Abstract
Background
There are ABO antigens on the surface of platelets, but whether ABO compatible platelets are necessary for transfusions is a matter of ongoing debate. We retrospectively reviewed the ABO matching of platelet transfusions in a subset of patients undergoing autologous haematopoietic progenitor cell transplantation during a 14-year period. Our aim was to analyse the characteristics and outcomes of patients who received platelet transfusions that were or were not ABO identical.
Material and methods
We analysed 529 consecutive patients with various haematological and non-haematological diseases who underwent 553 autologous progenitor stem cell transplants at the University Hospital la Fe between January 2000 and December 2013. We retrospectively analysed and compared transfusion and clinical outcomes of patients according to the ABO match of the platelet transfusions received. The period analysed was the time from transplantation until discharge.
Results
The patients received a total of 2,772 platelet concentrates, of which 2,053 (74.0%) were ABO identical and 719 (26.0%) ABO non-identical; of these latter 309 were compatible and 410 incompatible with the patients’ plasma. Considering all transplants, 36 (6.5%) did not require any platelet transfusions, while in 246 (44.5%) cases, the patients were exclusively transfused with ABO identical platelets and in 47 (8.5%) cases they received only ABO non-identical platelet transfusions. The group of patients who received both ABO identical and ABO non-identical platelet transfusions had higher transfusion needs and worse clinical outcomes compared to patients who received only ABO identical or ABO non-identical platelets.
Discussion
In our hospital, patients undergoing autologous haematopoietic stem cell transplantation who received ABO identical or ABO non-identical platelet transfusions had similar transfusion and clinical outcomes. The isolated fact of receiving ABO non-identical platelets did not influence morbidity or survival.
Keywords: platelet transfusion, autologous haematopoietic stem cell transplantation, transfusion outcome
Introduction
Platelet transfusions are an important therapy for a large number of haemato-oncological diseases, but are also a valuable resource of limited availability and their use should be optimised1. It has been demonstrated that there are ABO antigens on the surface of platelets, but the need for ABO compatibility in platelet transfusions remains a controversial issue and one that continues to be subject of debate2,3. A survey of a large number of North American laboratories showed that 17% of transfusion services did not have a clear policy regarding the use of ABO non-identical platelets4. Although transfusion policy varies between centres, platelet transfusions are usually given across the ABO barrier. This strategy has some clear advantages, such as greater availability and better response in emergency situations, avoiding wastage of platelets. However, some studies have demonstrated higher post-transfusion platelet increments after ABO identical platelet transfusions, supporting the practice of platelet matching5. The clinical significance of the greater platelet increment has not been elucidated6. In some reports, the transfusion of ABO-minor incompatible platelets has been associated with acute haemolytic transfusion reactions due to the exposure of recipients to ABO-incompatible plasma containing anti-A or anti-B isoagglutinins7. In order to prevent this and other adverse effects, many transfusion centres suspend platelets in additive solutions that significantly reduce the quantity of plasma8. Despite these considerations, there is little information about the relevance of ABO matching in platelet transfusions in current clinical practise.
We retrospectively reviewed the ABO matching of platelet transfusions in a subset of patients undergoing autologous haematopoietic progenitor cell transplantation (APCT) during a 14-year period. Our objective was to analyse the characteristics and outcomes of patients who received platelet transfusions that were or were not ABO identical.
Material and methods
Patients
We analysed 529 consecutive patients with various haematological and non-haematological diseases who underwent 553 APCT in the period from January 2000 until December 2013 at the University Hospital la Fe. The patients’ characteristics are shown in Table I.
Table I.
Characteristics of the patients divided according to whether they received ABO identical platelet transfusions, ABO non-identical platelets or both ABO identical and ABO non-identical platelet transfusions.
Results are shown as mean±SD. Differences in variables between the ABO identical and ABO non-identical groups are not statistically significant.
| Characteristics | Subsets of patients who received platelets | p | ||
|---|---|---|---|---|
|
| ||||
| ABO identical | ABO non-identical | Both | ||
| N. of patients/transplants | 238/246 | 45/47 | 246/260 | |
|
| ||||
| Sex | ||||
| Male/female | 129/113 | 27/18 | 153/107 | 0.238 |
|
| ||||
| Age (years) | 51.2±13.3 | 48.9±13.3 | 50.7±12.6 | 0.402 |
|
| ||||
| N. of transplants | ||||
| 1 | 234 | 45 | 241 | |
| 2 | 8 | 2 | 14 | 0.503 |
|
| ||||
| ABO blood group | 9 | |||
| O | 99 | 6 | 5 | |
| A | 129 | 21 | 119 | |
| B | 7 | 13 | 25 | |
| AB | 3 | 5 | 7 | <0.001 |
|
| ||||
| Diagnosis | ||||
| AML | 13 | 3 | 56 | |
| Lymphoma | 77 | 15 | 85 | |
| MM | 99 | 19 | 66 | |
| MS | 16 | 1 | 7 | |
| Other* | 33 | 6 | 32 | <0.001 |
|
| ||||
| Positive indirect antiglobulin test | 4 | 2 | 7 | 0.518 |
|
| ||||
| Days until neutrophils >1×109/L | 12.9±4.8 | 13.2±2.4 | 14.5±9.6 | 0.006 |
|
| ||||
| Days until platelets* >20×109/L | 17.3±15.6 | 19.6 ± 22.8 | 23.3±20.4 | <0.001 |
|
| ||||
| Fever up to 30 days after APCT | ||||
| Yes | 60 | 9 | 50 | |
| No | 161 | 33 | 179 | 0.117 |
|
| ||||
| Days in hospital | 26.3±6.7 | 26.7±5.7 | 31.5±14.7 | <0.001 |
|
| ||||
| Follow-up (days) | 1,071.4 ±1,176.3 | 1,662.1 ±1,673.7 | 1,006.9 ±1,174.2 | 0.059 |
|
| ||||
| Status 90 days after APCT | ||||
| Death | 7 | 0 | 23 | |
| Alive | 219 | 47 | 215 | 0.003 |
AML: acute myeloid leukaemia, MM: multiple myeloma, MS: multiple sclerosis.
acute lymphoblastic leukaemia, aplastic anaemia, chronic myeloid leukaemia, myelodysplastic syndrome.
APCT: autologous haematopoietic progenitor cell transplantation.
Transfusion policy
The policy regarding platelet transfusions in our centre followed the Standards developed by the Spanish Society of Blood Transfusion (SETS)9 and was based on international guidelines. The policy can be summarised as follows: (i) the threshold for prophylactic transfusion was a pre-transfusion platelet count ≤10×109/L. If the patient had a fever ≥38.5 ºC, sepsis, mucositis ≥ grade 2, uncontrolled high pressure, coagulopathy or acute promyelocytic leukaemia, the threshold was increased to a pre-transfusion platelet count ≤20×109/L; (ii) the threshold for platelet transfusions with the aim of controlling bleeding or increasing the platelet count before an invasive procedure was <50×109/L.A standard dose was considered to be one platelet concentrate, composed of platelets pooled from various buffy coats. Platelets for buffy coats were prepared from four to five donors per unit and suspended in platelet additive solution making up to two-thirds of the final volume10,11. Red blood cells were transfused if haemoglobin levels were <80 g/dL. All blood components were leucocyte-reduced and irradiated with 25 Gray. In our Institution, ABO non-identical platelets can be transfused if ABO identical products are not available.
ABO and Rh typing and the indirect antiglobulin test were performed in the automated system ORTHO Autovue Innova (Ortho Clinical Diagnostics, Inc., Raritan, NJ, USA). The specificity of sera of patients with positive tests was investigated using panels of red blood cells with known antigens (Ortho Clinical Diagnostics Inc.; Bio-Rad GmbH, Cressier-sur-Morat, Switzerland; Sanquin, Amsterdam, The Netherlands).
We retrospectively analysed and compared transfusion and clinical outcomes of patients according to the ABO match of the platelet transfusions administered: ABO identical, ABO non-identical, or both ABO identical and ABO non-identical. The period analysed was the time from transplantation until discharge.
Statistics
Computer software (SPSS, release 13.0, IBM Corporation, Armonk, NY, USA) was used to perform the statistical analyses. Descriptive statistics are presented for variables. Results are expressed as mean ± standard deviation (SD) or median and range for continuous variables and as numbers with percentages for categorical variables. The Kolmogorov-Smirnov test was employed to investigate the normality distribution of the variables. Categorical variables were compared by means of the chi square test or Fisher’s exact test. The Mann-Whitney U-test and Kruskall-Wallis test for continuous variables were used to compare the groups when applicable. Variables that were statistically significant in the univariate models were included in a stepwise binary logistic regression model to assess the risk of receiving ABO non-identical platelet transfusions. Univariate odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated. A survival analysis was performed using the Kaplan-Meier method and compared with the log-rank test.
Results
Table I shows the patients’ characteristics according to whether they received ABO identical, ABO non-identical or both types of platelet transfusions. As regards ABO blood group, 200 patients (37.8%) were O, 269 (50.8 %) were A, 45 (8.5 %) were B and 15 (2.8 %) were AB. Patients who underwent APCT received a median of two (range, 0–35) red blood cell concentrates and three (range, 0–86) platelet concentrates, of which two (range, 0–63) were ABO identical and one (range, 0–20) was ABO non-identical. The patients received a total of 2,772 platelet concentrates, of which 2,053 (74.0%) were ABO identical and 719 not ABO identical (26.0%); 309 were compatible and 410 incompatible with the patients’ plasma. Considering all transplants, in 36 cases (6.5%) no platelet transfusions were given, while in 246 cases (44.5%) the patients were exclusively transfused with ABO identical platelets and in 47 cases (8.5%) received only ABO non-identical platelet transfusions. In the 241 (43.6%) transplants for which patients received both ABO identical and ABO non-identical platelets, in 154 cases the patients received more ABO identical platelets, in 26 cases they received more ABO non-identical platelets, and in 61 cases they received the same number of ABO identical and ABO non-identical platelet transfusions. Table II shows the blood product requirements and clinical outcomes of patients according to whether they received ABO identical, ABO non-identical or both types of platelet transfusions. The group of patients who received both ABO identical and ABO non-identical platelet transfusions had higher transfusion needs and worse clinical outcomes compared to patients who received only ABO identical or ABO non-identical platelets. Patients with acute leukaemia had later platelet engraftment (36.4±32.0 vs 18.8±34.5 days after APCT), received more units of red blood cells (5.2±4.5 vs 3.1±4.1 units), more ABO non-identical platelet units (2.3±2.5 vs 1.0±1.9 units), a higher number of overall platelet transfusions (8.9±9.0 vs 4.0±6.8 units) and stayed longer in hospital (32.9±10.5 vs 28.1±11.1 days) than patients with other diagnoses (p<0.001 for all variables). In the subset of patients with leukaemia, there was no statistical difference in survival between patients who did or did not receive ABO non-identical platelets (p=0.364).
Table II.
Transfusion outcome of patients who received only ABO identical, only ABO non-identical and both ABO identical and ABO non-identical platelet transfusions. Results are shown as median and range.
| Patients who received platelet transfusions | p | |||
|---|---|---|---|---|
|
| ||||
| ABO identical | ABO non-identical | Both | ||
| Number | 246 | 47 | 260 | |
| Red blood cells | 2 (0–10) | 2 (0–10) | 4 (0–35) | |
| ABO identical platelets | 2 (1–8) | 3 (1–63) | <0.001 | |
| ABO non-identical platelets | 1 (0–9) | 1 (1–20) | ||
| Plasma compatible | 0 (0–4) | 1 (0–15) | ||
| Plasma incompatible | 1 (0–9) | 1 (0–17) | ||
| Total platelets | 2 (0–10) | 1 (0–9) | 4 (1–86) | <0.001 |
| Fresh frozen plasma | 0 (0–0) | 0 (0–0) | 0 (0–73) | 0.010 |
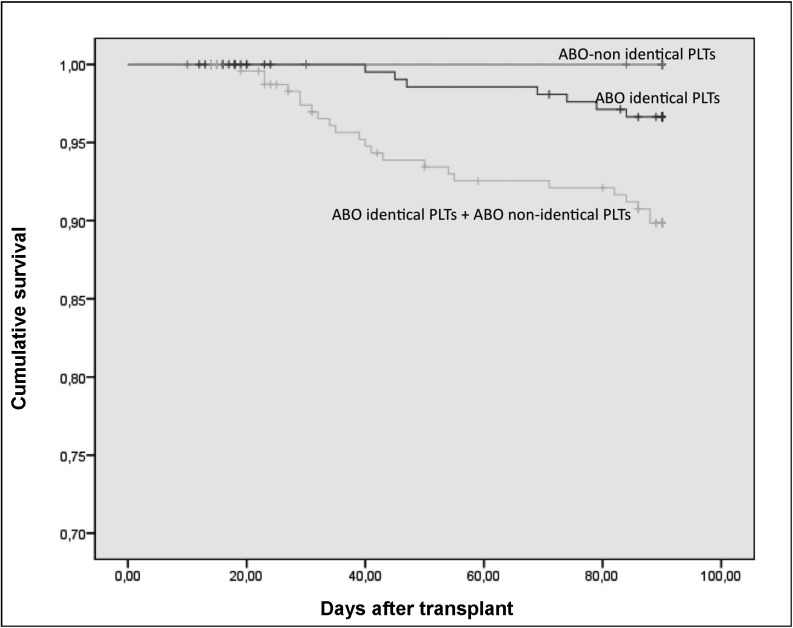
Figure 1 illustrates the cumulative survival to 90 days after APCT of all patients according to whether they received only ABO identical, only ABO non-identical or both types of platelet transfusions.
Figure 1.
Cumulative survival 90 days after APCT of all patients according to whether they received only ABO identical platelets, only ABO non-identical platelets or both ABO identical and ABO non-identical platelets.
p=0.03 (p=0.255 between the ABO identical platelet and ABO non-identical platelet curves).
PLTs: platelets.
Table III shows the clinical and transfusion outcomes of patients receiving three platelet transfusions, according to whether they did or did not receive ABO identical platelet transfusions. We performed this analysis to circumvent the problem that patients with higher transfusion requirements may be disproportionately represented in the group of ABO non-identical recipients.
Table III.
Clinical and transfusion outcomes of patients who received three platelet transfusions, according to whether they received only ABO identical or both identical ABO and non-identical ABO platelet transfusions. Results are shown as mean ± standard deviation.
| Characteristics | Patients who received platelet transfusions | p | |
|---|---|---|---|
|
| |||
| Only ABO identical | Both | ||
| Number | 43 | 45 | |
|
| |||
|
Sex (male/female) |
23/20 | 28/17 | 0.407 |
|
| |||
| Age (years) | 53.6±11.5 | 48.9±13.6 | 0.102 |
|
| |||
| Days until neutrophils >1×109/L | 12.5±1.8 | 13.5±3.0 | 0.111 |
|
| |||
| Days until platelets >20×109/L | 17.6±11.1 | 16.0±6.7 | 0.611 |
|
| |||
| RBC transfusions | 2.4±2.0 | 2.3±2.0 | 0.734 |
|
| |||
| ABO identical platelets | 3.0±0.0 | 1.7±0.6 | <0.001 |
|
| |||
| ABO non-identical platelets | 0 | 1.3±0.6 | <0.001 |
|
| |||
| Fever* | |||
| Yes | 10 | 12 | |
| No | 23 | 33 | 0.817 |
|
| |||
| Days at hospital | 28.0±9.3 | 26.0±7.6 | 0.062 |
|
| |||
| Status 90 days after APCT | |||
| Death | 1 | 0 | |
| Alive | 42 | 45 | 0.314 |
RBC: red blood cells;
fever in the first 30 days after transplantation; APCT: autologous haematopoietic progenitor cell transplantation.
Results of the multivariate analysis showed that a diagnosis of acute myeloid leukaemia was the only variable that significantly increased the risk of receiving ABO non-identical platelet transfusions (Table IV).
Table IV.
Variables influencing the risk of receiving ABO non-identical platelet transfusions: results of multivariate analysis.
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Age | 0.997 | 0.981–1.013 | 0.703 |
| Sex | 1.318 | 0.844–2.060 | 0.225 |
| ABO group O | 0.332 | 0.7061–1.804 | 0.201 |
| ABO group A | 0.382 | 0.071–2.049 | 0.261 |
| ABO group B | 2.352 | 0.343–16.133 | 0.384 |
| Diagnosis (AML) | 3.879 | 1.555–9.672 | 0.004 |
| Days until platelets >20×109/L | 1.008 | 0.991–1.025 | 0.345 |
| Days until neutrophils >1×109/L | 1.045 | 0.980–1.114 | 0.184 |
| Fever* | 1.062 | 0.658–1.716 | 0.805 |
| N. of transplants (2) | 1.654 | 0.546–5.008 | 0.373 |
OR: odds ratio; CI: confidence interval; AML: acute myeloid leukaemia. Fever*: fever up to 30 days after autologous haematopoietic stem cell transplantation.
Among 112 patients who were RhD negative, 66 received RhD positive platelet transfusions (median 3; range, 1–25). One patient developed anti-D + anti-E 10 months after one RhD positive platelet transfusion, and another patient developed anti-D 30 days after transfusion of 16 RhD positive platelet concentrates. The former patient received ABO identical platelet transfusions while the latter received ABO non-identical platelets.
Another three patients developed anti-erythrocyte antibodies (2 anti-E, 1 anti-Kpa); all three had received mostly ABO-identical platelets (p=0.318).
Among the recipients of ABO identical platelets, three had allergic reactions, while there were seven adverse reactions in the group of patients receiving ABO identical plus ABO non-identical platelets (4 allergic reactions, 2 febrile non-haemolytic reactions and 1 case of hypotension, p=0.139). In five cases, adverse reactions occurred after ABO identical platelet transfusions, and in another five cases after ABO non-identical platelet transfusions.
Discussion
ABO non-identical platelet transfusions have been associated with different adverse effects such as immune complex formation, platelet refractoriness and adverse clinical outcomes12. Results of some studies comparing ABO identical and ABO non-identical platelet transfusions in adult and childhood populations are controversial4,13. Our study provides information about clinical and transfusion outcomes of a large subset of patients undergoing APCT who did or did not receive ABO identical platelets.
It seems reasonable that transfusion of ABO identical platelets should be a goal to reach in blood banks, but the application of this strict policy in clinical practice is difficult because of the limited availability of platelet concentrates, especially in emergency circumstances. In spite of this, some groups have shown that it can be feasible to transfuse only ABO identical platelets by changing ordering and inventorying procedures, with a minimum increase in wastage14. In fact, following our centre’s transfusion policy most platelet transfusions performed in our patients were also ABO identical. However, the largest group of patients received both ABO identical and ABO non-identical platelet transfusions. This group comprised patients who engrafted neutrophils and platelets later, received more red blood cell and total platelet transfusions, and stayed longer in hospital than patients who received only ABO identical or only ABO non-identical platelets. This probably means that patients who receive ABO non-identical platelets are a selected population with greater transfusion requirements that cannot be met only by ABO identical platelets. In fact, these patients shared some characteristics that justified greater transfusion requirements, such as late neutrophil and platelet engraftment. This conclusion is also supported by the analysis of patients who received the same number of platelet transfusions. In this occasion, we failed to find any difference in engraftment, time spent in hospital or survival between patients who did or who did not receive ABO identical platelets.
However, it cannot be excluded that ABO non-identical platelet transfusions contribute to a worse outcome. The doubt also remains that the lower post-transfusion increments after ABO non-identical platelet transfusions, observed previously15,16, may have contributed to increase the number of total platelet transfusions in the group of patients who received ABO non-identical platelets. Unfortunately, we are unable to demonstrate whether this was the case because we did not systematically determine the corrected count increment after platelet transfusions.
We are well aware of the limitations of this analysis: this was a retrospective study of a large subset of patients with different clinical conditions. However, the number of patients who received ABO non-identical platelets was sufficiently large for comparison with the group of patients who received only ABO identical platelets.
Some literature data suggest that ABO non-identical platelet transfusions may contribute to allergic and febrile reactions and also increase the red cell immunisation rate12. Our results, however, are in concordance with those of another study which failed to show these associations17. Considering the number of platelet transfusions received in each group of patients, there was no difference in the incidence of adverse reactions to platelets in patients who did or did not receive ABO non-identical platelets. Consistently with this fact, the RBC alloimmunisation rate was not statistically different between the groups in our study.
There are controversial data about an association of ABO-mismatched platelet transfusions with morbidity and mortality5,18–23. In 153 patients undergoing cardiac surgery, recipients of ABO identical platelets had a lower mortality rate, fewer red blood cell transfusions, fewer mean days of antibiotics and a shorter stay in hospital19. In adult patients with leukaemia, a possible survival advantage has been reported for those who receive ABO identical platelet transfusions, without this having a clear explanation18. In the present study, red blood cell and platelet requirements, hospital stay and mortality were similar in patients who received ABO identical or ABO non-identical platelet transfusions. Our results agree with those published by Lin et al.20, showing similar transfusions and clinical outcomes for a large subset of patients undergoing cardiovascular surgery and transfused with ABO identical platelets or ABO mismatched platelets. However, their data have been questioned because all group O platelet transfusions had an anti-A or anti-B titre of 1 in 32 or less21. Exposure of ABO antigen platelets to high titres of anti-A or anti-B has been associated with alterations of platelet function and clot formation kinetics24. In contrast, we do not have the titres of anti-A or anti-B in group O platelet concentrates, so our results are more consistent than those of the study by Lin et al.20
Conclusion
In our hospital, patients undergoing APCT who received ABO identical or ABO non-identical platelet transfusions had similar transfusion and clinical outcomes. The isolated fact of receiving ABO non-identical platelet did not influence morbidity or survival. Prospective studies are needed in order to confirm our results and clarify whether a strategy of ABO identical platelet transfusions could reduce platelet transfusion requirements.
Footnotes
Authorship contributions
PS and NC participated in the study design, data collection, data analysis and paper writing. AB, SR and GI participated in data collection. IL participated in data analysis. The rest of the Authors participated in writing and critically reviewing the paper.
The Authors declare no conflicts of interest.
References
- 1.Cid J, Harm SK, Yazer MH. Platelet transfusion: the art and science of compromise. Transfus Med Hemother. 2013;40:160–71. doi: 10.1159/000351230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooling LL, Kelly K, Barton J, et al. Determinants of ABH expression on human blood platelets. Blood. 2005;15:3356–64. doi: 10.1182/blood-2004-08-3080. [DOI] [PubMed] [Google Scholar]
- 3.Curtis BR, Edwards JT, Hessner MJ, et al. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood. 2000;15:1574–81. [PubMed] [Google Scholar]
- 4.Lozano M, Heddle N, Williamson LM, et al. Practices associated with ABO-incompatible platelets transfusions: a BEST collaborative international survey. Transfusion. 2010;50:1743–8. doi: 10.1111/j.1537-2995.2010.02642.x. [DOI] [PubMed] [Google Scholar]
- 5.Shehata N, Tinmouth A, Naglie G, et al. ABO-identical versus nonidentical platelet transfusion: a systematic review. Transfusion. 2009;49:2442–53. doi: 10.1111/j.1537-2995.2009.02273.x. [DOI] [PubMed] [Google Scholar]
- 6.Dunbar NM, Ornstein DL, Dumont L. ABO incompatible platelets: risks versus benefits. Curr Opin Hematol. 2012;19:475–9. doi: 10.1097/MOH.0b013e328358b135. [DOI] [PubMed] [Google Scholar]
- 7.Cooling L. ABO and platelet transfusion therapy. Immunohematology. 2007;23:20–33. [PubMed] [Google Scholar]
- 8.Kacker S, Ness PM, Savage WJ, et al. The cost-effectiveness of platelet additive solution to prevent allergic transfusion reactions. Transfusion. 2013;53:2609–19. doi: 10.1111/trf.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sociedad Española de Transfusión Sanguínea y Terapia Celular. Guía sobre la transfusión de componentes sanguíneos y derivados plasmáticos. Barcelona: SETS; 2010. [Google Scholar]
- 10.Larsson S, Sandgren P, Sjodin A, et al. Automated preparation of platelet concentrates from pooled buffy coats: in vitro studies and experiences with the OrbiSac system. Transfusion. 2005;45:743–51. doi: 10.1111/j.1537-2995.2005.04096.x. [DOI] [PubMed] [Google Scholar]
- 11.Surowiecka M, Zantek N, Morgan S, et al. Anti-A and anti-B titers in group O platelet units are reduced in PAS C versus convencional plasma units. Transfusion. 2014;54:255–6. doi: 10.1111/trf.12427. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman RM. Platelet ABO matters. Transfusion. 2009;49:5–7. doi: 10.1111/j.1537-2995.2008.02011.x. [DOI] [PubMed] [Google Scholar]
- 13.Julmy F, Ammann RA, Taleghani BM, et al. Transfusion efficacy of ABO major-mismatched platelets (PLTs) in children is inferior to that of ABO-identical PLTs. Transfusion. 2009;49:21–33. doi: 10.1111/j.1537-2995.2008.01914.x. [DOI] [PubMed] [Google Scholar]
- 14.Henrichs KF, Howk N, Masel DS, et al. Providing ABO identical platelets and cryoprecipitate to (almost) all patients - approach, logistics and associated decreases in transfusion reaction and red cell alloimmunization incidence. Transfusion. 2012;52:635–40. doi: 10.1111/j.1537-2995.2011.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klumpp TR, Herman JH, Innis S, et al. Factors associated with response to platelet transfusion following hematopoietic stem cell transplantation. Bone Marrow Transplant. 1996;17:1035–41. [PubMed] [Google Scholar]
- 16.Triulzi DJ, Assmann SF, Strauss RG, et al. The impact of platelet transfusion characteristics on posttransfusion platelet increment and clinical bleeding in patients with hypoproliferative thrombocytopenia. Blood. 2012;119:5553–62. doi: 10.1182/blood-2011-11-393165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazer MH, Raval JS, Triulzi DJ, Blumberg N. ABO-mismatched transfusions are not over-represented in febrile non-haemolytic transfusion reactions to platelets. Vox Sang. 2012;102:175–7. doi: 10.1111/j.1423-0410.2011.01529.x. [DOI] [PubMed] [Google Scholar]
- 18.Heal JM, Kenmotsu N, Rowe JM, Blumberg N. A possible survival advantage in adults with acute leukemia receiving ABO-identical platelet transfusions. Am J Hematol. 1994;45:189–90. doi: 10.1002/ajh.2830450219. [DOI] [PubMed] [Google Scholar]
- 19.Heal JM, Rowe JM, McMican A, et al. The role of ABO matching in platelet transfusion. Eur J Haematol. 1993;50:110–7. doi: 10.1111/j.1600-0609.1993.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 20.Blumberg N, Heal JM, Hicks GL, Risher WH. Association of ABO-mismatched platelet transfusions with morbidity and mortality in cardiac surgery. Transfusion. 2001;41:790–3. doi: 10.1046/j.1537-2995.2001.41060790.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Callum JL, Coovadia AS, Murphy PM. Transfusion of ABO non-identical platelets is not associated with adverse clinical outcomes in cardiovascular surgery patients. Transfusion. 2002;42:166–72. doi: 10.1046/j.1537-2995.2002.00037.x. [DOI] [PubMed] [Google Scholar]
- 22.Blumberg N, Heal JM. ABO-mismatched platelet transfusions and clinical outcomes after cardiac surgery. Transfusion. 2002;42:1527–8. doi: 10.1046/j.1537-2995.2002.00263.x. [DOI] [PubMed] [Google Scholar]
- 23.Refaai MA, Fialkow LB, Heal JM, et al. An association of ABO non-identical platelet and cryoprecipitate transfusions with altered red cell transfusion needs in surgical patients. Vox Sang. 2011;101:55–60. doi: 10.1111/j.1423-0410.2010.01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Refaai MA, Carter J, Henrichs KF, et al. Alterations of platelet function and clot formation kinetics after in vitro exposure to anti-A and -B. Transfusion. 2013;53:382–93. doi: 10.1111/j.1537-2995.2012.03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]