Abstract
Background
Great interest has been raised recently by the design of new adoptive immunotherapeutic strategies based on the in vivo infusion of ex vivo-expanded and activated natural killer (NK) cells. The development of good manufacturing practice (GMP) methods for the efficient production of fully functional NK cells is mandatory for clinical application.
Materials and methods
Peripheral blood mononuclear cells were obtained by leukapheresis and processed in the GMP facility. For NK-cell enrichment, a two-step immunomagnetic procedure consisting of CD3+ T-cell depletion followed by CD56+ cell positive selection was used. Isolated NK cells were suspended in serum-free medium containing autologous plasma, interleukin (IL)-2 and IL-15 in the presence of irradiated autologous feeder cells and cultured for 14 days at 37 °C. IL-2 and IL-15 were also added during the last 24 hours of culture. Expanded cells underwent full quality control testing for cytogenetic characteristics, viability, sterility, phenotype and endotoxin status; functional tests, such as degranulation assays and cytotoxicity, were performed on expanded NK cells before cryopreservation and after thawing.
Results
NK-cell populations expanded on average 15.7±4.7 fold by day 14, with a viability of 96% ±0.5. At the end of the incubation period, 97% ±1.1 of the expanded population was CD56+ NK cells; these effector cells showed significant up-regulation of the activating receptors NKG2D and DNAM-1. Functional tests demonstrated that expanded NK cells are fully functional with no difference whether tested before cryopreservation or after thawing.
Discussion
These data provide the basis for developing new NK-cell-based immunotherapeutic strategies for the treatment of patients with cancer.
Keywords: NK cells, GMP expansion and activation, immunotherapy, anti-cancer activity
Introduction
Natural killer (NK) cells are a subset of peripheral blood lymphocytes immunophenotypically characterised by the expression of the CD56 surface antigen and the lack of CD3 and T-cell receptor proteins; these cells are able to recognise and kill a variety of tumour-transformed cells in a human leucocyte antigen (HLA)-unrestricted fashion1,2.
The cytotoxic activity of NK cells is finely regulated by the balance between activating and inhibitory signals derived from receptors expressed on the cell surface. Among the most important receptors are the killer-cell immunoglobulin-like receptors (KIR), which display either inhibitory (KIR2DL, KIR3DL) or activating (KIR2DS, KIR3DS) functions3, the natural cytotoxicity receptors (NCR: NKp30, NKp44 and NKp46)4, NKG2D5,6 and DNAM-17, the last three of them actively concurring to tumour recognition.
The possibility of using these cells in the design of new NK-based immunotherapeutic strategies for the treatment of cancer patients has recently gained considerable interest. The need for large quantities of highly activated effector cells to produce an anti-cancer effect translates into the necessity of developing good manufacturing practice (GMP)-compliant methods for the efficient production of fully functional NK cells for clinical application.
Several protocols for ex vivo NK-cell expansion and activation have been investigated, including long-term culture with cytokines8,9 and the use of different sources of allogeneic feeder cells10–15. Despite the excellent expansion, according to transfusion regulations the majority of these protocols are not acceptable for clinical use in many countries, either because they do not utilise clinical grade materials or because of the potential transmission of infectious diseases given the use of allogeneic feeders. In addition, the proportion of contaminating CD3+ T lymphocytes remains high in the majority of these approaches, with an associated risk of Graft-versus-Host disease (GVHD) in the allogeneic context16,17; the probability of developing such a complication becomes even higher when anti-CD3 monoclonal antibody is added to the culture system with the aim of maximally activating the feeder18,19, running the risk of expanding and activating residual CD3+ cells.
The aim of this study was to develop a new GMP-compliant method for the efficient ex vivo expansion and activation of highly pure NK cells from healthy donors.
Materials and methods
Donors
Four healthy volunteer donors, after having given their written informed consent to undergo a leukapheretic procedure and subsequent biological studies, in accordance with the Declaration of Helsinki, were enrolled in the study.
Leukapheresis
The leukapheretic procedures were performed with a Fresenius Com-Tech blood cell separator (Fresenius Kabi, Friedberg, Germany) using the White Blood Cell Set (P1YA) for the collection of mononuclear cell products according to the guidelines for the collection of these cells, established and tested by Fresenius. The inlet volume estimation tool was used to calculate the inlet volume to be processed, which was usually between 6 and 10 L or twice the total blood volume. The mean white blood cell count of the leukapheretic products was 77.8±14.4×109/L (range 62.7–95.9×109/L), with a mean percentage of lymphocytes of 59.8% ±6.1 (range 53.9–66.4%).
Natural killer-cell enrichment
The cellular product obtained with the leukapheretic procedure was then processed in the GMP facility of the Italian National Institute of Health (FaBioCell). For NK-cell enrichment, a two-step closed immunomagnetic system was used, consisting of CD3+ T-cell depletion followed by CD56+ cell positive selection on a CliniMACS (Miltenyi Biotec, Bergisch Gladbach, Germany). The CliniMACS device is compliant with European conformity requirements (CE-mark class 1). Only disposable, sterile, plastic materials, separation buffer and CliniMACS tubing sets produced according to GMP standards were used. The quality assurance protocol included microbial cultures, viability, recovery and functional analysis of target cells. Isolated cells were analysed immediately after purification for phenotypic markers and then expanded. CD3 and CD56 microbeads for NK-cell enrichment were provided by Miltenyi Biotec.
Natural killer-cell expansion
For ex vivo cell expansion, isolated NK cells (2×105/mL) were suspended in CellGro® SCGM serum-free medium (CellGenix, Freiburg, Germany) supplemented with 5% autologous plasma, 500 U/mL interleukin (IL)-2 (Aldesleuchina, Proleukin®, kindly provided by NovartisFarma S.p.A., Varese, Italy) and 50 ng/mL IL-15 (CellGro®, CellGenix, Freiburg, Germany) in the presence of irradiated (35 Gy) autologous monocytes, T and B cells as feeders (5×105/mL) and cultured for 14 days at 37 °C. IL-2 and IL-15 were also added to the culture medium during the last 24 hours of the expansion period. Only clinical grade materials were used.
NK-cell expansion was performed in 225 cm2 tissue culture flasks (Thermo Scientific Nalgene and Nunc, Rochester, NY, USA) with 50 mL of complete medium. After 7 days of culture, 25 mL of fresh CellGro SCGM medium with 5% autologous plasma were added to each flask and the flask position was changed from upright to horizontal. Total cell numbers were assessed by staining cells with trypan blue dye (Sigma, St Louis, MO, USA) on day 14. The fold expansion was calculated by dividing the absolute number of viable NK cells present at the end of the culture (day 14) by the absolute number of viable NK cells at the beginning of the culture (day 0).
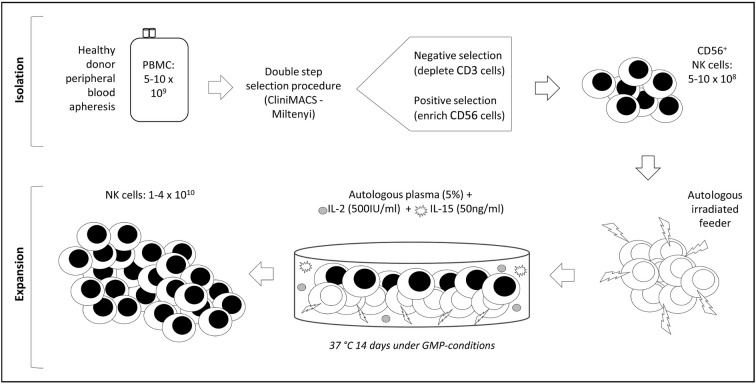
An outline of the expansion protocol is summarised in Figure 1.
Figure 1.
Clinical grade NK-cell isolation and expansion.
PBMC: peripheral blood mononuclear cells; NK: natural killer; GMP: good manufacturing practice.
Cryopreservation of expanded natural killer cells
Expanded NK cells were then harvested, counted and re-suspended in 5% human serum albumin (Baxter S.p.A, Rome, Italy) at a concentration of 40×106 cells/mL. The final product was counted and re-suspended in an equal volume of freezing medium which was prepared by mixing eight volumes of 5% human serum albumin with two volumes of dimethyl sulfoxide (WAK-Chemie Medical GmbH, Steinbach, Germany) at the final concentration of 20×106 cells/mL. The cellular product was then transferred into 2 mL cryovials (Thermo Scientific Nalgene and Nunc) that were deep-frozen under decreasing controlled temperature and stored in liquid nitrogen in vapour phase.
Characterisation of expanded natural killer cells
Expanded cells underwent full quality control testing for cytogenetic characteristics, viability, sterility, phenotype and endotoxin status. In addition, functional tests, such as degranulation assays and cytotoxicity, were performed on these cells both before cryopreservation and after thawing.
Immunofluorescence and flow cytometry
Freshly isolated and expanded NK cells were analysed by immunofluorescence using different combinations of the following monoclonal antibodies: FITC-conjugated anti-CD14 and anti-CD16; PE-conjugated anti-CD56 and anti-CD19; APC-conjugated anti-CD3, anti-Nkp30, anti-Nkp44 and anti-Nkp46 (BD Biosciences, San Jose, CA, USA); and FITC-conjugated anti-CD158a and anti-CD158e (R&D System, Minneapolis, MN, USA). NK cells were quantified as a percentage of CD56+/CD3− cells within the total population.
The expression of the NKG2D and DNAM-1 activating receptors on NK cells was evaluated using anti-NKG2D (R&D System, Minneapolis, MN, USA) or anti-DNAM-1 (AbD Serotec, Oxford, UK) unconjugated monoclonal antibodies, followed by secondary FITC-conjugated IgG1 (BioLegend, San Diego, CA, USA) staining. The level of expression of the markers analysed was quantified as the mean fluorescence intensity (MFI). Flow cytometry was carried out with a FACSCanto flow cytometer and the data were analysed using FACSDiva software (BD Bioscience, San Jose, CA, USA).
Degranulation assays
Expanded NK cells were analysed by flow cytometry before and after cryopreservation to measure CD107a expression on the surface of NK cells following activation and degranulation. The assay was performed in the presence of either the K562 target cells (final ratio NK:K562 of 10:1, 5:1, 2:1 and 1:1) or the non-specific stimulators phorbol-12-myristate-13-acetate (PMA) and ionomycin (both from Sigma, St Louis, MO, USA) at a final concentration of 2.5 μg/mL and 0.5 μg/mL, respectively. NK cells were incubated for 1 hour with FITC-conjugated anti-CD107a (BD Biosciences) at 37±1 °C; a 2 μM solution of monensin (BD Biosciences), a protein transport inhibitor, was then added to the medium for a further 3 hours. Finally, cells were analysed for CD56 and CD107a expression.
Cytotoxic capacity
The cytotoxic activity of expanded NK cells against the K562 cell line, before and after cryopreservation, was determined in a standard 4-hour 51chromium release assay. NK effector:target cell (E:T) ratios ranged from 50:1 to 0.39:1, using 2×103 target cells in triplicate wells. After incubation at 37 °C, plates were centrifuged and 25 μL of culture supernatant were transferred into Luma plates (Perkin Elmer, Ontario, Canada) and counted in a γ-counter (Top Count, Perkin Elmer). The percentage of specific lysis was calculated as follows:
Statistical analysis
Statistical analysis was performed using a Student’s paired t test. Statistical significance was set at p<0.05.
Results
The results obtained in this study are summarised in Table I.
Table I.
Data on GMP-production of ex vivo expanded NK cells for clinical use.
| Apheresis (n=4) | Batch n. 11009 | Batch n. 12001 | Batch n. 12005 | Batch n. 12007 |
|---|---|---|---|---|
| PBMC | 8.4×109 | 12×109 | 8.8×109 | 7×109 |
| CD56+ cells | 11% | 8% | 4.9% | 8% |
| PBMC for CD3 depletion | 3.9×109 | 8.62×109 | 5.58×109 | 6.22×109 |
| PBMC for CD56 enrichment | 2.4×109 | 3.76×109 | 1.925×109 | 2.73×109 |
| Selected CD56+ cells | 390×106 | 791×106 | 112×106 | 130×106 |
| CD56+ cell recovery | 33% | 68% | 26% | 23% |
|
| ||||
| Quality controls | ||||
| Viability | 100% | 99% | 100% | 100% |
| Culture of supernatant | Sterile | Sterile | Sterile | Sterile |
| Culture of expanded NK cells | Sterile | Sterile | Sterile | Sterile |
| Mycoplasma | Negative | Negative | Negative | Negative |
| Endotoxin | <1 EU/mL | <1 EU/mL | <1 EU/mL | <1 EU/mL |
| Cytogenetics | 46, XY | 46, XY | 46, XY | 46, XY |
|
| ||||
| Functional tests | ||||
| Degranulation assay (fresh NK cells) | NA | 81% (PMA-I) | 88% (PMA-I) | 83% (PMA-I) |
| Cytotoxicity vs K562 (50:1) (fresh NK cells) | 72% | 85% | 78% | 83% |
| Viability after thawing | 92% | 90% | 94% | 85% |
| Degranulation assay (cryopreserved NK cells) | 75% (PMA-I) | 88% (PMA-I) | 90% (PMA-I) | 85% (PMA-I) |
| Cytotoxicity vs K562 (50:1) (cryopreserved NK cells) | 80% | 85% | 70% | 78% |
NK: natural killer; PBMC: peripheral blood mononuclear cells; EU: Endotoxin Unit; NA: not available; PMA: phorbol-12-myristate-13-acetate.
Isolation of natural killer cells
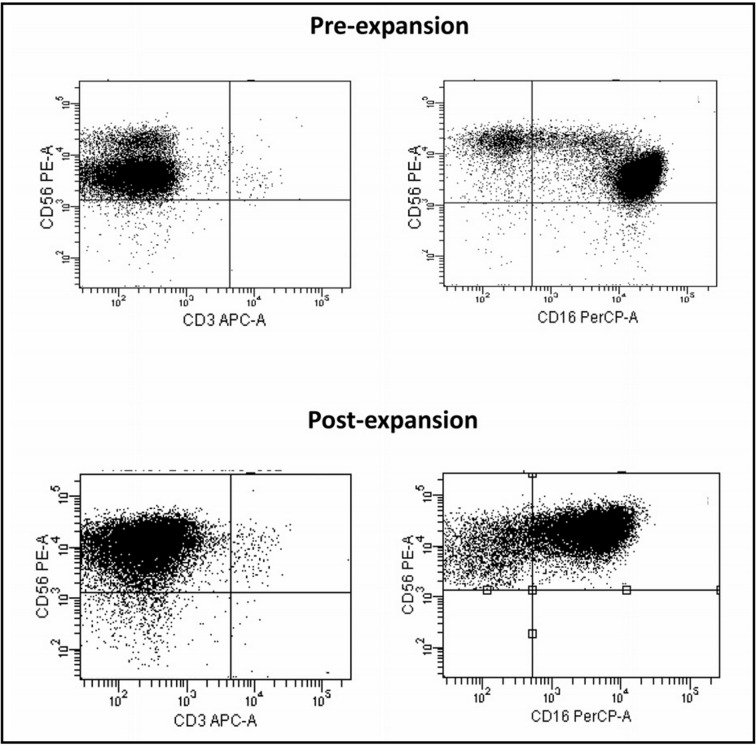
The mean percentage of NK cells in the leukapheretic products was 8% ±2.5 (range, 5–11%); at the end of the isolation process, an average of 94% ±2.4 CD56+ cells (range, 91–96%) was obtained. Upon selection, the mean percentage of the CD3+ cell population was 0.5% ±0.6 (Figure 2). A minimal contamination by CD19+ B cells, as well as CD14+ monocytes, was also observed (1.3% ±2.1 and 4.4% ±1.4, respectively).
Figure 2.
Phenotypic analysis of NK cells obtained after performing the isolation procedure compared to the phenotype after expansion (one representative case).
NK: natural killer.
Expansion of natural killer cells
The NK-cell populations expanded on average 15.7±4.7 fold (range, 9–19) by day 14, with a viability of 96% ±0.5; at this time point the expansion potential reached a plateau, thus translating into reduced fold expansion when measured later on (days 21 and 28) (data not shown). Neither the presence of feeder cells alone nor that of cytokines alone in the culture medium was associated with the same fold expansion. In particular, the presence of cytokines alone produced on average a 3.4±2.1 fold expansion of the NK-cell populations.
Immunophenotypic characterisation of expanded natural killer cells
The phenotypic analysis performed on healthy donor expanded cells revealed that CD56+ NK cells dominated the culture also at the end of the incubation period, reaching a mean percentage of 97% ±1.1 of CD56+ cells (range, 96–98%). There was no proliferation of the feeder cell population and both B lymphocytes and monocytes disappeared completely.
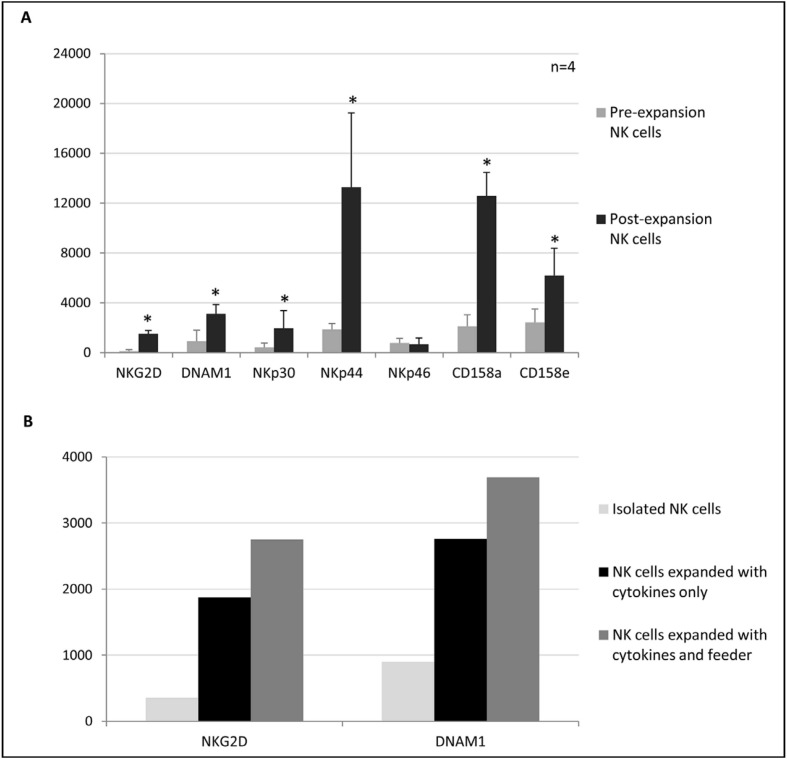
Expanded NK cells were mainly composed of a CD56bright population (94% ±2.6). During the culture period, CD56 MFI increased from 3,375±457 to 31,550±7,918 (p=0.003); 73.5% ±19.4 of the cells had a CD56brightCD16+ phenotype, probably originating from the CD56dimCD16+ population, while the remaining 26.5% ±19.3 was composed of a CD56brightCD16dim population already present in the culture after enrichment. After expansion, the mean percentage of the CD3+CD56− cell population was 0.2% ±0.3 (Figure 2). Expanded NK cells also showed a significant up-regulation of the activating receptors NKG2D (p=0.028), DNAM-1 (p=0.002), Nkp30 (p=0.0001) and Nkp44 (p<0.0001), and of the inhibitory KIR CD158a (p<0.0001) and CD158e (p=0.0005) (Figure 3A).
Figure 3.
(A) Changes in the phenotypic expression pattern on NK cells following expansion with cytokines plus autologous feeder.
Results are expressed as mean fluorescence intensity ± standard deviation.
(B) Levels of NKG2D and DNAM1 expression before and after expansion with cytokines alone or with cytokines plus autologous feeder (one representative case).
*p≤0.05. NK: natural killer.
Finally, no significant differences were observed when comparing the up-regulation of the activating receptors NKG2D and DNAM1 obtained after expansion with cytokines plus feeder or with cytokines alone (Figure 3B); nonetheless, a trend towards a higher expression of these markers with cytokines plus feeder was evident.
Degranulation capacity of expanded natural killer cells against the K562 cancer cell line
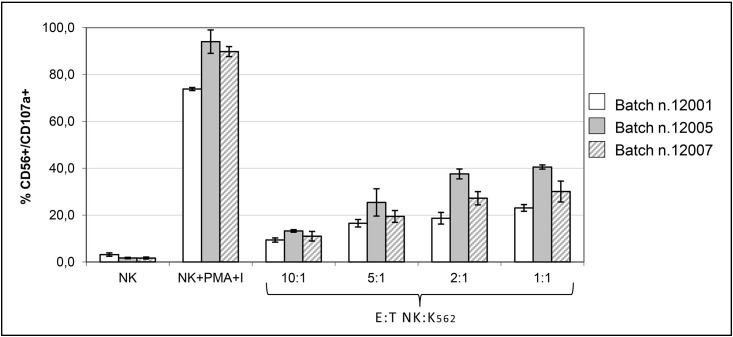
The degranulation capacity of fresh and cryopreserved expanded NK cells was evaluated by measuring the expression of CD107a on the cell surface.
Cryopreserved expanded NK cells were analysed in the presence of K562 target cells as well as after stimulation with PMA and ionomycin, whereas fresh expanded NK cells were analysed only in the presence of PMA and ionomycin. Fresh and cryopreserved expanded NK cells stimulated with PMA and ionomycin showed a similar degranulation capacity, with the mean percentage of CD107a expression being 84% ±3.6 and 87.6% ±2.5, respectively.
Cryopreserved expanded NK cells analysed in the presence of the K562 cancer cell line also showed significant CD107a expression with respect to unstimulated NK cells, with a mean percentage of 31.2% ±8.7 at an E:T ratio of 1:1 (Figure 4).
Figure 4.
CD107a expression on cryopreserved expanded NK cells after stimulation with PMA and ionomycin (I) and in the presence of the K562 cell line.
Each bar represents the mean percentage of triplicate measurements ± standard deviation.
NK: natural killer; PMA: phorbol-12-myristate-13-acetate; E:T: effector:target.
Cytolytic activity of expanded natural killer cells against the K562 cancer cell line
To evaluate the cytolytic properties of expanded NK cells against leukemic cells, we performed cytotoxic assays based on 51chromium release using the K562 cancer cell line as the target.
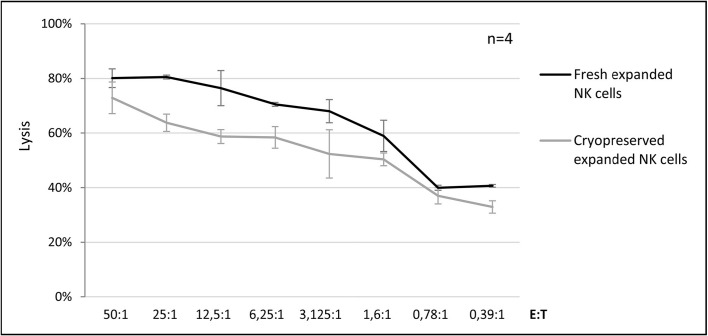
The results obtained revealed that both fresh and cryopreserved expanded NK cells mediated efficient lysis of the K562 cell line, with a mean percentage of killing of 80.1% ±3.4 and 72.9% ±5.8, respectively, at an E:T ratio of 50:1 (Figure 5).
Figure 5.
Comparison between the lytic activity against the K562 cell line of fresh and cryopreserved expanded NK cells.
Data are expressed as mean percentage of lysis ± standard deviation of four independent experiments. NK: natural killer; E:T: effector:target.
Discussion
Biological and targeted therapies have recently gained considerable interest for the treatment of cancer patients20. The onset of treatment resistance and the long-term toxic effects of current therapies are reasons why new therapeutic approaches are needed, particularly for the management of the many patients who due to age or comorbidities cannot be treated with conventional chemotherapy regimens.
The anti-neoplastic potential of NK cells is well documented1,2,21,22 and many reports have highlighted the anti-tumour activity of these effectors, mainly in the context of haematological malignancies23–27, both in paediatric and adult patients. NK cells are particularly promising candidates for adoptive immunotherapy also because they can be generated and infused in both autologous and allogeneic settings. For this purpose, the elaboration of a clinical grade method for the production of active effectors is essential.
In this study, we have developed and tested a GMP-compliant method to generate large numbers of highly enriched and activated NK cells from healthy donors for clinical use. The application of this method resulted in the efficient production of effector cells with significantly increased expression of activating receptors important in tumour cell recognition, including NKG2D, DNAM1, Nkp30 and Nkp44, when compared to freshly isolated NK cells. The two-step NK-cell selection procedure described here ensures very high purity of the final product, as demonstrated by a minimal contamination by CD3+ cells belonging to the T- and NK-T compartments; this is important in order to minimise the risk of inducing GVHD when the product is used in the allogeneic context. Furthermore, the high level of B-cell depletion achieved with this system is also an important condition to prevent Epstein-Barr virus reactivation, which may potentially trigger lymphoproliferative diseases in immunocompromised patients.
The expansion strategy utilised in this protocol has several other advantages, including: (i) the lack of in vivo cytokine infusion which can cause systemic toxicity; (ii) the use of clinical grade manufactured IL-2 and IL-15, which play essential roles in NK-cell development, expansion, homeostasis and activation28,29; (iii) the use of autologous feeder cells to prevent the unknown effects associated with the use of allogeneic feeders; and (iv) the absence of anti-CD3 monoclonal antibody in the culture system, thus reducing the risk of GVHD in the allogeneic setting. When anti-CD3 monoclonal antibody was added to a GMP expansion system otherwise similar to the one utilised in our work, despite further increases in the fold expansion, a high level of residual T-cell proliferation was observed, eventually obliging the authors to perform a CD3+ depletion step after expansion at the expense of significant NK cell loss19.
In our model, both cytokines and feeder cells are necessary to obtain optimal NK-cell proliferation, since less expansion was observed in the presence of cytokines or feeder cells alone. Under these conditions, the degree of NK-cell expansion was on average 15.7-fold. When tested in a cytotoxic assay against the K562 cell line, these expanded effector cells proved to be highly active. This confirms recently published data from our group documenting the ability of expanded NK cells to recognise and kill primary acute leukaemia blast cells23–24. Similar results were obtained when the expanded effectors were tested in a degranulation assay. Interestingly, these cells exerted a comparable cytotoxic activity when used prior to cryopreservation and after thawing; this is extremely important since ex vivo expanded cells must be cryopreserved before in vivo infusion in adoptive cell therapy protocols, in order to allow the necessary quality tests to be carried out before the batch of cells can be released for clinical use.
Conclusion
In conclusion, in the present study we describe a new method for expanding functional NK cells under GMP-suitable conditions from healthy donors, providing the basis for developing the design of innovative immunotherapeutic strategies for the management of patients with cancer.
Acknowledgements
The Authors thank Miltenyi Biotec (Bergisch Gladbach, Germany) for providing the CD3 and CD56 microbeads for the NK-cell enrichment.
Footnotes
Funding and resources
Research grant support: Associazione Italiana per la Ricerca sul Cancro (AIRC) special projects 5×1,000, Milan, Italy; Ministero della Salute, Fondazione Italiana di Ricerca in Medicina Sperimentale (FIRMS); Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR), Fondo per gli investimenti della ricerca di base (FIRB).
Authorship contributions
GFT designed the study, analysed the data and wrote the manuscript; CR designed the study, analysed the data, performed GMP expansion of NK cells and edited the manuscript; LS, GDA, EF, MRP, DMM and DC performed GMP expansion of NK cells, cytofluorimetric analyses and degranulation assays; NP designed the study, performed GMP expansion of NK cells, cytofluorimetric analyses and cytotoxic assays; PM and SP performed cytofluorimetric analyses and cytotoxic assays; MG, MSB and GG performed leukapheresis of donors; AG and FB designed the study, interpreted results and edited the manuscript; RF designed the study, interpreted results, wrote and edited the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–38. [PubMed] [Google Scholar]
- 2.Miller JS. The biology of natural killer cells in cancer, infection, and pregnancy. Exp Hematol. 2001;29:1157–68. doi: 10.1016/s0301-472x(01)00696-8. [DOI] [PubMed] [Google Scholar]
- 3.Thielens A, Vivier E, Romagné F. NK cell MHC class I specific receptors (KIR): from biology to clinical intervention. Curr Opin Immunol. 2012;24:239–45. doi: 10.1016/j.coi.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Moretta A, Bottino C, Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 5.Coudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol. 2006;16:333–43. doi: 10.1016/j.semcancer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007;7:737–44. doi: 10.1038/nri2144. [DOI] [PubMed] [Google Scholar]
- 7.Shibuya A, Campbell D, Hannum C, et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–81. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 8.Carlens S, Gilljam M, Chambers BJ, et al. A new method for in vitro expansion of cytotoxic human CD3-CD56+ natural killer cells. Hum Immunol. 2001;62:1092–8. doi: 10.1016/s0198-8859(01)00313-5. [DOI] [PubMed] [Google Scholar]
- 9.McKenna DH, Jr, Sumstad D, Bostrom N, et al. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–8. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 10.Torelli GF, Guarini A, Maggio R, et al. Expansion of natural killer cells with lytic activity against autologous blasts from adult and pediatric acute lymphoid leukemia patients in complete hematologic remission. Haematologica. 2005;90:785–92. [PubMed] [Google Scholar]
- 11.Luhm J, Brand JM, Koritke P, et al. Large-scale generation of natural killer lymphocytes for clinical application. J Hematother Stem Cell Res. 2002;11:651–7. doi: 10.1089/15258160260194794. [DOI] [PubMed] [Google Scholar]
- 12.Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–7. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perussia B, Ramoni C, Anegon I, et al. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat Immun Cell Growth Regul. 1987;6:171–88. [PubMed] [Google Scholar]
- 14.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–9. [PubMed] [Google Scholar]
- 15.Boissel L, Tuncer HH, Betancur M, et al. Umbilical cord mesenchymal stem cells increase expansion of cord blood natural killer cells. Biol Blood Marrow Transplant. 2008;14:1031–8. doi: 10.1016/j.bbmt.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Suck G, Koh MB. Emerging natural killer cell immunotherapies: large-scale ex vivo production of highly potent anticancer effectors. Hematol Oncol Stem Cell Ther. 2010;3:135–42. doi: 10.1016/s1658-3876(10)50024-4. [DOI] [PubMed] [Google Scholar]
- 17.Miller JS. Should natural killer cells be expanded in vivo or ex vivo to maximize their therapeutic potential? Cytotherapy. 2009;11:259–60. doi: 10.1080/14653240902888000. [DOI] [PubMed] [Google Scholar]
- 18.Ahn YO, Kim S, Kim TM, et al. Irradiated and activated autologous PBMCs induce expansion of highly cytotoxic human NK cells in vitro. J Immunother. 2013;36:373–81. doi: 10.1097/CJI.0b013e3182a3430f. [DOI] [PubMed] [Google Scholar]
- 19.Siegler U, Meyer-Monard S, Jörger S, et al. Good manufacturing practice-compliant cell sorting and large-scale expansion of single KIR-positive alloreactive human natural killer cells for multiple infusions to leukemia patients. Cytotherapy. 2010;12:750–63. doi: 10.3109/14653241003786155. [DOI] [PubMed] [Google Scholar]
- 20.Cramer P, Hallek M. Hematological cancer in 2011: new therapeutic targets and treatment strategies. Nat Rev Clin Oncol. 2012;9:72–4. doi: 10.1038/nrclinonc.2011.212. [DOI] [PubMed] [Google Scholar]
- 21.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 22.Knorr DA, Bachanova V, Verneris MR, Miller JS. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol. 2014;26:161–72. doi: 10.1016/j.smim.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peragine N, Torelli GF, Mariglia P, et al. Immunophenotypic and functional characterization of ex vivo expanded natural killer cells for clinical use in acute lymphoblastic leukemia patients. Cancer Immunol Immunother. 2015;64:201–11. doi: 10.1007/s00262-014-1614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torelli GF, Peragine N, Raponi S, et al. Recognition of adult and pediatric acute lymphoblastic leukemia blasts by natural killer cells. Haematologica. 2014;99:1248–54. doi: 10.3324/haematol.2013.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachanova V, Burns LJ, McKenna DH, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59:1739–44. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–9. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curti A, Ruggeri L, D’Addio A, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–9. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 28.Liao W, Lin J-X, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croce M, Orengo AM, Azzarone B, Ferrini S. Immunotherapeutic applications of IL-15. Immunotherapy. 2012;4:957–69. doi: 10.2217/imt.12.92. [DOI] [PubMed] [Google Scholar]