Prevalence of pre-operative anaemia
A recent study, using public data from 187 countries worldwide and World Health Organization (WHO) definitions of anaemia (Table I), found a significant decrement in the global prevalence of anaemia, which decreased from 40.2% in 1990 to 32.9% in 2010, though the prevalence varied widely across regions1. However, a lower prevalence of mild and moderate anaemia accounted for most of the reduction, while the prevalence of severe anaemia remained largely unchanged1.
Table I.
Haemoglobin levels to diagnose anaemia at sea level*.
| Population | No anaemia* | Anaemia | ||
|---|---|---|---|---|
|
| ||||
| Mild** | Moderate | Severe | ||
| Children 6–59 months of age | ≥11 | 10–10.9 | 7–9.9 | <7 |
| Children 5–11 years of age | ≥11.5 | 11–11.4 | 8–10.9 | <8 |
| Children 12–14 years of age | ≥12 | 11–11.9 | 8–10.9 | <8 |
| Non-pregnant women (15 years of age and above) | ≥12 | 11–11.9 | 8–10.9 | <8 |
| Pregnant women | ≥11 | 10–10.9 | 7–9.9 | <7 |
| Men (15 years of age and above) | ≥13 | 11–12.9 | 8–10.9 | <8 |
Haemoglobin in grams per decilitre;
“Mild” is a misnomer: iron deficiency is already advanced by the time anaemia is detected. The deficiency has consequences even when no anaemia is clinically apparent (WHO/NMH/NHD/MNM/11.1; http://www.who.int/vmnis/indicators/haemoglobin.pdf, accessed on 20/12/2014).
Previously, the third US National Health and Nutrition Examination Survey (NHANES III, Phases 1&2, 1988–1994; 26,372 individuals), showed an average prevalence of anaemia of 7% in the 1- to 64-year old age group, with the prevalence being slightly higher among females2. In people 65 years old or more, the prevalence of anaemia increased progressively with age (13% in subjects aged 75–84, 23% in those over 85 years) and the condition was more common among males2. However, analysing the distribution of haemoglobin (Hb) levels in men and women aged 65 years and older showed that 32.4% of women and 23.3% of men had Hb levels lower than 13 g/dL, indicating that the higher overall prevalence of anaemia among older men just results from the gender-specific WHO definitions of anaemia2. This progressive increase of anaemia prevalence with age was also noted in a meta-analysis of 34 studies (85,409 elderly individuals); the overall prevalence was 17%, but fell to 6% when considering cases with a Hb of ≤11 g/dL, which indicates that anaemia was mild in the majority of cases3.
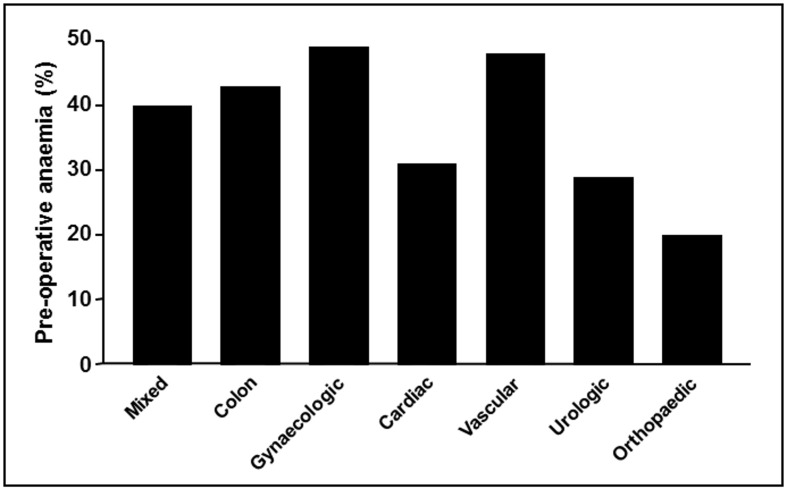
Do these figures of anaemia prevalence in individuals living in the community apply to hospitalised patients? In a cohort investigation of adult patients (n=232,440) hospitalised for surgical or medical pathologies between January 2009 and August 2011, 19% presented with anaemia upon admission, whereas 60% of those who were not anaemic upon presentation developed hospital-acquired anaemia4. Another retrospective study of patients of any age (n=2,234) hospitalised in the departments of digestive diseases, internal medicine, cardiology or respiratory diseases between September and October 2010 found an anaemia prevalence of 50%5. In cancer patients, the European cancer anaemia survey found a prevalence of anaemia (Hb cut-off 12 g/dL) at recruitment which varied between 25% in patients with head and neck cancer and 53% in those with a haematological cancer5. In addition, among patients receiving treatment, the mean anaemia prevalence was 53%, ranging from 29% for those being treated with radiotherapy to 75% for those given cis-platinum based chemotherapy6. In 18 large observational studies encompassing over 650,000 surgical patients, the mean prevalence of pre-operative anaemia was around 35%, varying between 10.5% and 47.9%7–24. There were marked differences in the prevalence of pre-operative anaemia according to the type of surgery (Figure 1), populations of patients and definitions of anaemia, though it was higher than among the general population.
Figure 1.
Prevalence of pre-operative anaemia in patients scheduled for major surgery, according to most frequent procedures (estimated from references7–24).
Causes of pre-operative anaemia
Iron deficiency is the commonest cause of anaemia worldwide, followed by parasitic infestation (hookworm, schistosomiasis, malaria), haemoglobinopathies, obstetric and gynaecological disorders, and anaemia associated with chronic renal failure, though the proportion of cases resulting from specific causes varies widely across regions1.
In regard to anaemia in people 65 years and older, data from the NHANES III (n=2,814,000) showed that, approximately, one-third of cases were caused by chronic inflammation, with or without chronic kidney disease, one-third by nutrient deficiency (iron, folic acid, vitamin B12), and one-third were classified as unexplained anaemia2. Interestingly, unexplained anaemia in older persons is characterised by low erythropoietin and low levels of pro-inflammatory markers (which are also seen in anaemia due to vitamin B12/folate deficiency), and low lymphocyte counts25.
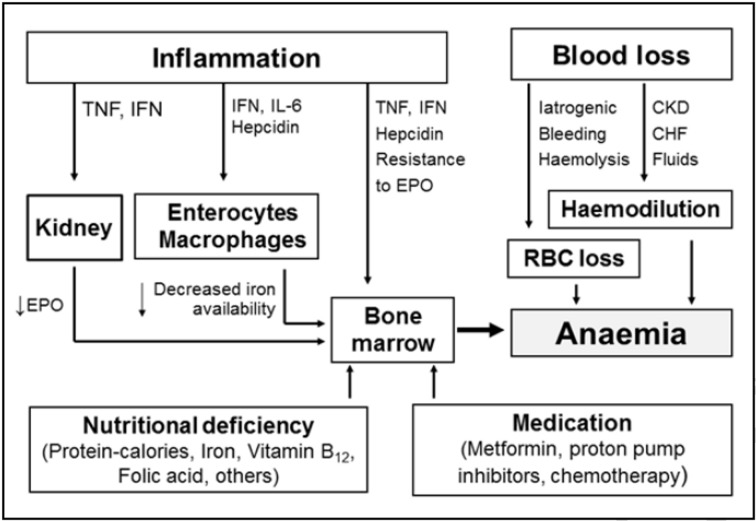
The aetiology of pre-operative anaemia may be multifactorial and complex (Figure 2). Nutritional deficiencies (e.g., iron, folate, vitamin B12, proteins), due to poor nutrition or malabsorption, and some drugs (e.g., inhibitors of angiotensin-converting enzyme, metformin) may contribute to reduced red blood cell (RBC) production. Other causes may be activation of the immune system by underlying processes as well as certain immune and inflammatory cytokines, including tumour necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), and interleukins (IL)-1, -6, -8 and -10. These inflammatory mediators participate in a variety of pathophysiological mechanisms: (i) decreased RBC half-life due to dyserythropoiesis with red cell damage and increased erythrophagocytosis (TNF-α); (ii) inadequate endogenous erythropoietin (EPO) response for the severity of anaemia; (iii) impaired responsiveness of erythroid cells to EPO (IFN-γ, IL-1, and TNF-α); (iv) inhibited proliferation and differentiation of erythroid cells (IFN-γ, IL-1, TNF-α, and a-1-antitrypsin); and (v) pathological iron homeostasis (IFN-g, TNF-a, IL-1, IL-6, IL-10, hepcidin)26,27. Repeated diagnostic phlebotomies, gastrointestinal or genitourinary blood loss, coagulopathies and haemodilution (due to renal failure or congestive cardiac failure) can also contribute significantly to the development of anaemia28.
Figure 2.
Most common causes and pathophysiological mechanisms of pre-operative anaemia.
TNF: tumour necrosis factor; IFN: interferon; IL: interleukin; EPO: erythropoietin; CKD: chronic kidney disease; CHF: chronic heart failure; RBC: red blood cell.
The proportion of cases of pre-operative anaemia resulting from specific causes has not been so extensively studied. In 1,142 consecutive admissions for orthopaedic surgery, 224 (19.6%) patients presented with anaemia, which was normochromic, suggesting anaemia of chronic inflammation, in 135 (64%), hypochromic, suggesting iron deficiency anaemia in 49 (23%), and was attributed to other causes in 26 (13%)13. In another cohort of orthopaedic surgical patients (n=715), the prevalence of anaemia was 10.5%, according to WHO definitions (20% for a Hb cut-off of 13 g/dL)15. The main causes of anaemia were nutrient deficiencies (30.8%) and anaemia of chronic inflammation with or without chronic kidney disease (30.8%), although 38.4% of cases were classified as anaemia of mixed or unexplained origin15. In a series of 437 patients scheduled for curative colorectal cancer resection, 242 (55%) presented with iron deficiency (with or without anaemia), most probably due to chronic blood loss29. However, pre-operative anaemia in colorectal cancer should not only be attributed to chronic haemorrhage, as neoadjuvant chemotherapy or radiotherapy, nutritional deficiencies, and activation of the immune system with release of inflammatory cytokines may also play a role.
Prescribed medical treatment should also be considered as a potential cause of anaemia in surgical patients. In a cohort of cardiac surgical patients (n=576), logistic regression analysis showed that age (odds ratio [OR]: 1.037), chronic kidney disease (OR: 3.161), loop diuretics (OR: 1.504), and proton pump inhibitors or histamine H2 receptor antagonists (OR: 1.531) were independent risk factors for pre-operative anaemia (prevalence 36.5%)24.
It seems that not only the prevalence but also the distribution of specific causes of anaemia among surgical patients vary according to the type of surgery.
Consequences of pre-operative anaemia
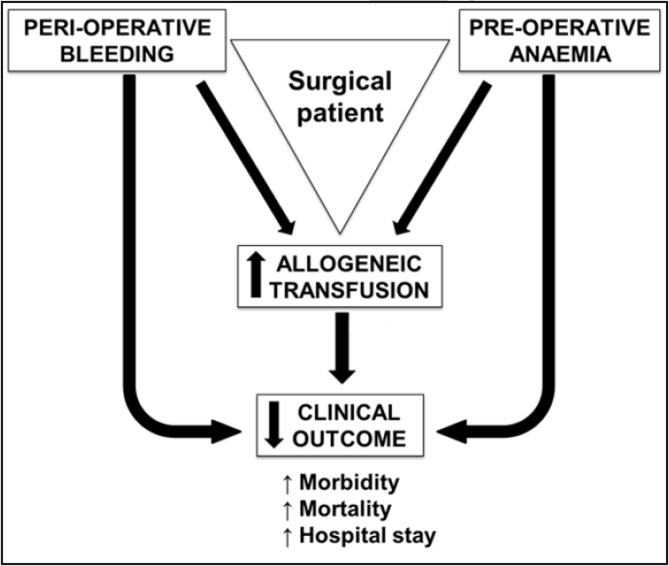
The significant association of pre-operative anaemia with poorer patients’ outcomes (length of hospital stay, post-operative complications and death) (Figure 3) was already described in 1970 by Lunn and Elwood30. However, pre-operative anaemia is frequently not considered a risk factor and it is not, therefore, corrected or improved prior to scheduled surgical procedures associated with possible major bleeding.
Figure 3.
Effects of anaemia, bleeding and allogeneic transfusion on clinical outcome in patients undergoing major surgery.
Generally, the rationale behind this thought is that preoperative anaemia just reflects the severity of the underlying condition that necessitates surgery and will be solved after it. However, several large observational studies, including over 600,000 patients, have confirmed the presence of an association between pre-operative anaemia and poorer post-operative outcomes. After adjusting for a number of potential confounders, using multivariate logistic regression analysis, preoperative anaemia remained an independent risk factor for increased post-operative morbidity and mortality, as well as for prolonged length of hospital stay (LOS)7,9,16,23,31. Moreover, these negative effects were present even for mild anaemia9,16,23. As for cardiac surgery, the incremental value of pre-operative anaemia in risk prediction with the EuroSCORE II model has been recently documented23.
In addition, pre-operative anaemia is a major independent predictive factor for the need of peri-operative allogeneic blood transfusion (ABT) (Figure 3). In major surgery, peri-operative blood loss and blunted post-operative erythropoiesis may lead to acute anaemia, especially in patients presenting with pre-operative anaemia or low Hb levels. ABT is usually given to avoid the detrimental effects of acute anaemia. However, although ABT produces a quick and effective, albeit transitory increase in Hb levels, there is a lack of evidence regarding its efficacy at increasing tissue oxygen consumption or reducing tissue oxygen debt in selected patients, and the use of ABT is frequently associated with poorer outcomes. In critically ill and surgical patients, it was recently found that transfusion of a single unit of packed RBC increased the multivariate risk of mortality, wound problems, pulmonary complications, post-operative renal dysfunction, systemic sepsis, composite morbidity, and prolonged post-operative LOS in comparison to propensity-matched patients who did not receive intra-operative ABT32,33 (Figure 3). Further concerns have been raised that ABT is associated with recurrence in cancer surgery34. Data from an observational cohort of 2,087,423 primary total hip arthroplasties showed that ABT was independently associated with a longer stay in hospital, increased costs and worse surgical and medical outcomes, without affecting in-hospital mortality35. Moreover, the negative effects of blood in loss, ABT and preoperative anaemia are synergistic. A single-centre, retrospective study including 16,154 consecutive adult patients undergoing cardiac surgery (2002–2010) showed that major bleeding is per se a risk factor for 30-day post-operative mortality, which is strongly enhanced by packed RBC transfusions and, to a lesser extent, pre-operative anaemia36 (Figure 3).
On the other hand, although packed RBC come from altruistic blood donations, processing, screening, conservation and distribution costs (acquisition costs), as well as administration costs, are high. The reported costs to buy a unit of packed RBC are quite similar across western countries (Spain, € 155; Switzerland, € 145; Austria, € 115; UK, € 150; USA, € 150–190), whereas the administration costs are more subject to country variations (from € 88 to € 700 per unit)37–39. From a systematic review of the literature it is estimated that the cost of a two-unit packed RBC transfusion in western Europe is around € 80040.
Despite evidence of its clinical and economic disadvantages, ABT continues to be the most frequently used treatment for acute intra- and post-operative anaemia. Two recent large studies showed that up to 30–35% of all units of allogeneic blood are used in the surgical setting41,42, and large inter-centre variability in the percentage of patients who receive ABT when undergoing a particular surgical procedure has also been observed11,12. In order to reduce variability in transfusion practice, both with regards to the proportions of patients receiving ABT and the volumes of blood administered per transfused patient, scientific societies have developed evidence-based guidelines and recommendations on the indications for ABT43–45. The final objective of these guidelines is a more rational, tailored and “restrictive” use of ABT in patients for whom pharmacological options are not available or cannot be implemented (e.g., acute severe anaemia). The implementation of transfusion indicators has also been shown to play a key role in reducing variability in transfusion practice, especially for packed RBC46,47, the percentage of patients transfused, the volume of transfused components, and transfusion-associated complications (e.g., nosocomial infection)48.
Nevertheless, even when pre-operative anaemia is a major risk factor for peri-operative ABT, blood circulating volume and total RBC mass may also play a role. For the same Hb level, female patients have a lower blood circulating volume and lower RBC mass than males. In the Austrian benchmark study, the mean relative peri-operative RBC loss in knee arthroplasty, hip arthroplasty and coronary artery bypass grafting was significantly higher in women than in men, and this was reflected in a significantly higher proportion of female patients receiving ABT11. Similarly, for a given pre-operative Hb level (e.g., 12 g/dL) in patients who underwent lower limb arthroplasty, ABT rates were higher in females (42%) than in males (30%)10.
Therefore, data suggest that WHO definitions of anaemia (Table I) may not be reliable for female patients undergoing major surgical procedures in which significant blood loss is expected, and a higher cut-off level would be desirable (Hb<13 g/dL).
Management of peri-operative anaemia
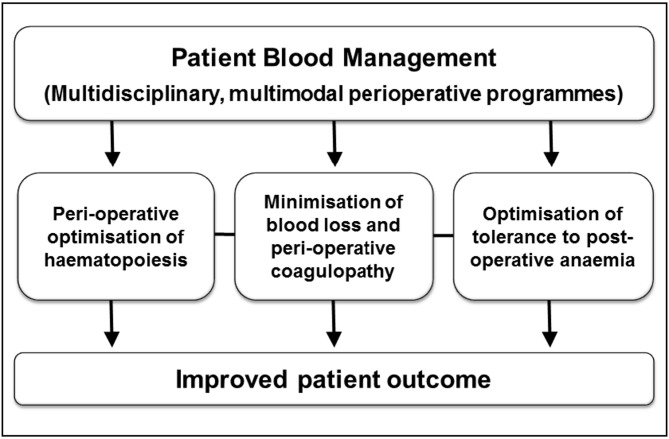
As both pre-operative anaemia and peri-operative ABT have been linked to clinical and economic disadvantages, there is a growing interest in multidisciplinary, multimodal, individualised strategies, collectively termed patient blood management (PBM), aimed at minimising ABT with the ultimate goal of improving patients’ outcomes (Figure 4). This new standard of care relies on detection and treatment of peri-operative anaemia, reduction of surgical blood loss and peri-operative coagulopathy and optimisation of physiological tolerance of anaemia, thus allowing restrictive use of ABT. PBM programmes are now being established for major elective surgery at high risk of bleeding and/or consistent anticipated blood loss in several European countries49,50. We will propose here three different approaches to the management of pre-operative anaemia.
Figure 4.
Key components of a Patient Blood Management programme.
The “orthodox” approach
Several consensus documents have recommended that patients scheduled for major surgery should have a full blood cell count (including reticulocyte count) and iron status (serum iron, ferritin, and transferrin saturation) tested preferably 30 days before the scheduled surgical procedure to allow the detection and classification of anaemia, as well as the implementation of appropriate treatment, if needed and available (grade 1C recommendation)44,45,51,52. The use of easy-to-follow sequential algorithms allows diagnosis of common causes of anaemia, avoids the need for patients to return for another blood test (draw one sample for a complete blood count and a sample for additional testing to “hold” for additional tests if needed), and eliminates unnecessary laboratory studies53. The safety aspects of the different treatment options have been discussed elsewhere54.
The diagnosis of unexpected anaemia in patients undergoing elective surgery, in which significant blood loss is anticipated, should be considered an indication for rescheduling the operation until the evaluation is completed52. Importantly, non-anaemic patients with low ferritin levels undergoing surgical procedures with moderate-to-high blood losses may also benefit from pre-operative iron administration, as they may not have enough stored iron to replenish their peri-operative Hb loss and maintain normal iron stores (grade 1C recommendation)52. Most cases of pre-operative anaemia can be successfully treated with iron and/or erythropoiesis stimulating agents (ESA), whereas the prescription of ABT should be restricted to individuals with severe anaemia, active bleeding and/or poor physiological reserve.
Should the patient present with IDA, if there is enough time and no contraindications, oral iron supplementation could be attempted (grade 2B recommendation)44. Some studies showed that administration of ferrous salts (100–200 mg/day; 2–4 weeks) to patients scheduled for different elective surgical procedures improved Hb levels, reduced transfusion rates and, in some cases, LOS55–59. On the contrary, Lachance et al. showed that it was not able to increase pre-operative Hb in patients scheduled for hip or knee arthroplasty60. Newer oral iron formulations, such as heme iron polypeptide or liposomal iron, seem to offer advantages over the traditional iron salts even in the context of inflammation, although more studies are needed to confirm these promising results61,62.
If there is poor absorption or poor tolerance of oral iron or an accelerated response to treatment is required, pre-operative intravenous iron supplementation should be considered, using one of the several formulations currently available (intramuscular iron administration is no longer recommended) (grade 2B recommendation)44. An intravenous iron course, starting 3–4 weeks prior to the scheduled procedure, increased Hb levels and/or corrected anaemia and reduced ABT requirements in patients presenting with iron deficiency anaemia with or without anaemia of chronic inflammation32,63–68. It is important to stress that administration of intravenous iron alone never results in supra-physiological Hb levels and/or thrombocytosis and will not, therefore, increase the risk for thromboembolic complications.
Should a patient present with anaemia of chronic inflammation and no contraindications, the administration of ESA could be considered, in addition to the treatment of the underlying disease, if possible44,45,52. In Europe, ESA administration is indicated for improving pre-operative Hb levels and reducing ABT rates in patients undergoing elective orthopaedic surgery with moderate anaemia (Hb between 10 and 13 g/dL) and expected to have moderate blood losses, in whom nutritional deficiencies have been ruled out, corrected or both (grade 2A recommendation)44,45,52. A large randomised controlled trial demonstrated that the administration of four doses of ESA (recombinant human erythropoietin [rHuEPO] 40,000 IU plus oral iron), starting 3 weeks prior to the scheduled procedure, significantly decreased ABT rates in patients undergoing lower limb arthroplasty or spinal surgery69. In contrast, in a more recent study with similar design, rHuEPO was found to significantly reduce the number of patients requiring ABT but not the number of ABT units transfused, at an unacceptably high costs (€ 7,300 per avoided transfusion)70, thus questioning the routine use of four rHuEPO doses. In this regard, data from several studies suggest that one or two rHuEPO doses could be sufficient to reach a target Hb level ≥13 g/dL, especially when co-adjuvant intravenous iron is administered65,68,71,72. In this population, it would, therefore, be advisable to adjust ESA dose individually, ensure iron supply to the bone marrow (administering adjuvant iron, preferably intravenously), and provide adequate pharmacological thromboembolic prophylaxis42.
The possible role of off-label use of ESA in cardiac and gastrointestinal cancer surgery has also been explored44. In cardiac surgery it seemed to reduce ABT, although there is some reluctance to use it in patients with coronary artery disease, due to the possible increased risk of ischaemic complications73. Recently, Cladellas et al.74 showed that pre-operative administration of rHuEPO plus intravenous iron to patients undergoing valve surgery not only significantly increased pre-operative Hb levels and reduced ABT requirements, but also decreased post-operative major cardiac events and renal failure, as well as the LOS, when compared with those in a historical control group.
The possible role of ESA in the treatment of gastrointestinal cancer-related anaemia remains unclear, as pooled data from six trials (621 patients) showed that peri-operative treatment with rHuEPO did not reduce ABT (33% vs 37%; OR: 0.89; p=0.206)75. However, reductions of both the percentage of transfused patients and the number of transfused units were observed for those receiving ESA with adjuvant intravenous iron, which allowed for a significant reduction of the total dose of the ESA75. Additionally, a recent systematic review and meta-analysis of four randomised controlled trials found no significant differences in post-operative mortality or thrombotic events between groups, but none of the studies included evaluated cancer recurrences, survival, or quality of life76. Therefore, until more safety data in gastrointestinal cancer-related anaemia are available, ESA should only be used in the approved indications and following the recommendations of international guidelines77.
Finally, as unexplained anaemia and vitamin B12/folate deficiency anaemia in older people are characterised by low levels of EPO and pro-inflammatory markers25, the use of ESA may also considered in this context, when an appropriate haematological response is not attained with specific vitamin supplementation.
The “pragmatic” approach
Anaemia should be viewed as a serious and treatable medical condition, rather than simply as an abnormal laboratory value52,53. Therefore, for adequate risk-stratification and risk reduction, standard pre-operative laboratory testing may not be enough, and additional laboratory parameters may be required in patients undergoing surgical procedures. However, the above described approach to the classification of pre-operative anaemia can be perceived as a discrepancy with the global reduction of pre-operative testing recommended by various guidelines45,78, might negatively affect hospital personnel work-load and might not be cost-effective. Could a more pragmatic approach be implemented?
Cuenca et al.79 assessed the requirements for ABT in 156 consecutive patients undergoing surgery for primary knee arthroplasty, included in a PBM protocol consisting of supplementation with iron ferrous sulphate (256 mg/day; 80 mg of Fe2+), vitamin C (1,000 mg/day), and folic acid (5 mg/day) during the 30–45 days preceding surgery. All patients were managed with using a restrictive transfusion threshold (Hb<8 g/dL and/or clinical signs/symptoms of acute anaemia/hypoxaemia). Compared to a previous series of 156 patients undergoing total knee replacement, who served as a control group, treated patients had a lower transfusion rate (5.8% vs 32%; p<0.01) and a lower transfusion index (1.8 vs 2.2 units per transfused patient; p<0.05). After stratification, 19% of treated patients with a pre-operative Hb<13 g/dL still needed ABT. This protocol does, therefore, seem to be effective for avoiding ABT in non-anaemic patients (highlighting the high prevalence of iron deficiency among non-anaemic patients)14, whereas for anaemic patients additional blood saving strategies, such as pre-operative intravenous iron or rHuEPO, should be added to increase its effectiveness further.
Recently, Theusinger et al.80 reported on the results of a pragmatic approach to PBM in major orthopaedic surgery, which was successfully implemented at the Balgrist University Hospital in Zurich (2009–2011; n=6,721). One fundamental pillar of their PBM programme was the detection of pre-operative anaemia by contacting the patients’ general practitioners upon patients presenting to the anaesthesiologist, at least 4 weeks prior to surgery. This emphasises the overwhelming importance of enhancing communication/collaboration between primary health care and specialised medical attention, by unifying and sharing all patients’ electronic records, and by reducing the high rate of patients not presenting to the anaesthesiologist or presenting too late (70% of patients).
As the most common types of anaemia among the orthopaedic surgical populations are iron-deficiency anaemia and anaemia of chronic inflammation, if there were no contraindications, patients presenting with Hb <13 g/dL were initially treated with 1,000 mg intravenous iron and 40,000 IU rHuEPO subcutaneously (approved rHuEPO indication). They also received pre-operative supplementation with vitamin B12 sc (1 mg) plus oral folic acid (5 mg/day) to treat or prevent any functional or absolute deficiency. In fact, especially in older patients, erythropoietic activity may need to be stimulated with rHuEPO14 and vitamin B12 and folate levels may not be routinely measured. After 14 days, patients whose Hb remained <13 g/dL received a second dose of intravenous iron, rHuEPO and vitamin B12. All treated patients presented with normal Hb levels (≥13 g/dL) on the day of surgery. The PBM programme also included meticulous surgical technique, optimal surgical blood-saving techniques (cell salvage and/or topical haemostatic agents), and standardised transfusion triggers. Overall, in comparison with the immediately preceding year (2008, n=2,150), this PBM programme was associated with a lower incidence of anaemia on the day of surgery, lower transfusion rate, and lower transfusion volume per transfused patient80. Thus, although further refinements are needed, this well-defined PBM programme seems to be in the right direction for fulfilling the aim of performing major surgical orthopaedic procedures without the use of ABT and without placing the patient at risk of complications. Whether this protocol could apply to other surgical procedures deserves further research.
The “opportunity” approach
What can we do when the recommended time-frame for detection, evaluation and treatment of pre-operative anaemia is not available, such as in case of non-elective surgery or in patients presenting just a few days before a scheduled procedure? Could peri-operative treatment with intravenous iron and/or rHuEPO be useful to improve peri-operative anaemia, hasten the recovery of post-operative anaemia and reduce transfusion requirements in patients undergoing elective and non-elective major surgery?51
A recent study pooled data on ABT, post-operative nosocomial infection, 30-day mortality and LOS from 2,547 patients undergoing elective lower limb arthroplasty or hip fracture repair81. Comparisons were made between patients who received very short-term peri-operative intravenous iron treatment (200–600 mg; n=1,538), with or without rHuEPO (40,000 IU), or standard treatment (n=1,009)81. In hip fracture patients (n=1,361), peri-operative intravenous iron significantly reduced the rates of ABT, post-operative nosocomial infection (10.7% vs 26.9%; p=0.001) and 30-day mortality (4.8% vs 9.4%; p=0.003), and shortened LOS (11.9 days vs 13.4 days; p=0.001) when compared to standard therapy79. These benefits were observed in both transfused and non-transfused patients, and have been corroborated, at least in part, by other authors82,83. It is important to recall that pre-operative rHuEPO was only administered to 351 out of 1,059 patients presenting with Hb ≤13 g/dL and no contraindication. Appropriate training, education, and awareness among the medical staff and nurses would be useful in increasing adherence to PBM, thus limiting the exposure of anaemic patients to ABT and ABT-related risks.
In patients undergoing elective arthroplasty (n=1,186), intravenous iron reduced ABT rates (8.9% vs 30.1%; p=0.001) and LOS (8.4 days vs 10.7 days; p=0.001), without causing differences in post-operative nosocomial infection rates (2.8% vs 3.7%; p=0.417), and there was no effect on 30-day mortality81. Similar results have been reported for patients undergoing cardiac procedures and receiving rHuEPO, with or without intravenous iron, shortly before surgery84–87.
Although large, prospective confirmatory studies are needed, these results suggest that very short-term peri-operative administration of intravenous iron and/or rHuEPO in patients undergoing major surgical procedures is associated with reduced ABT rates and LOS, without increasing post-operative morbidity or mortality (grade 2B recommendation)44,51. It does, therefore, seem that any time is a good time to take the opportunity of treating pre-operative anaemia.
The Authors’ perspective
From an analysis of the available information and the recommendations issued by several consensus documents, it seems fair to conclude that:
Pre-operative anaemia, which is more frequent in patients undergoing major elective surgical procedures than the general population, has been linked to increased rates of post-operative morbidity and mortality, as well as to longer stays in hospital and higher 30-day mortality. However, it is not clear whether anaemia is a modifiable risk factor for poorer outcomes and not simply a marker of other conditions that confer increased risk.
Pre-operative anaemia or suboptimal pre-operative Hb level (<13 g/dL) is one of the stronger predictors of the need for peri-operative ABT, which in turn is also associated with increased rates of postoperative morbidity and mortality and longer stays in hospital.
Whenever feasible, pre-operative anaemia should be detected and classified at least 4 weeks prior to the scheduled procedure, and appropriate pharmacological treatment implemented, if possible. ABT should be reserved for patients with severe anaemia, ongoing bleeding and/or poor physiological reserve (orthodox approach).
As the most common types of anaemia among surgical populations are iron-deficiency anaemia and anaemia of chronic inflammation, patients presenting with Hb<13 g/dL could be initially treated with intravenous iron and subcutaneous rHuEPO, adjusting dosages after 2 weeks according to the haematological response. Routine folic acid (5 mg/day, oral) and vitamin B12 (1 mg intramuscular) supplementation could be considered to prevent functional or absolute deficiency of these vitamins (pragmatic approach).
For patients undergoing non-elective procedures or presenting shortly before an operation, current evidence broadly supports treatment with intravenous iron and/or ESA to reduce ABT rates. In addition, the acceptable safety profile of this treatment and its ability to be administered without delaying surgery seems to further support its clinical use (opportunity approach).
Finally, it must be borne in mind that the aim of performing major surgical procedures without the use of ABT and without placing the patient at risk of complications may be better accomplished by combining several blood conservation strategies into a defined PBM algorithm, in which management of pre-operative anaemia is central.
Footnotes
Disclosure of conflicts of interest
Manuel Muñoz has received honoraria for consultancy or lectures and/or travel support from Wellspect HealthCare (Sweden), Roche (Spain), Vifor Pharma (Spain and Switzerland), PharmaCosmos (Denmark) and Zambon (Spain) but not for this work. Susana Gómez-Ramírez, Arturo Campos, Joaquín Ruiz, and Giancarlo M. Liumbruno have nothing to declare.
References
- 1.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–24. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 3.Gaskell H, Derry S, Andrew Moore R, McQuay HJ. Prevalence of anaemia in older persons: systematic review. BMC Geriatr. 2008;8:1. doi: 10.1186/1471-2318-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch CG, Li L, Sun Z, et al. From bad to worse: anemia on admission and hospital-acquired anemia. J Patient Saf. 2014 doi: 10.1097/PTS.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz M, Gómez-Ramírez S, Cobos A, Enguix A. Prevalence and severity of anaemia among medical patients from different hospital departments. Transfus Alternat Transfus Med. 2011;12:30. [Google Scholar]
- 6.Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40:2293–306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297:2481–8. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 8.Beattie WS, Karkouti K, Wijeysundera DN, Tait G. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology. 2009;110:574–81. doi: 10.1097/ALN.0b013e31819878d3. [DOI] [PubMed] [Google Scholar]
- 9.Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378:1396–407. doi: 10.1016/S0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- 10.Rosencher N, Kerkkamp HE, Macheras G, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43:459–69. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 11.Gombotz H, Rehak PH, Shander A, Hofmann A. Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47:1468–80. doi: 10.1111/j.1537-2995.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 12.Gombotz H, Rehak PH, Shander A, Hofmann A. The second Austrian benchmark study for blood use in elective surgery: results and practice change. Transfusion. 2014;54:2646–57. doi: 10.1111/trf.12687. [DOI] [PubMed] [Google Scholar]
- 13.Saleh E, McClelland DB, Hay A, et al. Prevalence of anaemia before major joint arthroplasty and the potential impact of preoperative investigation and correction on perioperative blood transfusions. Br J Anaesth. 2007;99:801–8. doi: 10.1093/bja/aem299. [DOI] [PubMed] [Google Scholar]
- 14.Bisbe E, Castillo J, Sáez M, et al. Prevalence of preoperative anemia and hematinic deficiencies in patients scheduled for elective major orthopedic surgery. Transfus Alternat Transfus Med. 2008;10:166–73. [Google Scholar]
- 15.Jans Ø, Jorgensen C, Kehlet H, Johansson PI. Role of preoperative anemia for risk of transfusion and postoperative morbidity in fast-track hip and knee arthroplasty. Transfusion. 2014;54:717–26. doi: 10.1111/trf.12332. [DOI] [PubMed] [Google Scholar]
- 16.Leichtle SW, Mouawad NJ, Lampman R, et al. Does preoperative anemia adversely affect colon and rectal surgery outcomes? J Am Coll Surg. 2011;212:187–94. doi: 10.1016/j.jamcollsurg.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Gupta PK, Sundaram A, Mactaggart JN, et al. Preoperative anemia is an independent predictor of postoperative mortality and adverse cardiac events in elderly patients undergoing elective vascular operations. Ann Surg. 2013;258:1096–102. doi: 10.1097/SLA.0b013e318288e957. [DOI] [PubMed] [Google Scholar]
- 18.David O, Sinha R, Robinson K, Cardone D. The prevalence of anaemia, hypochromia and microcytosis in preoperative cardiac surgical patients. Anaesth Intensive Care. 2013;41:316–21. doi: 10.1177/0310057X1304100307. [DOI] [PubMed] [Google Scholar]
- 19.Elmistekawy E, Rubens F, Hudson C, et al. Preoperative anaemia is a risk factor for mortality and morbidity following aortic valve surgery. Eur J Cardiothorac Surg. 2013;44:1051–5. doi: 10.1093/ejcts/ezt143. [DOI] [PubMed] [Google Scholar]
- 20.Karkouti K, Wijeysundera DN, Beattie WS. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation. 2008;117:478–84. doi: 10.1161/CIRCULATIONAHA.107.718353. [DOI] [PubMed] [Google Scholar]
- 21.Kim C, Connell H, McGeorge A, Hu R. Prevalence of preoperative anaemia in patients having first-time cardiac surgery and its impact on clinical outcome. A retrospective observational study. Perfusion. 2015;30:277–83. doi: 10.1177/0267659114542457. [DOI] [PubMed] [Google Scholar]
- 22.Kulier A, Levin J, Moser R, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116:471–9. doi: 10.1161/CIRCULATIONAHA.106.653501. [DOI] [PubMed] [Google Scholar]
- 23.Scrascia G, Guida P, Caparrotti SM, et al. Incremental value of anemia in cardiac surgical risk prediction with the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II model. Ann Thorac Surg. 2014;98:869–75. doi: 10.1016/j.athoracsur.2014.04.124. [DOI] [PubMed] [Google Scholar]
- 24.Muñoz M, Ariza D, Gómez-Ramírez S, et al. Preoperative anemia in elective cardiac surgery: prevalence, risk factors, and influence on postoperative outcome. Transfus Alternat Transfus Med. 2010;11:47–56. [Google Scholar]
- 25.Ferrucci L, Guralnik JM, Bandinelli S, et al. Unexplained anaemia in older persons is characterised by low erythropoietin and low levels of pro-inflammatory markers. Br J Haematol. 2007;136:849–55. doi: 10.1111/j.1365-2141.2007.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part 1: Molecular basis of iron homeostasis. J Clin Pathol. 2011;64:281–6. doi: 10.1136/jcp.2010.079046. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part II: Iron deficiency and iron overload. J Clin Pathol. 2011;64:287–96. doi: 10.1136/jcp.2010.086991. [DOI] [PubMed] [Google Scholar]
- 28.Muñoz M, García-Erce JA, Leal-Noval SR. Is there still a role for recombinant erythropoietin in the management of anemia of critical illness? Med Clin (Barc) 2009;132:749–55. doi: 10.1016/j.medcli.2008.11.019. [In Spanish] [DOI] [PubMed] [Google Scholar]
- 29.Díaz Espallardo C, Laso Morales MJ, Colilles Calvet C, et al. The multidisciplinary approach is useful for optimising preoperative haemoglobin in colorectal cancer surgery. Cir Esp. 2011;89:392–9. doi: 10.1016/j.ciresp.2011.01.013. [In Spanish] [DOI] [PubMed] [Google Scholar]
- 30.Lunn JN, Elwood OC. Anaemia and surgery. BMJ. 1970;3:71–3. doi: 10.1136/bmj.3.5714.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranucci M, Di Dedda U, Castelvecchio S, et al. Surgical and Clinical Outcome Research (SCORE) Group. Impact of preoperative anemia on outcome in adult cardiac surgery: a propensity-matched analysis. Ann Thorac Surg. 2012;94:1134–41. doi: 10.1016/j.athoracsur.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 32.Leal-Noval SR, Muñoz-Gómez M, Jiménez-Sánchez M, et al. Red blood cell transfusion in non-bleeding critically ill patients with moderate anemia: is there a benefit? Intensive Care Med. 2013;39:45–53. doi: 10.1007/s00134-012-2757-z. [DOI] [PubMed] [Google Scholar]
- 33.Ferraris VA, Davenport DL, Saha SP, et al. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg. 2012;147:49–55. doi: 10.1001/archsurg.2011.790. [DOI] [PubMed] [Google Scholar]
- 34.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery - systematic review and meta-analysis. Ann Surg. 2012;256:235–44. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 35.Saleh A, Small T, Chandran Pillai AL, et al. Allogenic blood transfusion following total hip arthroplasty: results from the nationwide inpatient sample, 2000 to 2009. J Bone Joint Surg Am. 2014;96:e155. doi: 10.2106/JBJS.M.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranucci M, Baryshnikova E, Castelvecchio S, Pelissero G Surgical and Clinical Outcome Research (SCORE) Group. Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Ann Thorac Surg. 2013;96:478–85. doi: 10.1016/j.athoracsur.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Muñoz M, Ariza D, Campos A, et al. The cost of post-operative shed blood salvage after total knee arthroplasty: an analysis of 1,093 consecutive procedures. Blood Transfus. 2013;11:260–71. doi: 10.2450/2012.0139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 39.Toner RW, Pizzi L, Leas B, et al. Costs to hospitals of acquiring and processing blood in the US: a survey of hospital-based blood banks and transfusion services. Appl Health Econ Health Policy. 2011;9:29–37. doi: 10.2165/11530740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Abraham I, Sun D. The cost of blood transfusion in Western Europe as estimated from six studies. Transfusion. 2012;52:1983–8. doi: 10.1111/j.1537-2995.2011.03532.x. [DOI] [PubMed] [Google Scholar]
- 41.Norgaard A, De Lichtenberg TH, Nielsen J, Johansson PI. Monitoring compliance with transfusion guidelines in hospital departments by electronic data capture. Blood Transfus. 2014;12:509–19. doi: 10.2450/2014.0282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion. 2014;54:2678–86. doi: 10.1111/trf.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 44.Leal-Noval SR, Muñoz M, Asuero M, et al. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: the 2013 update of the “Seville Document”. Blood Transfus. 2013;11:585–610. doi: 10.2450/2013.0029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30:270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]
- 46.Leal-Noval SR, Arellano-Orden V, Maestre-Romero A, et al. Impact of national transfusion indicators on appropriate blood usage in critically ill patients. Transfusion. 2011;51:1957–65. doi: 10.1111/j.1537-2995.2011.03091.x. [DOI] [PubMed] [Google Scholar]
- 47.Goodnough LT. Trends in blood utilization. Transfus Med. 2014;24:2. [Google Scholar]
- 48.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317–26. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shander A, Van Aken H, Colomina MJ, et al. Patient blood management in Europe. Br J Anaesth. 2012;109:55–68. doi: 10.1093/bja/aes139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Assembly. WHA 63.12 (resolution) Availability, safety and quality of blood products. 2010. [Accessed on 27/08/2013]. Available at: http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R12-en.pdf.
- 51.Beris P, Muñoz M, García-Erce JA, et al. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100:599–604. doi: 10.1093/bja/aen054. [DOI] [PubMed] [Google Scholar]
- 52.Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Society for the Advancement of Blood Management (SABM) Anemia in the pre-surgical patient. Recognition, diagnosis, and management. [Accessed on 12/02/2015]. Available at: http://www.sabm.org/publications.
- 54.Muñoz M, Gómez-Ramirez S, Campos A. Iron supplementation for perioperative anaemia in patient blood management. EMJ Hema. 2014;1:123–132. [Google Scholar]
- 55.Hallet J, Hanif A, Callum J, et al. The impact of perioperative iron on the use of red blood cell transfusions in gastrointestinal surgery: a systematic review and meta-analysis. Transfus Med Rev. 2014;28:205–11. doi: 10.1016/j.tmrv.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Rogers BA, Cowie A, Alcock C, Rosson JW. Identification and treatment of anaemia in patients awaiting hip replacement. Ann R Coll Surg Engl. 2008;90:504–7. doi: 10.1308/003588408X301163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lidder PG, Sanders G, Whitehead E, et al. Pre-operative oral iron supplementation reduces blood transfusion in colorectal surgery - a prospective, randomised, controlled trial. Ann R Coll Surg Engl. 2007;89:418–21. doi: 10.1308/003588407X183364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okuyama M, Ikeda K, Shibata T, et al. Preoperative iron supplementation and intraoperative transfusion during colorectal cancer surgery. Surg Today. 2005;35:36–40. doi: 10.1007/s00595-004-2888-0. [DOI] [PubMed] [Google Scholar]
- 59.Quinn M, Drummond RJ, Ross F, et al. Short course pre-operative ferrous sulphate supplementation – is it worthwhile in patients with colorectal cancer? Ann R Coll Surg Engl. 2010;92:569–72. doi: 10.1308/003588410X12699663904277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lachance K, Savoie M, Bernard M, et al. Oral ferrous sulfate does not increase preoperative hemoglobin in patients scheduled for hip or knee arthroplasty. Ann Pharmacother. 2011;45:764–70. doi: 10.1345/aph.1P757. [DOI] [PubMed] [Google Scholar]
- 61.Nagaraju SP, Cohn A, Akbari A, et al. Heme iron polypeptide for the treatment of iron deficiency anemia in non-dialysis chronic kidney disease patients: a randomized controlled trial. BMC Nephrol. 2013;14:64. doi: 10.1186/1471-2369-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pisani A, Riccio E, Sabbatini M, et al. Effect of oral liposomal iron versus intravenous iron for the treatment of iron deficiency anaemia in CKD patients: a randomized trial. Nephrol Dial Transplant. 2015;30:645–52. doi: 10.1093/ndt/gfu357. [DOI] [PubMed] [Google Scholar]
- 63.Theusinger OM, Leyvraz PF, Schanz U, et al. Treatment of iron deficiency anemia in orthopedic surgery with intravenous iron: efficacy and limits: a prospective study. Anesthesiology. 2007;107:923–7. doi: 10.1097/01.anes.0000291441.10704.82. [DOI] [PubMed] [Google Scholar]
- 64.Díez-Lobo AI, Fisac-Martín MP, Bermejo-Aycar I, Muñoz M. Preoperative intravenous iron administration corrects anemia and reduces transfusion requirement in women undergoing abdominal hysterectomy. Transfus Alternatives Transfus Med. 2007;9:114–9. [Google Scholar]
- 65.Gonzalez-Porras JR, Colado E, Conde MP, et al. An individualized pre-operative blood saving protocol can increase pre-operative haemoglobin levels and reduce the need for transfusion in elective total hip or knee arthroplasty. Transfus Med. 2009;19:35–42. doi: 10.1111/j.1365-3148.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- 66.Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M Anaemia Working Group España. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011;107:477–8. doi: 10.1093/bja/aer242. [DOI] [PubMed] [Google Scholar]
- 67.Muñoz M, Campos A, García-Erce JA. Intravenous iron in colo-rectal cancer surgery. Semin Hematol. 2006;34(Suppl 6):S36–8. [Google Scholar]
- 68.Basora M, Colomina MJ, Tio M, et al. Optimizing preoperative haemoglobin with intravenous iron. Br J Anaesth. 2013;110:488–90. doi: 10.1093/bja/aes587. [DOI] [PubMed] [Google Scholar]
- 69.Weber EW, Slappendel R, Hémon Y, et al. Effects of epoetin alfa on blood transfusions and postoperative recovery in orthopaedic surgery: the European Epoetin Alfa Surgery Trial (EEST) Eur J Anaesthesiol. 2005;22:249–57. doi: 10.1017/s0265021505000426. [DOI] [PubMed] [Google Scholar]
- 70.So-Osman C, Nelissen RG, Koopman-van Gemert AW, et al. Patient blood management in elective total hip- and knee-replacement surgery (Part 1): a randomized controlled trial on erythropoietin and blood salvage as transfusion alternatives using a restrictive transfusion policy in erythropoietin-eligible patients. Anesthesiology. 2014;120:839–51. doi: 10.1097/ALN.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 71.Karkouti K, McCluskey SA, Evans L, et al. Erythropoietin is an effective clinical modality for reducing RBC transfusion in joint surgery. Can J Anesth. 2005;52:363–8. doi: 10.1007/BF03016277. [DOI] [PubMed] [Google Scholar]
- 72.Rosencher N, Poisson D, Albi A, et al. Two injections of erythropoietin correct moderate anemia in most patients awaiting orthopedic surgery. Can J Anesth. 2005;52:160–5. doi: 10.1007/BF03027722. [DOI] [PubMed] [Google Scholar]
- 73.Alghamdi AA, Albanna MJ, Guru V, Brister SJ. Does the use of erythropoietin reduce the risk of exposure to allogeneic blood transfusion in cardiac surgery? A systematic review and meta-analysis. J Card Surg. 2006;21:320–6. doi: 10.1111/j.1540-8191.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- 74.Cladellas M, Farré N, Comín-Colet J, et al. Effects of preoperative intravenous erythropoietin plus iron on outcome in anemic patients after cardiac valve replacement. Am J Cardiol. 2012;110:1021–6. doi: 10.1016/j.amjcard.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 75.Muñoz M, Gómez-Ramírez S, Martín-Montañez E, Auerbach M. Perioperative anemia management in colorectal cancer patients: a pragmatic approach. World J Gastroenterol. 2014;20:1972–85. doi: 10.3748/wjg.v20.i8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Devon KM, McLeod RS. Pre and peri-operative erythropoietin for reducing allogeneic blood transfusions in colorectal cancer surgery. Cochrane Database Syst Rev. 2009:CD007148. doi: 10.1002/14651858.CD007148.pub2. [DOI] [PubMed] [Google Scholar]
- 77.Bennett CL, Spiegel DM, Macdougall IC, et al. A review of safety, efficacy, and utilization of erythropoietin, darbepoetin, and peginesatide for patients with cancer or chronic kidney disease: a report from the Southern Network on Adverse Reactions (SONAR) Semin Thromb Hemost. 2012;38:783–96. doi: 10.1055/s-0032-1328884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Society of Thoracic Surgeons Blood Conservation Guideline Task Force; Ferraris VA, Ferraris SP, Saha SP, et al. Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion. Spiess BD, Shore-Lesserson L, Stafford-Smith M, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83(Suppl 5):S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 79.Cuenca J, Garcia-Erce JA, Martinez F, et al. Preoperative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Int J Surg. 2007;5:89–94. doi: 10.1016/j.ijsu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Theusinger OM, Kind SL, Seifert B, et al. Patient Blood Management in Orthopaedic surgery – a four year follow up from 2008 to 2011 at the Balgrist University Hospital in Zurich, Switzerland on transfusion requirements and blood loss. Blood Transfus. 2014;12:195–203. doi: 10.2450/2014.0306-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muñoz M, Gómez-Ramírez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54:289–99. doi: 10.1111/trf.12195. [DOI] [PubMed] [Google Scholar]
- 82.Serrano-Trenas JA, Ugalde PF, Cabello LM, et al. Role of perioperative intravenous iron therapy in elderly hip fracture patients: a single-center randomized controlled trial. Transfusion. 2011;51:97–104. doi: 10.1111/j.1537-2995.2010.02769.x. [DOI] [PubMed] [Google Scholar]
- 83.Kateros K, Sakellariou VI, Sofianos IP, Papagelopoulos PJ. Epoetin alfa reduces blood transfusion requirements in patients with intertrochanteric fracture. J Crit Care. 2010;25:348–53. doi: 10.1016/j.jcrc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 84.Ootaki Y, Yamaguchi M, Yoshimura N, et al. The efficacy of preoperative administration of a single dose of recombinant human erythropoietin in pediatric cardiac surgery. Heart Surg Forum. 2007;10:E115–9. doi: 10.1532/HSF98.20061183. [DOI] [PubMed] [Google Scholar]
- 85.Weltert L, D’Alessandro S, Nardella S, et al. Preoperative very short-term, high-dose erythropoietin administration diminishes blood transfusion rate in off-pump coronary artery bypass: a randomized blind controlled study. J Thorac Cardiovasc Surg. 2010;139:621–6. doi: 10.1016/j.jtcvs.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 86.Yoo YC, Shim KK, Kim JC, et al. Effect of single recombinant human erythropoietin injection on transfusion requirements in preoperatively anemic patients undergoing valvular heart surgery. Anesthesiology. 2011;115:929–37. doi: 10.1097/ALN.0b013e318232004b. [DOI] [PubMed] [Google Scholar]
- 87.Weltert L, Rondinelli B, Bello R, et al. A single dose of erythropoietin reduces perioperative transfusions in cardiac surgery: results of a prospective single-blind randomized controlled trial. Transfusion. 2015 doi: 10.1111/trf.13027. [DOI] [PubMed] [Google Scholar]