The debate over whether or not oocytes are regenerated in mammalian ovaries during adulthood as a way to maintain the ovarian follicle pool and fertility has been reignited with the recent proposals that female germline stem cells (FGSCs) or oogonial stem cells (OSCs) can be found in the mammalian ovary.1,2 Many researchers believe that the claims of OSCs were made too hastily, and a distinguished team of reproductive biologists stated that rigorous scientific standards should be applied to the in vitro-derived female germ cells.3 For example, an intriguing question is how the proposed OSCs can enter meiosis, which is a critical step in the development of mammalian oocytes. This process has been demonstrated in primordial germ cells (PGCs), but has never been shown for the proposed OSCs.
Although the in vitro-obtained OSCs and FGSCs have not been accurately identified,4 it is still an interesting question to ask if mammalian oocytes renew themselves in adult life at all. It is tempting to think that because male spermatogonia are constantly regenerated, it must be the same way in females. As a matter of fact, the female germ cells do regenerate themselves, but only within a limited period of time and only in the form of migrating and proliferating female PGCs.5 Any philosophical belief that OSCs must exist in females, however, requires strong experimental evidence before such cells can be considered biologically relevant.
In 2013, a study by Lei et al. used a sensitive lineage-labeling system to determine whether oocyte regeneration is needed in female adult mice to compensate for follicular losses.6 After labeling the primordial follicles in early life and tracing the stability of the follicular pool at different time points in adulthood in mice, the authors showed that the primordial follicles formed during early life is sufficient to sustain the entire reproductive life without renewal. This research provided compelling evidence that adult female mice neither require nor contain active germline stem cells nor do they produce new oocytes in vivo.
Further scientific evidence was provided in a recent study published by Zhang et al. that was based on very simple and straightforward approaches.7 The authors used an inducible labeling system with the Foxl2-CreERT2 mouse model where all follicles in the mice were labeled at a young age with green fluorescence. When the animals reached adult age and to the end of the reproductive life, all of the remaining follicles were still labeled with green fluorescence (Fig. 1a). The conclusion of this follicle lineage tracing is straightforward: all of the follicles at the end of the mouse's reproductive life were from the original follicle pool that developed in the mouse right after birth.7
Figure 1.
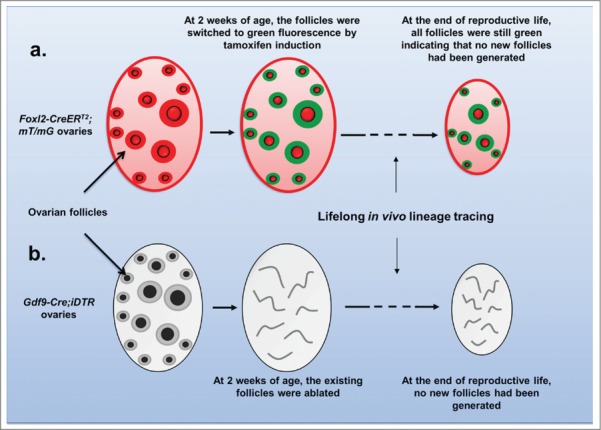
Lifelong in vivo tracing of follicle lineages in mouse ovaries. Using a Foxl2 -CreERT2;mT/mG mouse model (A) or Gdf9-Cre;iDTR mouse model (B), the existing follicle pool is selectively labeled or ablated in early life in vivo. At the end of the reproductive lives of the animals, no follicular regeneration is observed.
It has been argued that when oocytes are depleted from the ovary, such as by chemotherapy drugs, new oocytes can be regenerated from OSCs.1 Zhang et al. also tackled this question using the Gdf9-Cre;iDTR mouse model where all oocytes were selectively ablated in the early life of the mouse (Fig. 1b).7 At the end of the mouse's reproductive life, not a single regenerated oocyte or follicle was observed. This result indicates that under pathological conditions where oocytes were depleted from the ovary, there is also no adult oogenesis7 (Fig. 1b).
Based on the 2 studies by Lei et al.6 and Zhang et al.,7 it can now be concluded that oocytes are not regenerated in the mouse during adult life. The proposed OSCs and FGSCs are not functional in contributing oocytes for natural female fertility or for regenerating follicles under pathological conditions. The purified OSCs and FGSCs, regardless of where they originate from, be it the bone marrow, the ovarian epithelium, or the blood circulation, are not stem cells for the oocytes that contribute to natural female fertility because ovaries do not appear capable of regenerating oocytes in adult life.
References
- 1. Zou K, et al. Nat Cell Biol 2009; 11:631-6; PMID:19363485; http://dx.doi.org/ 10.1038/ncb1869 [DOI] [PubMed] [Google Scholar]
- 2. White YA, et al. Nat Med 2012; 18(3):413-21; PMID:22366948; http://dx.doi.org/ 10.1038/nm.2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Handel MA, et al. Cell 2014; 157:1257-61; PMID:24906145; http://dx.doi.org/ 10.1016/j.cell.2014.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang H, et al. Reproduction 2013; 146:R229-233; PMID:24006465; http://dx.doi.org/ 10.1530/REP-13-0202 [DOI] [PubMed] [Google Scholar]
- 5. McLaren A. 2003; Dev Biol 262:1-15; PMID:14512014; http://dx.doi.org/ 10.1016/S0012-1606(03)00214-8 [DOI] [PubMed] [Google Scholar]
- 6. Lei L, Spradling AC. Proc Natl Acad Sci U S A 2013; 110(21):8585-90; PMID:23630252; http://dx.doi.org/ 10.1073/pnas.1306189110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang H, et al. Proc Natl Acad Sci U S A 2014; 111:17983-8; PMID:25453063; http://dx.doi.org/ 10.1073/pnas.1421047111 [DOI] [PMC free article] [PubMed] [Google Scholar]


