Abstract
Esophageal adenocarcinoma (EAC) is one of the fastest growing malignancies in the US and needs newer therapeutic and diagnostic strategies. Chronic inflammation plays a role in the pathogenesis of EAC and contributes to the dysplastic conversion of normal esophageal epithelium to Barrett's esophagus and frank adenocarcinoma. Chemokines play important roles in mediating inflammation and recent evidence implicates these ligands and their receptors in the development and spread of various tumors. We demonstrated that the chemokines IL8, CXCL1 and CXCL3 are significantly overexpressed during esophageal carcinogenesis and accompanied by amplification and demethylation of the chr4q21 gene locus. We also demonstrated that IL8 levels can be detected in serum of patients with EAC and can serve as potential biomarkers. We now demonstrate that inhibition of IL8 receptor, CXCR2, leads to decreased invasiveness of esophageal adenocarcinoma derived cells without affecting cellular proliferation. Taken together, these studies reveal the important roles that chemokines play in development of esophageal cancer and demonstrate that these pathways can serve as potential therapeutic targets.
Keywords: barrett's esophagus, chemokines, CXCR2, esophageal adenocarcinoma, IL8
Introduction
Esophageal cancer is the fastest growing cancer in the United States and other industrialized nations. Esophageal adenocarcinoma like many other malignancies arises in the setting of chronic inflammation and is preceded by a premalignant condition called Barrett's esophagus. Many studies have pointed to a role of chemokines and chemokine receptors in cancer growth and metastasis. Our work and other recent findings have shown that chemokine signaling is also very important in esophageal carcinogenesis and can act as a potential therapeutic target in this disease.
Chemokines and Chemokine Receptors
Chemokines are a group of small (∼8–14 kDa), structurally related, mostly basic, molecules that act through 7-transmembrane G protein-coupled receptors (GPCRs).1 They play important roles in growth, differentiation and activation of various cells including those involved in immune responses.2
Since the identification of IL-8 (CXCL8) and MCP1 (CCL2) in the late 1980s, the chemokine family has significantly expanded with identification of over 40 ligands and their receptors (Table 1); helping us understand their role not only in inflammatory responses and allergic phenomenon but also in cancers, tissue homeostasis and wound healing.
Table 1.
Chemokine ligands and receptors
| Chemokine |
Receptor |
|
|---|---|---|
| Ligands | Agonistic | Antagonistic |
| CXC Subfamily | ||
| CXCL1 | CXCR2 | |
| CXCL2 | CXCR2 | |
| CXCL3 | CXCR2 | |
| CXCL4 | CXCR3-B | |
| CXCL4L1 | CXCR3-B | |
| CXCL5 | CXCR2 | |
| CXCL6 | CXCR1, CXCR2 | |
| CXCL7 | CXCR1, CXCR2 | |
| CXCL8 | CXCR1, CXCR2 | |
| CXCL9 | CXCR3 | CCR3 |
| CXCL10 | CXCR3 | CCR3 |
| CXCL11 | CXCR3, CXCR7 | CCR3, CCR5 |
| CXCL12 | CXCR4, CXCR7 | |
| CXCL13 | CXCR5, CXCR3 | |
| CXCL14 | UNKNOWN | |
| CXCl16 | CXCR6 | |
| CXCL17 | UNKNOWN | |
| CC subfamily | ||
| CCL1 | CCR8 | |
| CCL2 | CCR2 | |
| CCL3 | CCR1, CCR5 | |
| CCL3L1 | CCR1, CCR3, CCR5 | |
| CCL3L3 | ||
| CCL4 | CCR5 | |
| CCL4L1 | ||
| CCL4L2 | ||
| CCL5 | CCR1, CCR3, CCR5 | |
| CCL7 | CCR1, CCR2, CCR3 | CCR5 |
| CCL8 | CCR1, CCR2, CCR5 | |
| CCL11 | CCR3, CCR5 | CXCR3, CCR2 |
| CCL13 | CCR2, CCR3 | |
| CCL14 | CCR1, CCR3, CCR5 | |
| CCL15 | CCR1, CCR3 | |
| CCL16 | CCR1, CCR2, CCR5, CCR8, H4 | |
| CCL17 | CCR4 | |
| CCL18 | PITPNM3 | |
| CCL19 | CCR7 | |
| CCL20 | CCR6 | |
| CCL21 | CCR7 | |
| CCL22 | CCR4 | |
| CCL23 | CCR1, FPRL-1 | |
| CCL24 | CCR3 | |
| CCL25 | CCR9 | |
| CCL26 | CCR3, CX3CR1 | CCR1, CCR2, CCR5 |
| CCL27 | CCR10 | |
| CCL28 | CCR10, CCR3 | |
| XC Subfamily | ||
| XCL1 | XCR1 | |
| XCL2 | XCR1 | |
| CX3C Subfamily | ||
| CX3CL1 | CX3CR1 | |
Chemokine ligands
Structurally chemokines are classified into 4 subfamilies namely CC, CXC, CX3C and (X)C, depending on the arrangement of N-terminal 2 cysteine residues.1 Functionally they can be seen as inflammatory, homeostatic (regulating bodily functions) or dual function chemokines (i.e., they are homeostatic and also get upregulated during the inflammatory response).3 This functional classification is rather operational than mutually exclusive. Inflammatory chemokines are chemo-attractants and the CXC chemokines attract neutrophils and lymphocytes while CC chemokines attract lymphocytes and monocytes.4 Some inflammatory CXC chemokines have an ELR (Glu-Leu-Arg) motif just prior to first cysteine residue and these exert angiogenic effects through the CXCR1 and CXCR2 receptors. On the other hand chemokines like CXCL4, L9-L10 that lack this ELR motif, are angiostatic.5 Thus chemokines contributes in new vessel formation at the site of inflammation depending on the molecular signal.
Homeostatic chemokines are constitutively expressed in the lymphoid and other tissues and helps in migration and homing of various cells like lymphocytes and dendritic cells. The inflammatory chemokines are relatively new in evolutionary history and hence show variation between the species while homeostatic chemokines are ancient, well conserved and function in a more predictable manner.6
Chemokine receptors
The chemokine receptors are Class A GPCRs coupled with Gαi heterotrimeric G protein. They are also grouped in 4 subfamilies.7 The inflammatory chemokines are more in number than their receptors and chemokine ligands are shared by multiple receptors.8 This raises the possibility of functional redundancy also likely to be modulated by both spatial and temporal control of expression. For example natural antagonism is seen between the ligands of CXCR3 and CCR3, thus CXCL9, CXCL10 and CXCL11 are natural antagonists for CCR3 whereas CCL11 is a natural antagonist for CXCR3.9 The chemokine GPCRs signal through heterotimeric G-proteins which in turn regulate a diversity of signal transduction pathways involved in chemotaxis that include mitogen-activated protein (MAP) kinases, phospholipase-cβ, phospholipase 3-kinase (PI3K) and RAS or Rho GTPases.10 Interestingly the receptors can also bind with non-chemokine ligands such as Macrophage migration inhibitory factor (MIF) (to CXCR2 and CXCR4),11 anti-microbial peptides such as β-defensins (to CCR6)12 and extracellular ubiquitin (to CXCR4).13 Receptors for the Dual function and homeostatic chemokines on the other hand show a more restricted ligand usage with one or 2 ligands acting on a particular receptor in a specific manner.6
In addition to the above-mentioned “typical” receptors, certain atypical receptors are also known namely D6, Duffy antigen receptor for chemokines (DARC) and CCX-CKR (ChemoCentryx, chemokine receptor).13 These receptors are also heptahelical but do not transduce the signals due to the lack of DYR motif in the second intracellular loop needed for interaction with Gαi class of G-proteins. These probably function as decoy receptors, scavengers or as transporters for the ligands.14
Chemokines also interact with glycosaminoglycans (GAGs) and this binding is essential for presentation of chemokines over the endothelial layers and for migration of leukocytes.15
Role of chemokines in cancers
Chronic inflammation plays a key role in the initiation or progression of cancers of the lung, colon, liver, breast, cervix, prostate, bladder, ovary, esophagus, skin and lymphatics.16-19 Dynamic interaction between the tumor cells and the cells of the tumor microenvironment facilitates tumor growth and spread. Both the tumor cells and stromal cells elaborate chemokines, thereby recruiting different cell types, namely tumor-associated macrophages (TAMs), Tumor-associated neutrophils (TANs) and lymphocytes, cancer-associated fibroblasts (CAFs), mesenchymal stem cells (MSCs) and endothelial cells, to the tumor microenvironment. These infiltrating cells provide additional sources of chemokines that affects, tumor growth, survival, aging, angiogenesis, metastasis to distant sites and immune evasion.20
Tumor proliferation and immune evasion
CXCL12 secreted by stromal fibroblasts from the tumor microenvironment can bind CXCR4 on tumor cells and stimulate cell motility/chemotaxis.21 Interaction of CXCL12 with CXCR7 mediates cellular proliferation.22 The CXCR4 driven pathways have been shown to drive malignant growth in multiple tumor models.18-20 The chemokine CCL2, is widely expressed in many carcinomas and its production corresponds to macrophage recruitment.23,24 For instance, in esophageal and breast cancer cells, CCL2 expression is correlated with high TAM influx, lymph node metastasis and a poor prognosis.25 TAMs and CCL2 may have pro-tumorigenic role. TAMs stimulated by chemokines produce growth factors such as epidermal growth factor (EGR) and transforming growth factor (TGF-β), benefitting tumor cell proliferation25 Pro-inflammatory chemokines (specially CCL2)26 produced by infiltrating leukocytes and the neoplastic tissue may recruit Th2 cells and regulatory-T cell (Treg).4 Treg (CD4+CD25+) cells, suppress immune attack against self antigen and avoid autoimmunity. Macrophages stimulated by these Th2 T-lymphocytes (M2 macrophages), promote tumor growth and progression by elaborating the immunosuppressive cytokine, IL-10.20 Also TAM derived TGF-β can convert infiltrating CD4+CD25− T cells to CD4+CD25+ T cells, allowing immune evasion by the tumor cells thereby helping proliferation.27
Fibroblasts also play important roles in secretion of chemokines in the tumor microenvironment. Normal or resting fibroblasts in the tumor microenvironment get activated via effects of TGF-β to become TAFs.28-30 TAFs form bulk of the tumor stroma and are also the main source of CXCL12; the chemokine implicated in promoting tumor proliferation. TAFs also increase angiogenesis by recruiting endothelial cells.31 Within the tumor milieu the TGF-β stimulation also polarizes the infiltrating neutrophils to N2 state (increased expression of arginase and chemokines such as CCL2 and CCL5). These N2 TANs display pro-tumoral properties.31
Angiogenesis and metastasis
Formation of blood vessels and blood vessel density is correlated with higher incidence of metastases and more rapid disease recurrence.32,33 ELR+ Chemokines such as CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7 and CXCL8, that are promoters of tumor angiogenesis, bind to the CXCR2 and CXCR1 recptors.34-36 CXCR2 is the primary receptor for angiogenesis, and is required for endothelial cell chemotaxis.37,38 This receptor binds to all the ELR+ chemokines and regulates the response of endothelial cells to CXCL8.39 In-vitro studies have shown a link between prostaglandins and chemokines in promoting angiogenesis. Prostaglandin E2 (PGE2) increases expression of CXCL1 in a MAPK-dependent manner thus favoring endothelial cell migration and tube formation.40 Similarly, CXCL8 down-regulation using prolylhydroxylase (PHD)2 was shown to reduce angiogenesis.41 Knockdown of PHD2 in colon cells increased tumor growth and angiogenesis, possibly mediated by an increased NF-κB activity as well as by induction of CXCL8 and angiogenin.41 Decoy receptors for the chemokines such as D6 and Duffy antigen/receptor for chemokines (DARC) have been shown to have inverse relationship with angiogenesis. D6 has been shown to reduce CCL chemokine recruitment in a mouse model of skin inflammation.42 Studies using the TRAMP transgenic model of prostate cancer showed that mice with null DARC showed increased tumor growth and vascularization compared to TRAMP mice with DARC expression, probably due to defect in clearing of angiogenic chemokines in DARC null-mice.43 DARC overexpression has been inversely correlated to microvessel density, lymph node status and distant metastasis.44 CXCL12 although not an ELR+ CXC chemokine is also involved in promoting angiogenesis. It increases the expression of vascular endothelial growth factor (VEGF) by endothelial cells. VEGF in turn up-regulates CXCR4 expression over endothelial cells,45 the receptor implicated in metastasis. Tumor angiogenesis thus can be viewed as impaired balance of pro- and anti- angiogenic factors between normal and cancer tissues. In tumor angiogenesis there is thus, not only an increase in angiogenic chemokines levels, but also decrease in decoy receptors and other triggers, further favoring angiogenic switch.
Metastatic tumors express embryonic stem cell transcription factors and utilize stromal cell-derived factor-1 (SDF-1) /CXCR4-mediated migration,46 as seen in migration of embryonic and adult stem cells.47 Concentration gradient based metastasis to distant sites was described for the binding pairs such as CXCR4/CXCL12 (bone metastasis), CCR9/CCL9-CCL21 (lymph node metastasis) and CCR10/CCL27 (skin metastasis).48 Certain other chemokine receptor/ligand pairs that favor tumor metastasis to specific sites based on concentration gradient of the ligands has been discovered such as CX3CR1 producing pancreatic ductal carcinoma metastasizing to neurons and nerve fibers (higher concentration of CX3CL1)49; CCR9 positive melanoma to small intestines (higher levels of CCL25)50 and CXCR2 positive breast cancer cells to lungs (higher CXCL1 levels).51 Studies have proposed that tumors might be generating the gradient and actively promoting their own metastasis52 and tropism, as observed during cell migration toward lymphatic endothelia in a CCR7-dependent manner; more pronounced in slow interstitial flow conditions.53 This raises the possibility that cancer cells may control their own rolling capacity by affecting overall expression of surface molecules as they flow toward the specific organs.54
Metastasizing cells leave the favorable tumor microenvironment and face hostile conditions. It has been shown that CCR7 and CXCR4 could possibly help cancer cells in surviving anoikis (detachment-induced cell death) by down-regulating pro-apoptotic Bcl2-modifying factor (Bmf) thereby assisting metastasis.55
Reflux disease, Barrett's esophagus (BE) and esophageal adenocarcinoma (EAC)
Esophageal cancer is the eighth most common cancer worldwide.56 Lifetime risk of esophageal cancer in United States is ∼1 in 125 men and 1 in 400 women.57
There has been a steady increase in the incidence of EAC in past 2–3 decades in US, (Surveillance Epidemiology and End Results –SEER program).58 The rate of increase in EAC in the last 25yrs is greater than that of any other solid tumor in the US over the same time interval.59 Similarly increase in the incidence has been noted in the European60 and Australian61 populations. EAC has a very high male:Female ratio ∼7:1 and higher incidence among whites compared with blacks.62,63 EAC is commonly a disease of mature; peaking around 55–65years,64 obese65 males with gastroesophageal reflux disease (GERD).66 Other risk factors implicated in disease development are tobacco smoking,67 high calorie, fat and red meat diet68-69; medications that relax lower esophageal sphincter (LES)70 and hiatus hernia.71 Genetic and familial preponderance is also sought.
GERD is defined by recurrent heartburn, cardinal symptoms and acid regurgitation occurring at least weekly72,73 and has long been regarded as important risk factor of several upper gastrointestinal cancers.74,75 In a Swedish nationwide case-control study, GERD and obesity were identified as strong and independent risk factors for EAC.76
Barrett's esophagus
Long standing GERD is strongly associated with development of Barrett's esophagus; the transformation of esophageal mucosal lining from normal stratified squamous to “intestine like” columnar epithelium with goblet cells (intestinal metaplasia).77 Case control studies have shown that subjects with heartburn are around 6–10 times more likely to have BE than those without it. Furthermore the more frequent and chronic the GERD is, the more likely for them to have BE.78
The progression of BE to EAC is a multiple step process where this metaplastic epithelium is thought to sequentially undergo low-grade dysplasia (LGD), high-grade dysplasia (HGD), early EAC (non invasive disease) and eventually invasive carcinoma79-81. Virchow first linked inflammation to carcinogenesis in 1863.82 Up to 25% of human cancers are considered inflammation-related,83 gastroenterological organs in particular have a notably strong association, viz. colon cancer and inflammatory bowel disease,84 chronic Helicobacter pylori gastritis and gastric cancer,85 hepatitis B & C and liver cancer86 and reflux esophagitis/BE and EAC.87 Inflammation can act as a classical tumor promoter, increasing the risk and tumor progression.88 In addition inflammation also generates tumor-initiating DNA alterations.89,90 Reactive oxygen species-mediated DNA damage is a critical factor in carcinogenesis,91 leading to altered transcription, genomic instability and replication errors.92,93 Microsatellite instability with defect in mismatch repair genes94 and nucleotide excision repair pathways,95 genetic instability with allelic loss and ploidy abnormalities leading to loss of heterozygosity (LOH) viz. p53 LOH, p16LOH,96 copy number alterations and deletions at fragile sites of the genome,97,98 spindle checkpoint function failure like APC gene inactivation by promoter methylation,99 pro-inflammatory cytokines and nitric oxide induced suppression of p53 activity,100,101 increased human telomerase reverse transcriptase and human telomerase-associated RNA expression,102 have all been demonstrated in patients progressing from BE to dysplasia and EAC. Alterations in p53 and p16 are early events in the metaplasia-dysplasia-adenocarcinoma sequence, followed by loss of cell cycle checkpoints.103
Chronic inflammation is also pivotal in triggering epigenetic alterations in addition to DNA damage and genetic alterations as described above. These epigenetic events occur early on in tumorigenesis and not restricted only to malignant tumors, but also seen in premalignant lesions. Most important epigenetic alterations are aberrant DNA methylation and histone modifications.104-106 We conducted an analysis of genome-wide DNA methylation on endoscopic biopsies of dysplastic and malignant lesions to understand the role of epigenetic events associated with the progression of Barrett esophagus. We observed that the previously reported global hypomethylation phenomenon in cancer has its origins at the earliest stages of epithelial carcinogenesis and was seen in low grade dysplasia. Integration of methylation analysis with copy number analysis demonstrated that promoter demethylation synergizes with gene amplification and leads to significant upregulation of a chr4q21 chemokine cluster and other transcripts during Barrett neoplasia. This chromosomal region contains the genes for CXCL1, CXCL3 and IL8 chemokines. We observed that these ligands are significantly upregulated in Barrett's and dysplastic lesions. Importantly we were also able to show that IL8 levels in the serum are elevated in patients with esophageal cancer and can potentially serve as biomarkers of disease.107 Likewise molecular signatures are being sought for many other malignancies, which can help us diagnose or monitor the treatment of specific cancers.108
Other studies have also suggested that chemokines are involved in esophageal carcinogenesis. Fitzgerald et al.109 found that, in patients with reflux esophagitis, the inflammation is maximal at squamous-columnar junction and was accompanied by an increased expression of IL-8 and IL-1β. CXCR1 and CXCR2, the receptors for IL8, have also been shown to be constitutively expressed in esophageal mucosa.110-112 Infiltrating neutrophils have also been shown to contribute to the pool of IL8 thereby facilitating the progression of BE and EAC.113 Nguyen et al. in their study demonstrated a relation between increased IL8 expression and poorer prognosis in esophageal cancer confirming the pro-tumoral role of IL8.114 Thus our previous work and these studies promoted us to further examine the effect of IL8 pathway inhibition in esophageal cancer.
Chemokine receptor (CXCR2) blockade can inhibit invasiveness of esophageal cancer cells
To determine the role of CXCR2 in esophageal adenocarcinoma we used a specific inhibitor of the receptor. SB332235 is a small molecule inhibitor that has been shown to be a specific inhibitor of CXCR2.115 Esophageal adenocarcinoma derived OE33 cells were exposed to CXCR2 inhibitor and proliferation was assessed by a MTT assay. We observed that CXCR2 inhibition did not result in any significant effect on proliferation (Fig. 1A). Since invasiveness is the hallmark of cancer, we next used matrigel invasion assay to test the role of CXCR2 in this process. We observed a dose dependent decrease in cancer cell invasion with CXCR2 inhibition (P Value < 0.05, T Test)(Fig. 1B, C).
Figure 1.
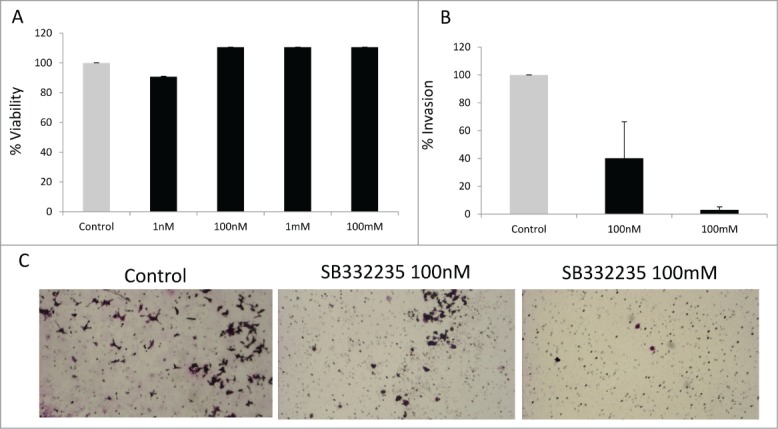
CXCR2 inhibition leads to reduced invasiveness of esophageal adenocarcinoma cells. Treatment with CXCR2 inhibitor, SB332235, did not lead to inhibition of proliferation of OE33 cell lines at 48 hrs by MTT assay (Mean +/− s.e.m of 3 independent experiments is shown) (A). Treatment with CXCR2 inhibitor, SB332235, led to inhibition of matrigel invasion of OE33 cell lines at 48 hrs at different dose levels (Mean +/− s.e.m of 3 independent experiments is shown; TTest with P value < 0.05 (*) (B). Representative pictographs shows decreased invasion after CXCR2 inhibition (C).
Taken together, we have shown that IL8 is significantly upregulated during esophageal carcinogenesis; can be detected in the serum of patients with adenocarcinoma and the IL8-CXCR2 pathway is a potential therapeutic target in this disease.
Conclusions
Chemokines and chemokine receptors play pivotal role in tumorigenesis and metastasis of many malignancies including esophageal adenocarcinoma. Therapeutic targeting the chemokines or their receptors may represent novel strategies to prevent tumor development at an early stage.
Materials and Methods
OE-33 cell proliferation
OE-33 (ATCC) were passaged in DMEM containing 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 ug/mL streptomycin. Growth of cells was measured after 3 days using MTT assay. CXCR2 inhibitor SB332235 was provided by GSK pharmaceuticals.
Matrigel invasion assay
The invasiveness of the OE33 cells were assessed with the modified Boyden chamber assay. Matrigel invasion chambers (BD BioCoat™ BD Matrigel™ Invasion Chamber) with 8 μm pore sizes in a 24-well plate format were used as per the manufacturer's recommendation. After the cells were allowed to invade, the matrigel was wiped off the membrane, and then it was fixed with 4% paraformaldehyde, stained with 0.2% crystal violet and the number of cells that had invaded through to the other side of the membrane were counted.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in Immunity. Immunity 2000; 12(2):121-7; PMID:10714678; http://dx.doi.org/ 10.1016/S1074-7613(00)80165-X [DOI] [PubMed] [Google Scholar]
- 2. Borisch LC, Steinke JW. Cytokines and chemokines. J Allergy Clin Immunol 2003; 111(2 suppl):S460-75; PMID:12592293; http://dx.doi.org/ 10.1067/mai.2003.108 [DOI] [PubMed] [Google Scholar]
- 3. Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends immunol 2004; 25:75-84; PMID:15102366; http://dx.doi.org/ 10.1016/j.it.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 4. Balkwill F, Mantovani A. Inflammation and cancer: back to virchow? Lancet 2001; 357:539-45; PMID:11229684; http://dx.doi.org/ 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 5. Kiefer F, Siekmann AF. The role of chemokines and their receptors in angiogenesis. Cell Mol Life Sci 2011; 68:2811-30; PMID:21479594; http://dx.doi.org/ 10.1007/s00018-011-0677-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity 2012; 36(5):705-16; PMID:22633458; http://dx.doi.org/ 10.1016/j.immuni.2012.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol 2006; 7:243; PMID:17201934; http://dx.doi.org/ 10.1186/gb-2006-7-12-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nomiyama H, Osada N, Yoshie O. A family tree of vertebrate chemokine receptors for a unified nomenclature. Dev Comp Immunol 2011; 35:705-15; PMID:21295066; http://dx.doi.org/ 10.1016/j.dci.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 9. Loetscher P, Pellegrino A, Gong JH, Mattioli I, Loetscher M, Bardi G, Baggiolini M, Clark-Lewis I. The ligands of CXC chemokine receptor 3, I-TAC, Mig and IP10, are natural antagonists for CCR3. J Biol Chem 2001; 276:2986-91; PMID:11110785; http://dx.doi.org/ 10.1074/jbc.M005652200 [DOI] [PubMed] [Google Scholar]
- 10. Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol 2008; 9:953-9; PMID:18711432; http://dx.doi.org/ 10.1038/ni.f.207 [DOI] [PubMed] [Google Scholar]
- 11. Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, et al. . MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 2007; 13:587-96; PMID:17435771; http://dx.doi.org/ 10.1038/nm1567 [DOI] [PubMed] [Google Scholar]
- 12. Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schröder JM, Wang JM, Howard OM, et al. . Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999; 286:525-8; PMID:10521347; http://dx.doi.org/ 10.1126/science.286.5439.525 [DOI] [PubMed] [Google Scholar]
- 13. Saini V, Marchese A, Majestschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem 2010; 285:15566-76; PMID:20228059; http://dx.doi.org/ 10.1074/jbc.M110.103408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol 2006; 6:907-18; PMID:17124512; http://dx.doi.org/ 10.1038/nri1964 [DOI] [PubMed] [Google Scholar]
- 15. Proudfoot AE. The biological relevance of chemokine-proteoglycan interactions. Biochem Soc Trans 2006; 34:422-6; PMID:16709177; http://dx.doi.org/ 10.1042/BST0340422 [DOI] [PubMed] [Google Scholar]
- 16. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005; 7:211-7; PMID:15766659; http://dx.doi.org/ 10.1016/j.ccr.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 17. Montovani A. Cancer: inflammation metastasis. Nature 2009; 457:36-7; PMID:19122629; http://dx.doi.org/ 10.1038/457036b [DOI] [PubMed] [Google Scholar]
- 18. Vindrieux D, Escobar P, Lazennec G. Emerging role of chemokines in prostate cancer. Endocr Relat Cancer 2009; 16:663-73; PMID:19556286; http://dx.doi.org/ 10.1677/ERC-09-0109 [DOI] [PubMed] [Google Scholar]
- 19. Ali S, Lazennec G. Chemokines: Novel targets for breast cancer metastasis. Cancer Metastasis Rev 2007; 26:401-20; PMID:17717637; http://dx.doi.org/ 10.1007/s10555-007-9073-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer lett 2007; 256(2): 137-65; PMID:17629396; http://dx.doi.org/ 10.1016/j.canlet.2007.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The CXC chemokine SDF-1 is the ligand for LESTRFusin and prevents infection by T-cell-line-adapted HIV-1. Nature 1996; 382:833-5; PMID:8752280; http://dx.doi.org/ 10.1038/382829a0 [DOI] [PubMed] [Google Scholar]
- 22. Burns JM. Summers BC, Wang Y. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion and tumor development. J Exp Med 2006; 203:2201-13; PMID:16940167; http://dx.doi.org/ 10.1084/jem.20052144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer 2004; 4:540-50; PMID:15229479; http://dx.doi.org/ 10.1038/nrc1388 [DOI] [PubMed] [Google Scholar]
- 24. Biswas Sk, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, et al. . A distinct and unique transcriptional program expressed by tumor-asociated macrophages (defective NF-kappaB and enhanced IRF-3STAT1 activation). Blood 2006; 107:2112-22; PMID:16269622; http://dx.doi.org/ 10.1182/blood-2005-01-0428 [DOI] [PubMed] [Google Scholar]
- 25. Sica A, Schioppa T, Mantovani A, Allavena P. Tumor-associated macrophages are a distinct M2 polarised population promoting tumour progression:potential targets of anti-cancer therapy. Eur J Cancer 2006; 42:717-27; PMID:16520032; http://dx.doi.org/ 10.1016/j.ejca.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 26. Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 2000; 404:407-11; PMID:10746730; http://dx.doi.org/ 10.1038/35006097 [DOI] [PubMed] [Google Scholar]
- 27. Liu VC, wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25- Tcells into CD4+CD25+ T regulatory cells:role of tumor-derived TGF-beta. J Immunol 2007; 178:2883-92; PMID:17312132; http://dx.doi.org/ 10.4049/jimmunol.178.5.2883 [DOI] [PubMed] [Google Scholar]
- 28. Ronnov-Jessen L, Peterson OW, Koteliansky VE, Bissell MJ. the origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest 1995; 95:859-73; PMID:7532191; http://dx.doi.org/ 10.1172/JCI117736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bierie B, Moses HL. Under pressure: stromal fibroblasts change their ways. Cell 2005; 123:985-7; PMID:16360028; http://dx.doi.org/ 10.1016/j.cell.2005.11.029 [DOI] [PubMed] [Google Scholar]
- 30. Kalluri R, Zaisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006; 6:392-401; PMID:16572188; http://dx.doi.org/ 10.1038/nrc1877 [DOI] [PubMed] [Google Scholar]
- 31. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1CXCL12 secretion. Cell 2005; 121:335-48; PMID:15882617; http://dx.doi.org/ 10.1016/j.cell.2005.02.034 [DOI] [PubMed] [Google Scholar]
- 32. Shchors K, Evan G. Tumor angiogenesis:cause or consequence of cancer? Cancer Res 2007; 67:7059-61; PMID:17671171; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-2053 [DOI] [PubMed] [Google Scholar]
- 33. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002; 29:15-8; PMID:12516034; http://dx.doi.org/ 10.1053/sonc.2002.37263 [DOI] [PubMed] [Google Scholar]
- 34. Mehrad B, Keane MP, Strieter RM. Chemokines as mediators of angiogenesis. Thromb Haemost 2007; 97:755-62; PMID:17479186 [PMC free article] [PubMed] [Google Scholar]
- 35. Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of tumor angiogenesis and neovascularization. Exp Cell Res 2011; 317(5):685-90; PMID:21040721; http://dx.doi.org/ 10.1016/j.yexcr.2010.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sarvaiya PJ, Guo D, Ulasov L, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget 2013; 4(12):2171-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2, CXCR2 is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol 2000; 16:5269-77; PMID:11046061; http://dx.doi.org/ 10.4049/jimmunol.165.9.5269 [DOI] [PubMed] [Google Scholar]
- 38. Murdoch C, Monk PN, Finn A. CXC chemokine receptor expression on human endothelial cells. Cytokine 1999; 11:704-12; PMID:10479407; http://dx.doi.org/ 10.1006/cyto.1998.0465 [DOI] [PubMed] [Google Scholar]
- 39. Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, et al. . Angiogenic effects of Interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem 2003; 278:8508-15; PMID:12496258; http://dx.doi.org/ 10.1074/jbc.M208231200 [DOI] [PubMed] [Google Scholar]
- 40. Wang D, Wang H, Brown J, Daikoku T, Ning W, Shi Q, Richmond A, Strieter R, Dey SK, DuBois RN. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med 2006; 203:941-51; PMID:16567391; http://dx.doi.org/ 10.1084/jem.20052124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chan DA, Kawahara TL, Sutphin PD, Chang HY, Chi JT, Giaccia AJ. Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell 2009; 15:527-38; PMID:19477431; http://dx.doi.org/ 10.1016/j.ccr.2009.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, Alcami A, Lira SA, Wiekowski M, Graham GJ. The chemokine receptor D6 Limits the inflammatory response in vivo. Nat Immunol 2005; 6:403-11; PMID:15750596; http://dx.doi.org/ 10.1038/ni1182 [DOI] [PubMed] [Google Scholar]
- 43. Shen H, Schuster R, Stringer KF, Waltz SE, Lentsch AB. The duffy antigenreceptor for chemokines (DARC) regulates prostate tumor growth. Faseb J 2006; 20:59-64; PMID:16394268; http://dx.doi.org/ 10.1096/fj.05-4764com [DOI] [PubMed] [Google Scholar]
- 44. Lazennec G, Rischmond A. Chemokines and chemokine receptors:new insights into cancer-related inflammation. Trends Mol Med 2010; 16(3):133-144; PMID:20163989; http://dx.doi.org/ 10.1016/j.molmed.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salcedo R, Wasserman K, Young H, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells:in vivo neovascularization induced by stromal-derived factor-1a. Am J Pathol 1999; 154:1125-35; PMID:10233851; http://dx.doi.org/ 10.1016/S0002-9440(10)65365-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Belton A, Gabrovsky A, Bae YK, Reeves R, Iacobuzio-Donahue C, Huso DL, Resar LM. HMGA1 induces intestinal polyposis in transgenic mice and drives tumor progression and stem cell properties in colon cancer cells. PLoS One 2012; 7:e30034; PMID:22276142; http://dx.doi.org/ 10.1371/journal.pone.0030034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nian WQ, Chen FL, Ao XJ, Chen ZT. CXCR4 positive cells from Lewis lung carcinoma cell line have cancer metastatic stem cell characteristics. Mol Cell Biochem 2011; 355:241; PMID:21553023; http://dx.doi.org/ 10.1007/s11010-011-0860-z [DOI] [PubMed] [Google Scholar]
- 48. Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis 2008; 25:345-56; PMID:17891505; http://dx.doi.org/ 10.1007/s10585-007-9097-3 [DOI] [PubMed] [Google Scholar]
- 49. Marchesi F, Piemonti L, Fedele G, Destro A, Roncalli M, Albarello L, Doglioni C, Anselmo A, Doni A, Bianchi P, et al. . The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res 2008; 68:9060-9; PMID:18974152; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1810 [DOI] [PubMed] [Google Scholar]
- 50. Amersi FF, Terando AM, Goto Y, Scolyer RA, Thompson JF, Tran AN, Faries MB, Morton DL, Hoon DS. Activation of CCR9CCL25 in cutaneous melanoma mediates preferential metastesis to the small intestine. Clin cancer Res 2008; 14:638-45; PMID:18245522; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature 2005; 436:518-24; PMID:16049480; http://dx.doi.org/ 10.1038/nature03799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maxwell PJ, Neisen J, Messenger J, Waugh DJ. Tumor-derived CXCL8 signaling augments stroma-derived CCL2-promoted proliferation and CXCL12-mediated invasion of PTEN-deficient prostate cancer cells. Oncotarget 2014; 5(13):4895-908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 2007; 11:526-38; PMID:17560334; http://dx.doi.org/ 10.1016/j.ccr.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 54. Miles FL, Pruitt FL, van Golen KL, Cooper CR. Stepping out of the flow:capillary extravasation in cancer metastesis. Clin Exp Metastasis 2008; 25:305-24; PMID:17906932; http://dx.doi.org/ 10.1007/s10585-007-9098-2 [DOI] [PubMed] [Google Scholar]
- 55. Kochetkova M, Kumar S, McColl SR. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ 2009; 16:664-73; PMID:19136936; http://dx.doi.org/ 10.1038/cdd.2008.190 [DOI] [PubMed] [Google Scholar]
- 56. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008:GLOBOCAN 2008. Int J Cancer 2010; 127:2893-917; PMID:213520676867 1269; http://dx.doi.org/ 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 57. American Cancer Society Cancer Facts and Figures 2011. Atlanta, GA: American Cancer Society; 2011; 1-60. [Google Scholar]
- 58. seer.cancer.gov . [Google Scholar]
- 59. Vial M, Grande L, Pera M. Epidemiology of adenocarcinoma of the esophagus, gastric cardia and upper gastric third. Recent Results Cancer Res 2010; 182:1-17; PMID:20676867; http://dx.doi.org/ 10.1007/978-3-540-70579-6_1 [DOI] [PubMed] [Google Scholar]
- 60. Bosetti C, Levi F, Ferlay J. Trends in oessophageal cancer incidence and mortality in Europe. Int J Cancer 2008; 122:1118-29; PMID:17990321; http://dx.doi.org/ 10.1002/ijc.23232 [DOI] [PubMed] [Google Scholar]
- 61. Nguyen AM, Luke CG, Roder D. Comparative epidemiological characteristics of oesophageal adenocarcinoma and other cancers of the oesophagus and gastric cardia. Asia Pac J Cancer Prev 2003; 4:225-31; PMID:14507243 [PubMed] [Google Scholar]
- 62. Powell J, McConkey CC, Gillison EW, Spychal RT. Continuing rising trend in oesophageal adenocarcinoma. Int J Cancer 2002; 102:422-7; PMID:12402314; http://dx.doi.org/ 10.1002/ijc.10721 [DOI] [PubMed] [Google Scholar]
- 63. Wu X, Chen VW, Andrews PA, Ruiz B, Correa P. Incidence of esophageal and gastric cancers among Hispanics, non- Hispanics whites and non-Hispanic blacks in the United States: subsite and histology differences. Cancer Causes Control 2007b; 18:585-93; PMID:17406989; http://dx.doi.org/ 10.1007/s10552-007-9000-1 [DOI] [PubMed] [Google Scholar]
- 64. Yang PC, Davis S. Incidence of cancer of the esophagus in the US by histologic type. Cancer 1988; 61:612-7; PMID:3338027; http://dx.doi.org/ 10.1002/1097-0142(19880201)61:3%3c612::AID-CNCR2820610332%3e3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- 65. Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systemic reviw and meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:872-8; PMID:16702363; http://dx.doi.org/ 10.1158/1055-9965.EPI-05-0860 [DOI] [PubMed] [Google Scholar]
- 66. Chai J, Jamal MM. Esophageal malignancy: A growing concern. World J Gastroenterol 2012; 18(45):6521-6; PMID:23236223; http://dx.doi.org/ 10.3748/wjg.v18.i45.6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Whiteman DC, Sadeghi S, Pandeya N, Smithers BM, Gotley DC, Bain CJ, Webb PM, Green AC, Australian Cancer Study. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut 2008; 57:173-80; PMID:17932103; http://dx.doi.org/ 10.1136/gut.2007.131375 [DOI] [PubMed] [Google Scholar]
- 68. Bahmanyar S, Ye W. Dietary patterns and risk of squamous-cell and adenocarcinoma of the esophagus and adenocarcinoma of the gastric cardia: a population-based case-control study in Sweden. Nutr Cancer 2006; 54:171-8; PMID:16898861; http://dx.doi.org/ 10.1207/s15327914nc5402_3 [DOI] [PubMed] [Google Scholar]
- 69. Navarro Silvera SA, Mayne ST, Risch H. Food group intake and risk of subtypes of esophageal and gastric cancer. Int J Cancer 2008; 123:852-60; PMID:18537156; http://dx.doi.org/ 10.1002/ijc.23544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lagergren J, Bergstrom R, Adami HO, Nyrén O. Association between medications that relax the lower esophageal sphincter and risk for esophageal adenocarcinoma. Ann Intern Med 2000a; 133:227-9 [DOI] [PubMed] [Google Scholar]
- 71. Wu AH, Tseng CC, Bernstein L. Hiatal hernia, reflux symptoms, body size and risk of esophageal and gastric adenocarcinoma. Cancer 2003b; 98:940-8; http://dx.doi.org/ 10.1002/cncr.11568 [DOI] [PubMed] [Google Scholar]
- 72. Klauser AG, Schindlbeck NE, Muller-Lissner SA. Symptoms in gastro-oesophageal reflux disease. Lancet 1990; 335:205-8; PMID:1967675; http://dx.doi.org/ 10.1016/0140-6736(90)90287-F [DOI] [PubMed] [Google Scholar]
- 73. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus Group. The montreal definition and classification of gastroesophageal reflux disease:a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900-20; quiz 43; PMID:16928254; http://dx.doi.org/ 10.1111/j.1572-0241.2006.00630.x [DOI] [PubMed] [Google Scholar]
- 74. Lagergren J, Bergstrom R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340:825-31; PMID:10080844; http://dx.doi.org/ 10.1056/NEJM199903183401101 [DOI] [PubMed] [Google Scholar]
- 75. Thrift AP, Pandeya N, Whiteman DC. Current status and future perspectives on the etiology of esophageal adenocarcinoma. Front Oncol 2012; 2:11; PMID:22655259; http://dx.doi.org/ 10.3389/fonc.2012.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lagergren J. [Increased incidence of adenocarcionoma of the esophagus and cardia. Reflus and obesity are strong and independent risk factors according to the SECC study]. Lakartidningen 2000; 97(16):1950-3; PMID:10826353 [PubMed] [Google Scholar]
- 77. Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ, American Gastroenterological Association. American Gastroenerological association technical review on the management of Barrett's esophagus. Gastroenterology 2011; 140(3):e13-8; PMID:21376939; http://dx.doi.org/ 10.1053/j.gastro.2011.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Eloubeidi MA, Provenzale D. Clinical and demographic predictors of barrett's esophagus among patients with gastroesophageal refluxdisease:a multivariate analysis in veterans. J Clin Gastroenterol 2001; 33:306-9; PMID:11588545; http://dx.doi.org/ 10.1097/00004836-200110000-00010 [DOI] [PubMed] [Google Scholar]
- 79. Falk GW. Barrett's esophagus. Gastroenterology 2002; 122:1569-91; PMID:12016424; http://dx.doi.org/ 10.1053/gast.2002.33427 [DOI] [PubMed] [Google Scholar]
- 80. Wild CP, Hardie IJ. Reflux, Barrett's oesophagus and adenocarcinoma:burning questions. Nat Rev Cancer 2003; 3:676-84; PMID:12951586; http://dx.doi.org/ 10.1038/nrc1166 [DOI] [PubMed] [Google Scholar]
- 81. Phillips WA, Lord RV, Nancarrow DJ, Watson DI, Whiteman DC. Barrett's esophagus. J of Gstroenterol and Hepatol 2011; 26:639-48; PMID:21166712; http://dx.doi.org/ 10.1111/j.1440-1746.2010.06602.x [DOI] [PubMed] [Google Scholar]
- 82. Balkwill F, Mantovani A. Inflammation and cancer:back to virchow. Lancet 2001; 357:539-45; PMID:11229684; http://dx.doi.org/ 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 83. Hussain SP, Harris CC. Inflammation and cancer;an ancient link with novel potentials. Int J Cancer 2007; 121:2372-80 [DOI] [PubMed] [Google Scholar]
- 84. Itzkowitz SH, Yio X. Inflammation cancer IV. Colorectal cancer in inflammatory bowel disease:the role of inflammation. Am J Physiol Gastrointes Liver Physiol 2004; 287:G7-17; http://dx.doi.org/ 10.1152/ajpgi.00079.2004 [DOI] [PubMed] [Google Scholar]
- 85. Bornschein J, Selgrad M, Warnecke M, Kuester D, Wex T, Malfertheiner P. H. pylori infection is a key risk factor for proximal gastric cancer. Dig Dis Sci 2010; 55:3124-31; PMID:20668939; http://dx.doi.org/ 10.1007/s10620-010-1351-x [DOI] [PubMed] [Google Scholar]
- 86. Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H, et al. . Risk factors for hepatocellular carcinoma among patients with hronic liver disease. N Engl J Med 1993; 328:1797-801; PMID:7684822; http://dx.doi.org/ 10.1056/NEJM199306243282501 [DOI] [PubMed] [Google Scholar]
- 87. Zhang HY, Spechler SJ, Souza RF. Esophageal adenocarcinoma arising in Barrett's esophagus. Cancer Lett 2009; 275:170-7; PMID:18703277; http://dx.doi.org/ 10.1016/j.canlet.2008.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436-44; PMID:18650914; http://dx.doi.org/ 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 89. Hofseth LJ, Khan MA, Ambrose M, Nikolayeva O, Xu-Welliver M, Kartalou M, Hussain SP, Roth RB, Zhou X, Mechanic LE, et al. . The adaptive imbalance in base excision-repair enzymes generates microsatellite instability in chronic inflammation. J Clin Invest 2003; 112:1887-94; PMID:14679184; http://dx.doi.org/ 10.1172/JCI19757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, et al. . DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest 2008; 118:2516-25; PMID:18521188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kawanishi S, Hiraku Y. Oxidative and nitrative DNA damage as biomarket for carcinogenesis with special reference to inflammation. Antioxid Redox Signal 2006; 8:1047-58; PMID:16771694; http://dx.doi.org/ 10.1089/ars.2006.8.1047 [DOI] [PubMed] [Google Scholar]
- 92. De BR, Van LN. Endogenous DNA damage in humans:a review of quantitative data. Mutagenesis 2004; 19:169-85; PMID:15123782; http://dx.doi.org/ 10.1093/mutage/geh025 [DOI] [PubMed] [Google Scholar]
- 93. Marnett LJ, Plastaras JP. Endogenous DNA damage and mutation. Trends Genet 2001; 17:214-21; PMID:11275327; http://dx.doi.org/ 10.1016/S0168-9525(01)02239-9 [DOI] [PubMed] [Google Scholar]
- 94. Falkenback D, Johansson J, Halvarsson B, Nilbert M. Defective mismatch-repair as a minor tumorigenic pathway in Barrett's esophagus-associated adenocarcinoma. Cancer Genet Cytogenet 2005; 157:82-86; PMID:15676154; http://dx.doi.org/ 10.1016/j.cancergencyto.2004.08.003 [DOI] [PubMed] [Google Scholar]
- 95. Pan J, Lin J, Izzo JG, Liu Y, Xing J, Huang M, Ajani JA, Wu X. Genetic susceptibility to esophageal cancer: the tole of the nucleotide excision repair pathway. Carcinogenesis 2009; 30:785-92; PMID:19270000; http://dx.doi.org/ 10.1093/carcin/bgp058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sanz-Ortega J, Hernandez S, Saez MC, Sierra E, Sanz-Ortega G, Torres A, Balibrea JL, Sanz-Esponera J, Merino MJ. 3p21, 5q21, 9p21 and 17p13.1 allelic deletions are potential markers of individuals with a high risk of developing adenocarcinoma in Barrett's epithelium without dysplasia. Hepatogastroenterology 2003; 50:404-7; PMID:12749233 [PubMed] [Google Scholar]
- 97. Lai LA, Kostadinov R, Barrett MT, Peiffer DA, Pokholok D, Odze R, Sanchez CA, Maley CC, Reid BJ, Gunderson KL, et al. . Deletion at fragile sites is a common and early event in Barrett's esophagus. Mol Cancer Res 2010; 8:1084-94; PMID:20647332; http://dx.doi.org/ 10.1158/1541-7786.MCR-09-0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Paulson TG, Maley CC, Li X, Li H, Sanchez CA, Chao DL, Odze RD, Vaughan TL, Blount PL, Reid BJ. Chromosomal instability and copy number alterations in Barrett's esophagus and esophageal adenocarcinoma. Clin Cancer Res 2009; 15:3305-14; PMID:19417022; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Clement G, Braunschweig N, Pasquier N, Bosman FT, Benhattar J. Alterations of the Wnt signaling pathway during the neoplastic progression of Barrett's esophagus. Oncogene 2006; 25:3084-92; PMID:16407829; http://dx.doi.org/ 10.1038/sj.onc.1209338 [DOI] [PubMed] [Google Scholar]
- 100. Calmels S, Hainaut P, Ohshima H. Nirtic oxide induces conformational and functional modifications of wild-type p53 tumor suppressor protein. Cancer Res 1997; 57:3365-9; PMID:9269997 [PubMed] [Google Scholar]
- 101. Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A Proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med 1999; 190:1375-82; PMID:10562313; http://dx.doi.org/ 10.1084/jem.190.10.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gertler R, Doll D, Maak M, Feith M, Rosenberg R. Telomere length and telomerase subunits as diagnostic and prognostic biomarkers in Barrett carcinoma. Cancer 2008; 112:2173-80; PMID:18348304; http://dx.doi.org/ 10.1002/cncr.23419 [DOI] [PubMed] [Google Scholar]
- 103. Kopper LB, Wijnhoven BP, Dekken H, Tilanus HW, Dinjens WN. The molecular biology of esophageal adenocarcinoma. J Surg Oncol 2005; 92(3):169-90; PMID:16299787; http://dx.doi.org/ 10.1002/jso.20359 [DOI] [PubMed] [Google Scholar]
- 104. Herceg Z, Ushijima T. Introduction:epigenetics and cancer. Adv Genet 2010; 70:1-23. [DOI] [PubMed] [Google Scholar]
- 105. Herceg Z, Ushijima T. Epigenetics is a fascinating field of modern biology. Preface Adv Genet 2010; 71:Xi-Xii; PMID:20933123; http://dx.doi.org/ 10.1016/B978-0-12-380864-6.00014-6 [DOI] [PubMed] [Google Scholar]
- 106. Gatti L, Sevko A, De Cesare M, Arrighetti N. et, al. Histone deacetylase inhibitor-temozolomide co-treatment inhibits melanoma growth through suppression of chemokine (C-C motif) ligand 2-driven signals. Oncotarget 2014; 5(12):4516-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Alvarez H, Opalinska J, Zhou L, Sohal D, Fazzari MJ, Yu Y, Montagna C, Montgomery EA, Canto M, Dunbar KB, et al. . Widespread Hypomethylation Occurs Early and Synergizes with Gene Amplification during Esophageal Carcinogenesis. PLoS Genet 2011; 7(3):e1001356; PMID:21483804; http://dx.doi.org/ 10.1371/journal.pgen.1001356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang H, Lin W, Kannan K, Luo L, Li J, Chao PW, Wang Y, Chen YP, Gu J, Yen L. Aberrant chimeric RNA GOLM1-Mak10 encoding a secreted fusion protein as a molecular signature for human esophageal squamous cell carcinoma. Oncotarget 2013; 4(11):2135-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fitzgerald RC, Abdalla S, Onwuegbusi BA, Sirieix P, Saeed IT, Burnham WR, Farthing MJ. Inflammatory gradient in Barrett's oesophagus:implications for disease complications. Gut 2002; 51:316-22; PMID:12171950; http://dx.doi.org/ 10.1136/gut.51.3.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Isomoto H, Kanazawa Y, Nishi Y. Expression of CXC receptor 1 and 2 in esophageal mucosa of patients with reflux esophagitis. World J Gastroenterol 2005; 11:1793-7; PMID:15793866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kanazawa Y, Isomote H, Wen CY, Wang AP, Saenko VA, Ohtsuru A, Takeshima F, Omagari K, Mizuta Y, Murata I, et al. . Impact of endoscopically minimal involvement on IL-8 mRNA expression in esophageal mucosa of patients with non-erosive reflux disease. World J Gastroenterol 2003; 9:2801-4; PMID:14669337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zachrisson K, Neopikhanov V, Samali A, Uribe A. Interleukin-1, interleukin-8, tumor necrosis factor alpha and interferon gamma stimulate DNA synthesis but have no effect on apoptosis in small-intestinal cell lines. Eur J Gastroenterol Hepatol 2001; 13:551-9; PMID:11396536; http://dx.doi.org/ 10.1097/00042737-200105000-00015 [DOI] [PubMed] [Google Scholar]
- 113. Hannelien V, Karel G, Jo VD, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochemica et Biophysica Acta-Reviews On Cancer 2012; 1:117-29; PMID:22079531; http://dx.doi.org/ 10.1016/j.bbcan.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 114. Nguyen GH, Schetter AJ, Chou DB, Bowman ED, Zhao R, Hawkes JE, Mathé EA, Kumamoto K, Zhao Y, Budhu A, et al. . Inflammatory and microRNA gene expression as prognostic classifiers of Barrett's associated esophageal adenocarcinoma. Clin Cancer Res 2010; 16:5824-34; PMID:20947516; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Singh S, Sadanandam A, Nannuru KC, Varney ML, Mayer-Ezell R, Bond R, Singh RK. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival and angiogenesis. Clin Cancer Res 2009; 15:2380-6; PMID:19293256; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-2387 [DOI] [PMC free article] [PubMed] [Google Scholar]


