Abstract
Background
There is increasing evidence indicating an association between red blood cell (RBC) transfusions and necrotising enterocolitis (NEC) in preterm infants, especially late-onset NEC. This phenomenon is referred to as transfusion-related acute gut injury (TRAGI). One theory as to a pathophysiological mechanism is that transfusion may result in an ischemia-reperfusion injury to intestinal tissue. We tested the hypothesis that there is significantly greater variability during transfusion in splanchnic tissue oxygen saturation (SrSO2) than in cerebral tissue oxygen saturation (CrSO2).
Materials and methods
This was a prospective, observational study using near-infrared spectroscopy to monitor SrSO2 and CrSO2 in preterm neonates undergoing RBC transfusion for symptomatic anaemia. Mean, standard deviation, highest and lowest SrSO2 and CrSO2 values during each transfusion were determined. The greatest difference in SrSO2 and CrSO2 during each transfusion was calculated, along with the coefficient of variation.
Results
We studied 37 subjects. Throughout all transfusions, the mean SrSO2 was 45.6% ±13.8 and the mean CrSO2 was 65.4% ±6.9 (p<0.001). The variability of SrSO2 was significantly greater than that of CrSO2. Averaging data from all subjects, the greatest difference in SrSO2 was 43.8% ±13.4 compared with 23.3% ±7.6 for CrSO2 (p<0.001). The mean coefficient of variation in all transfusions was 20.5% for SrSO2 and 6.0% for CrSO2 (p<0.001). Increasing post-conceptional age did not affect SrSO2 variability (R2 =0.022; p=0.379), whereas CrSO2 variability during transfusion decreased with increasing post-conceptional age (R2=0.209; p=0.004).
Discussion
In preterm infants, there is a large degree of tissue oxygenation variability in splanchnic tissue during RBC transfusion and this does not change with increasing maturity. We speculate that these findings, combined with lower average tissue oxygenation, may demonstrate susceptibility of the preterm gut to TRAGI.
Keywords: blood transfusion, necrotising enterocolitis, near-infrared spectroscopy
Introduction
Red blood cell (RBC) transfusion remains a mainstay in the medical management of premature infants1. A majority of very-low birth weight infants will receive at least one RBC transfusion during their time in the neonatal intensive care unit (NICU)2. Necrotising enterocolitis (NEC) is a common gastrointestinal disease affecting preterm infants in the NICU and is associated with significant morbidity and mortality3. Its exact pathogenesis is unknown, but NEC is believed to be a multifactorial process that probably includes intestinal ischaemic injury combined with an infectious process and/or the activation of an inflammatory cascade4. Premature infants are especially vulnerable to this condition, because, in addition to having decreased gut barrier function, an immature immune system, and decreased gut motility, they also have poor circulatory regulation of their splanchnic organ system5.
Near-infrared spectroscopy (NIRS) is a well-described technique that can be used to determine both the regional splanchnic tissue oxygen saturation (SrSO2) and the regional cerebral tissue oxygen saturation (CrSO2) of a preterm infant6. Not only do these values provide information about the flow of blood to the intestines and brain, they also reflect the adequacy of the balance between tissue perfusion and oxygenation vs metabolic demand7.
Until recently, blood transfusions and NEC were thought to be unrelated. However, there is now growing evidence demonstrating a possible association between RBC transfusions and NEC8. A particular concern is developing regarding anaemic, yet stable, “feeding and growing” premature infants who develop late-onset NEC within days after receiving a transfusion8,9. This transfusion-related NEC that may occur in preterm infants has been termed transfusion-related acute gut injury (TRAGI), and several theories exist as to how this can occur. One explanation is that the splanchnic circulation is less capable of vascular regulation than other organ systems, such as the cerebral circulatory system, and an immature splanchnic circulation has especially poor vascular control capabilities5,10. Some, therefore, speculate that RBC transfusions can lead to such variability in perfusion and oxygen delivery to splanchnic tissue, which is already maintained in a relatively low blood flow environment to begin with, as to result in some degree of ischaemic injury9. This, in turn, could create a situation in which the gut becomes more prone to developing TRAGI.
In order to examine this as a possible mechanism for RBC transfusions increasing an infant’s risk of NEC, we set out to test the hypothesis that not only is mean SrSO2 lower than mean CrSO2 during preterm infant RBC transfusions, but that there is also greater variability in SrSO2 during the transfusion than in CrSO2. Our primary goal was to account for the extremes of tissue oxygenation (both high and low) that intestinal tissue may experience during a RBC transfusion, as we theorised that this could be an important factor related to TRAGI. We also sought to determine whether this finding would be seen consistently throughout the neonatal period, as TRAGI is thought to occur even in older preterm infants who generally do not manifest NEC.
Materials and methods
Patients
Preterm neonates in the NICU, who were at least 5 days old and born at New York University Langone Medical Center and Bellevue Hospital Center (New York, NY, USA), were eligible for the study. The exclusion criteria were a 5-minute Apgar score of 3 or less, a known chromosomal abnormality, a congenital cardiac malformation, grade 2 or greater intraventricular haemorrhage, a current diagnosis of NEC prior to enrolment, or a need for either vasopressor support or high frequency ventilation. This study received ethical approval by the hospital institutional review board and parental consent was obtained prior to any subject’s participation.
Study design
To monitor SrSO2 and CrSO2 we used the INVOS 5100C Cerebral/Somatic Oximeter (Somanetics, Troy, MI; now Covidien, Mansfield, MA, USA). This NIRS device uses light emitted from a skin sensor in the near-infrared wavelength (730 nm and 810 nm) to penetrate both soft tissue and bone in order to determine the amount of oxygenated haemoglobin (oxy-Hb) and deoxygenated haemoglobin (deoxy-Hb) in the organ tissue below. The result is displayed on the monitor as a percentage [oxy-Hb/(oxy-Hb + deoxy-Hb)] which represents the regional tissue oxygen saturation (rSO2) of that particular tissue. This technology has been demonstrated to be capable of measuring both SrSO2 and CrSO2 accurately in preterm neonates6.
Investigators were contacted when a transfusion was ordered by the primary NICU team because a study eligible infant had developed symptomatic anaemia. NIRS sensors were then placed on the subject if parental consent was obtained and monitoring could begin prior to starting the transfusion so that adequate baseline rSO2 values could be established. SrSO2 data were collected from an abdominal NIRS sensor placed just left of the umbilicus and CrSO2 data were collected from a forehead NIRS sensor. The INVOS display screen was covered to blind the NICU staff to the information collected. Single SrSO2 and CrSO2 data points were measured and recorded every 30 seconds. These data were then electronically stored on a hard drive for later analysis.
All monitored RBC transfusions were ordered to be 15 mL/kg with a goal transfusion time of 4 hours. All patients received either directed donor or random donor leucocyte-reduced, irradiated, Cytomegalovirus-negative blood. Feeds were withheld only during the transfusion period, as is the standard practice in our NICU. Feeds were resumed as soon as transfusions had been completed.
Data analysis
The mean SrSO2 and CrSO2 during the complete RBC transfusion period were calculated for each individual subject. In addition, the highest and lowest SrSO2 and CrSO2 values recorded from the exact time that the transfusion started until the time that the transfusion was completed were also determined for each subject. From these highest and lowest values, the range of SrSO2 and CrSO2 that patients were exposed to was established, and represents the greatest variability in oxygenation that each subject’s gut and brain experienced during the RBC transfusion. As an additional marker of general variability found in tissue oxygenation during a transfusion, a coefficient of variation analysis was also conducted examining both SrSO2 and CrSO2 for each transfusion. The coefficient of variation was calculated using the ratio: standard deviation divided by the mean. We then multiplied this value by 100 to obtain a percentage [coefficient of variation=(SD/mean)×100]. This calculation was done for SrSO2 and CrSO2 using all data points measured by NIRS during each individual transfusion. This has been found useful when comparing NIRS signal variability between two separate site measurements that may have very different mean values11.
The Student’s t test was used to examine for a difference in the mean SrSO2 compared with the mean CrSO2 that subjects experienced during their transfusion. This test was also used to determine any difference in the amount of variability in SrSO2 vs CrSO2 during our subjects’ monitored transfusions, both looking at the greatest degree of variability as well as the coefficient of variation. The Mann-Whitney test was used to compare median SrSO2 and CrSO2 values. Pearson’s correlation analysis was used to determine the relationship between post-conceptional age at the time of transfusion and the greatest variability in SrSO2 and CrSO2 occurring throughout the transfusion. All statistical calculations were performed using SPSS 17.0 Software for Windows (SPSS Inc., Chicago, IL, USA). Two-tailed tests were used to determine statistical significance, which was established by a p-value of less than 0.05.
Results
Patients’ characteristics
Thirty-seven patients were included in this study. Their demographic information is displayed in Table I. Our patients had a mean gestational age of 28.4 weeks and, at the time of their monitored transfusion, had a mean post-conceptional age of 32.7 weeks. The respiratory support needs of our study population were varied. Most of the infants were receiving feeds and 59% of all subjects were being treated with caffeine.
Table I.
Subjects’ demographics and clinical characteristics (n=37).
| Gender | |
|
| |
| Male | 65% (24/37) |
| Female | 35% (13/37) |
|
| |
| Twin gestation | 19% (7/37) |
|
| |
| Ethnicity | |
| Caucasian | 43% (16/37) |
| African American | 14% (5/37) |
| Hispanic | 35% (13/37) |
| Asian | 8% (3/37) |
|
| |
| Measurements at birth | |
| Gestational age (weeks) | |
| Mean±SEM | 28.4±0.48 |
| Median; IQR | 270/7; 260/7–300/7 |
| Birth weight (g) | |
| Mean±SEM | 1,112±69.4 |
| Median; IQR | 1,000; 805–1,202 |
|
| |
| Measurements at transfusion | |
| Post-conceptional age at transfusion (weeks) | |
| Mean±SEM | 32.7±0.58 |
| Median; IQR | 326/7; 301/7–353/7 |
| Mean weight at transfusion (g) | |
| Mean±SEM | 1,403±78.2 |
| Median; IQR | 1,370; 1,045–1,780 |
|
| |
| Ventilation support status at transfusion | |
| Room air | 38% (14/37) |
| Nasal cannula | 30% (11/37) |
| Nasal continuous positive airway pressure | 16% (6/37) |
| Conventional ventilation | 16% (6/37) |
| Inspired fraction of oxygen, mean±SD | 0.26±0.06 |
|
| |
| Feeds and caffeine at transfusion | |
| Receiving enteral feeds prior to transfusion | 78% (29/37) |
| Volume of feeds if receiving (mL/kg/day), mean±SD | 114±53.3 |
| Receiving caffeine at time of transfusion | 59% (22/37) |
|
| |
| Transfusion information | |
| Pre-transfusion Hb level (g/dL), mean±SD | 9.3±1.2 |
| Post-transfusion Hb level (g/dL), mean±SD | 13.0±3.3 |
| Volume of transfusion (mL/kg), mean±SD | 15.3±1.4 |
| Transfusion duration (h), mean±SD | 3.8±0.5 |
SEM: standard error of the mean; IQR: interquartile range; SD: standard deviation; Hb: haemoglobin.
Blood transfusions
The mean Hb value for which our subjects received a transfusion was 9.3 g/dL, as shown in Table I. Transfusions were given to subjects because of apnoea, bradycardia, or desaturation events (22/37), tachycardia (2/37), increased respiratory support requirements (10/37) or feeding intolerance (3/37), in addition to a low Hb level. The mean transfusion volume and transfusion period remained close to the targeted 15 mL/kg over 4 hours.
All transfusions were tolerated well and there were no reports of transfusion reaction. No patients had a major change in their cardiac or ventilatory status during their transfusion. In addition, there were no reported cases of NEC within 1 week after each transfusion having taken place.
Splanchnic and cerebral tissue oxygen saturation during transfusion
The mean SrSO2 throughout all monitored transfusions was found to be significantly less than the mean CrSO2 (mean±SD: 45.6% ±13.8 vs 65.4% ±6.9; p<0.001). In every case, but one, the mean SrSO2 throughout the transfusion period was always lower than the mean CrSO2 that each individual subject experienced. In the one exception, the mean SrSO2 was 57.8% and the mean CrSO2 was 56.7%. The median SrSO2 during transfusions was 49.5% compared to 66.0% for CrSO2 (p<0.01).
Variability in splanchnic and cerebral tissue oxygen saturation during transfusion
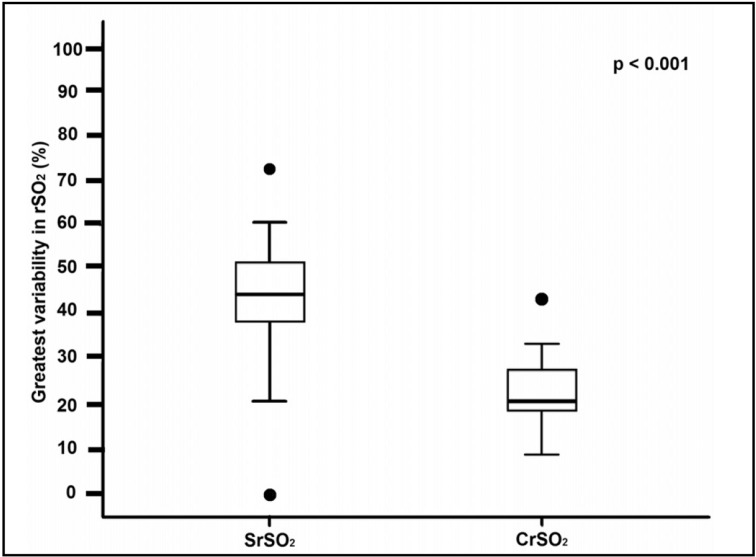
Figure 1 displays the highest and lowest SrSO2 and CrSO2 values recorded during each transfusion for every subject who was monitored. It is apparent that, during the transfusion period, there is a much wider range of SrSO2 values than CrSO2 values. From these data points, the greatest variability that each patient experienced in SrSO2 and CrSO2 was determined. These values are shown in Figure 2, demonstrating a significantly greater variability in SrSO2 than in CrSO2 during the transfusion periods (mean±SD: 43.8% ±13.4 vs 23.3% ±7.6; p<0.001).
Figure 1.
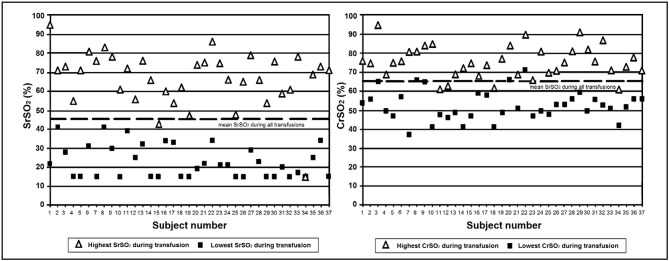
Graphical representation of the highest and lowest SrSO2 and CrSO2 values that each subject experienced during their RBC transfusion (n=37).
Figure 2.
Box plot demonstrating the mean and interquartile range for the amount of variability in SrSO2 and CrSO2 during all RBC transfusions (n=37).
The average coefficient of variation for SrSO2 in all individual transfusions was 20.5%, whereas that for CrSO2 was 6.0%. When comparing these means, NIRS SrSO2 monitoring had more than three times greater variability than NIRS CrSO2 monitoring (p<0.001). In addition, the mean standard deviation in SrSO2 monitoring for individual subjects was 8.8% compared with 3.9% for CrSO2 (p<0.001).
Variability in tissue oxygen saturation based on post-conceptional age
There was no decrease in the maximum amount of SrSO2 variability that each subject experienced during their blood transfusion as they increased in post-conceptional age (p=0.379) (Figure 3). However, a significant inverse correlation was found between increasing post-conceptional age at transfusion and the greatest degree of variability in CrSO2 during that blood transfusion (p=0.004). Increasingly mature subjects were more likely to experience lesser variability in brain tissue oxygenation.
Figure 3.
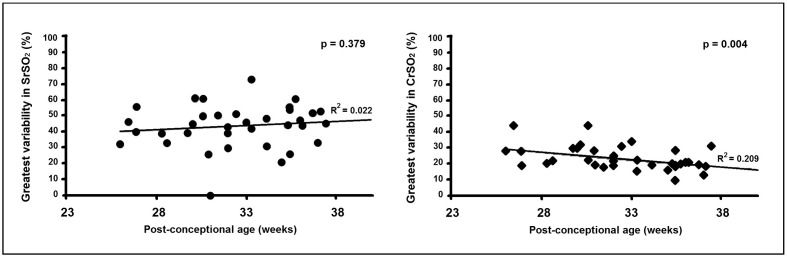
Correlation between post-conceptional age and the variability in tissue oxygen saturation experienced during a RBC transfusion (n=37).
Discussion
We found that splanchnic tissue oxygenation was significantly lower and had significantly greater variability during a preterm infant RBC transfusion when compared to tissue oxygenation of the brain. We also discovered that increasing maturity had no effect on the degree of splanchnic tissue oxygenation control during a transfusion, which was in contrast to the increasingly tightly controlled auto-regulation of cerebral tissue oxygen levels. These results support the theoretical possibility that RBC transfusions could lead to TRAGI secondary to intestinal mucosal damage from a combination of both hypoxia and ischaemia-induced gut injury, and that this could occur throughout the preterm neonatal period.
We decided to compare relative SrSO2 values to CrSO2 values for two reasons. To begin with, blood flow to the brain is considered relatively well controlled and consistent in delivering a high amount of constant oxygen to cerebral tissue12,13. In addition, to our knowledge, there is currently only limited published data suggesting a risk to the preterm brain resulting directly from a RBC transfusion and many feel that RBC transfusion is likely to be neuroprotective14. Therefore, it seemed appropriate to use CrSO2 as a control to compare both SrSO2 levels and variability. We chose to focus on the highest and lowest points in rSO2 during a transfusion for our primary analysis because we theorised that it may be the extremes of tissue perfusion status that make intestinal tissue most vulnerable to perfusion-ischaemia-reperfusion injury, which has been thought of as a potential factor that can play a role in the pathogenesis of NEC.
Conditions that lead to intestinal tissue hypoxia have been shown to be risk factors for developing NEC15. Examples include maternal-foetal placental insufficiency, umbilical vessel catheterisation occluding mesenteric blood flow, and congenital cardiac disease16. In fact, NEC is one of the primary complications of cardiac lesions associated with lower baseline arterial oxygen saturation17.
In addition to simply low tissue oxygen levels, a change from a low blood flow state back to a high blood flow state has been shown in animal models to cause intestinal injury that could theoretically lead to NEC18. Several different biochemical reactions that develop as a result of ischaemia followed by reperfusion have been demonstrated as possible pathways to injury. These include an increased production of reactive oxygen free radical species and the accumulation of potentially toxic metabolites19. The substantial changes in splanchnic blood flow that we demonstrated could potentially lead to these mechanisms of injury.
NIRS appears capable of monitoring for both low oxygen states and changes in blood flow, which are both potentially responsible for TRAGI. NIRS values provide information about the adequacy of tissue oxygen supply which is primarily based on the balance between organ blood flow vs the metabolic demands of that organ7. If a patient remains relatively stable without rapid changes in metabolic demands, then rSO2 provides a good representation of tissue perfusion. There have been case reports of patients with cardiac lesions with low SrSO2 values monitored with NIRS who later developed NEC17. Moreover, Fortune et al. demonstrated that low SrSO2 measured with NIRS in preterm infants were strongly associated with NEC20.
Ultrasound with Doppler analysis has been able to monitor the altered mesenteric blood flow velocity response in the major vessels that can occur immediately following a blood transfusion21. While the NIRS method that we employed does not examine the bulk flow of blood, it may provide more information about changes in blood flow, tissue perfusion, and oxygen delivery occurring in the microcirculation, which ultrasound cannot provide. In this regard, NIRS may be more advantageous than ultrasound for predicting which infants may be at risk of subsequently developing TRAGI.
There were limitations to this study. First, the lowest rSO2 value that the commercially available NIRS device we used can detect is 15%. If lower values could have been detected, we might have seen even greater variability in SrSO2. Secondly, to assess the amount of change in intestinal and cerebral tissue perfusion and oxygenation that occurred, our primary analysis was based on highest and lowest SrSO2 and CrSO2 values during the transfusion period. Although we feel this provides a good assessment of the degree of variability in tissue oxygenation, it does not describe well acceleration or deceleration of tissue oxygenation, which could play a role in transfusion-related gut injury. The analysis showing that SrSO2 had a much higher coefficient of variation than CrSO2 does, however, offer more insight into this as it also showed the overall relative variability in gut tissue oxygenation was much greater.
In addition, no patients monitored actually developed TRAGI (NEC in the post-transfusion period). It would have been helpful to determine whether there was either a lower baseline SrSO2 or an increased amount of variability in SrSO2 during transfusion in subjects who developed TRAGI. However, in this pilot study it would have been unlikely, as the incidence of transfusion-related NEC is reported to be very low8.
Even if changes in splanchnic tissue oxygenation do not represent the primary mechanism through which transfusion-related NEC can develop, this study still provides insight into the vascular regulation that occurs in preterm neonates. The degree of variability in CrSO2 was much less than that in SrSO2, demonstrating the auto-regulatory capabilities of the cerebral circulatory system. The decreasing variability in CrSO2 associated with increasing post-conceptional age shows that this mechanism continues to develop throughout the third trimester. This is likely in this district, in contrast to the blood vessels supplying the splanchnic circulation.
Conclusion
While further studies are needed to define the incidence and possible causes of TRAGI more clearly, the results from this study demonstrate that variability in intestinal tissue oxygen levels remains a potential pathophysiological mechanism for this condition. Larger studies performed in a prospective manner are needed to validate these results in order to establish whether SrSO2 variability predisposes the intestines to develop TRAGI in the post-transfusion period.
Acknowledgements
This study received support from a KiDS of NYU research grant and the NYU School of Medicine Division of Neonatology. We thank Dr. John Wells for his prior intellectual support. We are also thankful to all of our patients and their parents, as well as to our nursing staff at both NYU Langone Medical Center and Bellevue Hospital.
Footnotes
Authorship contributions
SMB wrote the initial draft of the manuscript and contributed to the development and design of the project. In addition, he recruited subjects, performed the NIRS monitoring, and collected and analysed data. KDH-M contributed to the development of the project and assisted in writing the manuscript. PVM contributed to the development and design of the project and assisted in writing the final version of the manuscript.
Disclosure of conflicts of interest
None of the Authors has a conflict of interest to report pertaining to this manuscript. The Neonatal Intensive Care Units at NYU Langone Medical Center and Bellevue Hospital were previously Somanetics Neonatal NIRS sensor beta-test sites.
References
- 1.Ohls RK. Transfusions in the preterm infant. NeoReviews. 2007;8:e377–86. [Google Scholar]
- 2.Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal vs restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–91. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holman RC, Stoll BJ, Clarke MJ, Glass RI. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health. 1997;87:2026–31. doi: 10.2105/ajph.87.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–83. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 5.Schnabl KL, Van Aerde JE, Thomson AB, Clandinin MT. Necrotizing enterolocolitis: a multifactorial disease with no cure. World J Gastroenterol. 2008;14:2142–61. doi: 10.3748/wjg.14.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dave V, Brion LP, Campbell DE, et al. Splanchnic tissue oxygenation, but not brain tissue oxygenation, increases after feeds in stable feeds in stable preterm neonates tolerating full bolus orogastric feeding. J Perinatol. 2009;29:213–8. doi: 10.1038/jp.2008.189. [DOI] [PubMed] [Google Scholar]
- 7.Wolfberg AJ, Plessis AJ. Near-infrared spectroscopy in the fetus and neonate. Clin Perinatol. 2006;33:707–28. doi: 10.1016/j.clp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Christensen RD, Lambert DK, Henry E, et al. Is “transfusion-associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion. 2010;50:1106–12. doi: 10.1111/j.1537-2995.2009.02542.x. [DOI] [PubMed] [Google Scholar]
- 9.Blau J, Calo JM, Dozor D, et al. Transfusion-related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J Pediatr. 2011;158:403–9. doi: 10.1016/j.jpeds.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Nankervis CA, Giannone PJ, Reber KM. The neonatal intesital vasculature: contributing factors to necrotizing enterocolitis. Semin Perinatol. 2008;32:83–91. doi: 10.1053/j.semperi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Mintzer JP, Parvez B, Chelala M, et al. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J Neonatal Perinatal Med. 2014;7:199–206. doi: 10.3233/NPM-14814035. [DOI] [PubMed] [Google Scholar]
- 12.Liem KD, Greisen G. Monitoring of cerebral haemodynamics in newborn infants. Early Hum Dev. 2010;86:155–8. doi: 10.1016/j.earlhumdev.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Greisen G. Cerebral blood flow and energy metabolism in the newborn. Clin Perinatol. 1997;24:531–46. [PubMed] [Google Scholar]
- 14.Andersen CC, Collins CL. Poor circulation, early brain injury, and the potential role of red cell transfusion in premature newborns. Pediatrics. 2006;117:1464–6. doi: 10.1542/peds.2005-3197. [DOI] [PubMed] [Google Scholar]
- 15.Vanderhoof JA, Zach TL, Adrian TE. Gastrointestinal disease. In: MacDonald MG, Mullet MD, Seshia MK, editors. Avery’s Neonatology: Pathophysiology and management of the newborn. 6th edition. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 949–50. [Google Scholar]
- 16.Stevenson DK, Blakely ML. Historical perspective: necrotizing enterocolitis: an inherited or acquired condition? NeoReviews. 2006;7:e125–34. [Google Scholar]
- 17.McElhinney DB, Hedrick HL, Bush DM, et al. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics. 2000;106:1080–7. doi: 10.1542/peds.106.5.1080. [DOI] [PubMed] [Google Scholar]
- 18.Papparella A. Ischemia-reperfusion injury in the intestines of newborn pigs. Pediatr Res. 1997;42:180–8. doi: 10.1203/00006450-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Hammerman C, Goldschmidt D, Caplan MS, et al. Amelioration of ischemia-reperfusion injury in rat intestine by pentoxifylline-mediated inhibition of xanthine oxidase. J Pediatr Gastroenterol Nutr. 1999;29:69–74. doi: 10.1097/00005176-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Fortune PM, Wagstaff M, Petros AJ. Cerebro-splanchnic oxygenation ratio (SCOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med. 2001;27:1401–7. doi: 10.1007/s001340100994. [DOI] [PubMed] [Google Scholar]
- 21.Krimmel GA, Baker R, Yanowitz TD. Blood transfusion alters the superior mesenteric artery blood flow velocity response to feeding in premature infants. Am J Perinatol. 2009;26:99–106. doi: 10.1055/s-0028-1090595. [DOI] [PubMed] [Google Scholar]