF, Invernizzi F, Lamperti C, Mora M, D'Adamo P, Novara F, Zuffardi O, Uziel G, Zeviani M. Severe X-linked mitochondrial encephalomyopathy associated with a mutation in Apoptosis-Inducing factor. The American journal of human Genetics 2010; 86:639-49; http://dx.doi.org/ 10.1016/j.ajhg.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modjtahedi N, Giordanetto F, Kroemer G. A human mitochondriopathy caused by AIF mutation. Cell Death Differ 2010; 17:1525-8; PMID:20835254; http://dx.doi.org/ 10.1038/cdd.2010.88 [DOI] [PubMed] [Google Scholar]
- 21.Rinaldi C, Grunseich C, Sevrioukova IF, Schindler A, Horkayne-Szakaly I, Lamperti C, Landouré G, Kennerson ML, Burnett BG, Bönnemann C, et al.. Cowchock syndrome is associated with a mutation in apoptosis-inducing factor. Am J Hum Genet 2012; 91:1095-102; PMID:23217327; http://dx.doi.org/ 10.1016/j.ajhg.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger I, Ben-Neriah Z, Dor-Wolman T, Shaag A, Saada A, Zenvirt S, Raas-Rothschild A, Nadjari M, Kaestner KH, Elpeleg O. Early prenatal ventriculomegaly due to an AIFM1 mutation identified by linkage analysis and whole exome sequencing. Mol Genet Metab 2011; 104:517-20; PMID:22019070; http://dx.doi.org/ 10.1016/j.ymgme.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 23.Zong L, Guan J, Ealy M, Zhang Q, Wang D, Wang H, Zhao Y, Shen Z, Campbell CA, Wang F, et al.. Mutations in apoptosis-inducing factor cause X-linked recessive auditory neuropathy spectrum disorder. J Med Genet 2015; PMID:25986071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hangen E, Féraud O, Lachkar S, Mou H, Doti N, Fimia GM, Lam NV, Zhu C, Godin I, Muller K, et al.. Interaction between AIF and CHCHD4 regulates respiratory chain biogenesis. Mol Cell 2015; 58:1001-14; PMID:26004228; http://dx.doi.org/ 10.1016/j.molcel.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 25.Hofmann S, Rothbauer U, Muhlenbein N, Baiker K, Hell K, Bauer MF. Functional and mutational characterization of human MIA40 acting during import into the mitochondrial intermembrane space. J Mol Biol 2005; 353:517-28; PMID:16185709; http://dx.doi.org/ 10.1016/j.jmb.2005.08.064 [DOI] [PubMed] [Google Scholar]
- 26.Chacinska A, Guiard B, Muller JM, Schulze-Specking A, Gabriel K, Kutik S, Pfanner N. Mitochondrial biogenesis, switching the sorting pathway of the intermembrane space receptor Mia40. J Biol Chem 2008; 283:29723-9; PMID:18779329; http://dx.doi.org/ 10.1074/jbc.M805356200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature 2002; 419:367-74; PMID:12353028; http://dx.doi.org/ 10.1038/nature01034 [DOI] [PubMed] [Google Scholar]
- 28.Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, et al.. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 2007; 131:476-91; PMID:17981116; http://dx.doi.org/ 10.1016/j.cell.2007.08.047 [DOI] [PubMed] [Google Scholar]
- 29.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 2012; 337:587-90; PMID:22700657; http://dx.doi.org/ 10.1126/science.1223560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, et al.. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013; 154:430-41; PMID:23870130; http://dx.doi.org/ 10.1016/j.cell.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhuang J, Wang PY, Huang X, Chen X, Kang JG, Hwang PM. Mitochondrial disulfide relay mediates translocation of p53 and partitions its subcellular activity. Proc Natl Acad Sci U S A 2013; 110:17356-61; PMID:24101517; http://dx.doi.org/ 10.1073/pnas.1310908110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yahagi N, Shimano H, Matsuzaka T, Najima Y, Sekiya M, Nakagawa Y, Ide T, Tomita S, Okazaki H, Tamura Y, et al.. p53 Activation in adipocytes of obese mice. J Biol Chem 2003; 278:25395-400; PMID:12734185; http://dx.doi.org/ 10.1074/jbc.M302364200 [DOI] [PubMed] [Google Scholar]
- 33.Molchadsky A, Ezra O, Amendola PG, Krantz D, Kogan-Sakin I, Buganim Y, Rivlin N, Goldfinger N, Folgiero V, Falcioni R, et al.. p53 is required for brown adipogenic differentiation and has a protective role against diet-induced obesity. Cell Death Differ 2013; 20:774-83; PMID:23412343; http://dx.doi.org/ 10.1038/cdd.2013.9 [DOI] [PMC free article] [PubMed] [Google Scholar]


