Abstract
The introduction of transcatheter aortic valve replacement (TAVR in US - TAVI in Europe) has resulted in a paradigm shift in the treatment of patients with severe aortic stenosis. Although three randomized trials and multiple single-center and multicenter registry studies have been published, the profile and longer-term outcome of patients undergoing transcatheter aortic valve replacement (TAVR) in patients with severe aortic stenosis have been limited. The recently published reports from the United Kingdom (UK)1 and United States (US)2 TAVR registries add tremendously to the currently available literature. These studies provide an excellent model to clinicians that would aid in the proper patient selection and help in guiding discussions with patients who are undergoing TAVR. The present review discusses the recently published UK and US TAVR registry data.
Introduction
Transcatheter aortic valve replacement (TAVR) emerged just over a decade ago and changed the landscape of structural interventional cardiology. Following the publication of three randomized trials,3–5 TAVR has been used with increasing frequency for the treatment of severe calcific aortic stenosis in patients who have been deemed inoperable or are at high risk for surgical aortic valve replacement. However, introducing new medical devices into routine practice raises concerns because patients and outcomes may differ from those in randomized trials. For that reason, several TAVR registries in different countries have been established to represent the “real world” experience with this new technology. This present review discusses two recent registry results, reflecting the TAVR experience in the United Kingdom (UK) and the United States (US).
UK TAVI registry
In this paper, the authors reported on data from the UK TAVI registry, examining trends and predictors of outcome for 3,980 TAVR procedures performed at 33 centers in the United Kingdom from 2007 through 2012. The patients, who account for all TAVR cases performed in the country, received either Sapien/Sapien XT (n = 2,036; Edwards Lifesciences) or CoreValve (n = 1,897; Medtronic devices) devices. A minority of patients received Portico (n = 35; St. Jude), Direct Flow device (n = 3; Direct Flow Medical), or JenaValve (n = 3, JenaValve) devices. The majority of cases (71.2%) were performed via femoral access.
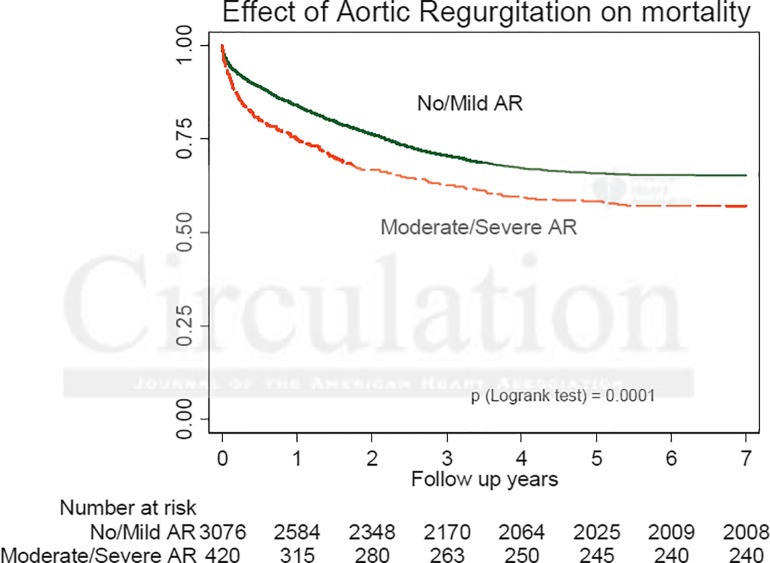
The authors carried out an excellent analysis of trends, risk factors for mortality and results. Over a 6-year follow up period, there was very little change in the clinical profile of the patients treated with the only demographic shift being treating patients with lower left ventricular ejection fraction < 30% (10.2% vs 5.9% in earlier years). There was no change in the mean logistic EuroSCORE, however, fewer patients with previous stroke and peripheral vascular disease underwent the procedure in later years. There was consistent association between particular patient characteristics and poorer outcomes. On multivariate analysis, logistic EuroSCORE > 40 was the only independent predictor of 30-day mortality. Predictors of 1-year mortality were pre-procedural atrial fibrillation, chronic obstructive pulmonary disease, renal insufficiency (creatinine >200 μmol/L) and diabetes mellitus. Predictors of 2-year mortality were the same with the exception of diabetes and the addition of coronary artery disease. Patients who could be treated by the femoral route had a lower mortality than those for whom an alternative route was needed. Unadjusted survival for the direct aortic and the transapical approach were similar at 1 to 2 years. The presence of post procedural aortic regurgitation (moderate or severe) was associated with lower long term survival on multivariate analysis at 1 and 2 years (Figure 1). There was no difference in survival at any time point between Sapien and CoreValve devices, however, CoreValve was associated with a higher incidence of post TAVR aortic regurgitation (p < 0.0001) and need for pacemaker implantation (p < 0.0001). Implantation of pacemaker decreased markedly with CoreValve from 29% to 15% in the more recent years (p < 0.0001).
Figure 1.
Kaplan-Meier Survival curves according to degree of aortic regurgitation following TAVR.
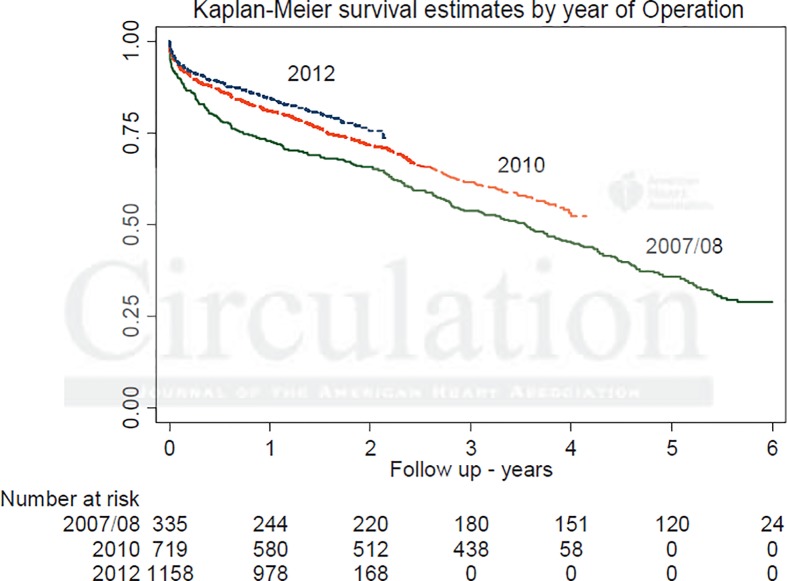
There was an observed trend towards higher survival in the more recent TAVR cohorts. The 4-year survival was 55% for those treated in 2009 and 65% for those treated in 2011. Furthermore, survival at 1, 2, and 3 years was higher for patients treated in 2012 than for those treated in all previous years (Figure 2). As expected, the survival fell from 81.7% at 1 year following TAVR to 37.3% at 6 years. In an effort to address the issue of a potential learning curve, the first 50 and subsequent cases were compared at every center with no significant difference observed in the thirty-day mortality (6.5% vs 6.2%; p = 0.70). Unlike open-heart surgery, there was no relationship seen between a center's total case volume and thirty-day mortality. This finding is of interest and may play a role in the dissemination of this technology.
Figure 2.
Kaplan-Meier survival curve comparing patients treated in 2007/8, 2010 and 2012.
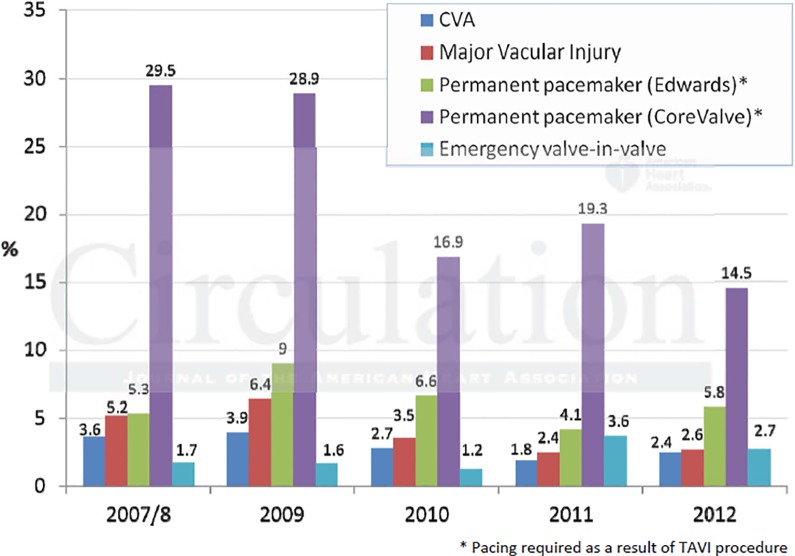
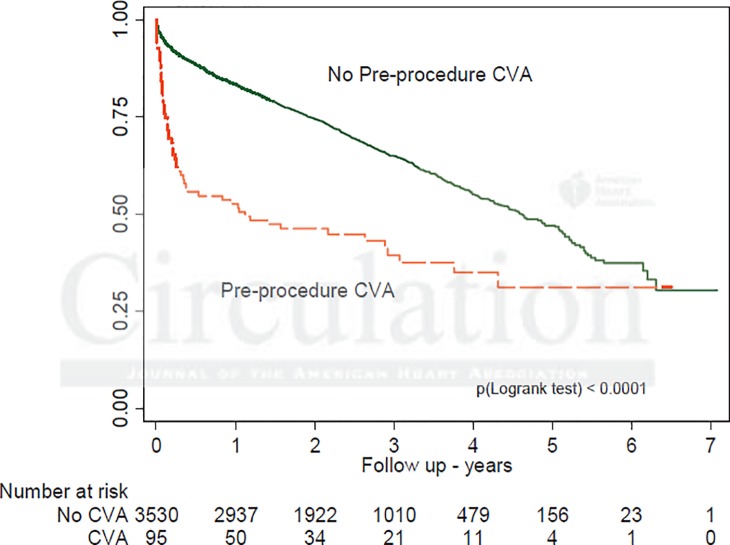
Median post-procedural length of stay decreased from 10 to 8 days over the study period with the number of patients who could be discharged by the fifth day nearly doubling (from 16.7% to 28.5%). Procedural complications also showed temporal decrease (Figure 3), with stroke dropping from 3.6% to 2.4% (p = 0.022) and major vascular injury falling from 5.2% to 2.6% (p < 0.0001). Periprocedural stroke had the strongest independent association with early and late mortality (p < 0.0001) (Figure 4). Similarly, vascular complications were associated with increased mortality at 2-year follow up.
Figure 3.
Procedural complications by annual cohort.
Figure 4.
Kaplan-Meier survival curves according to peri-procedural stroke.
US TVT registry
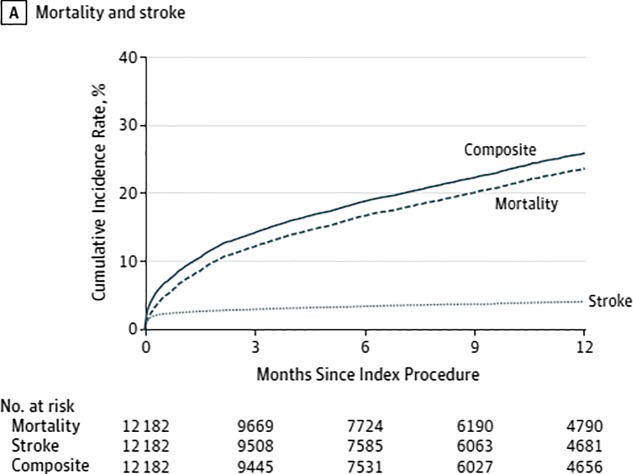
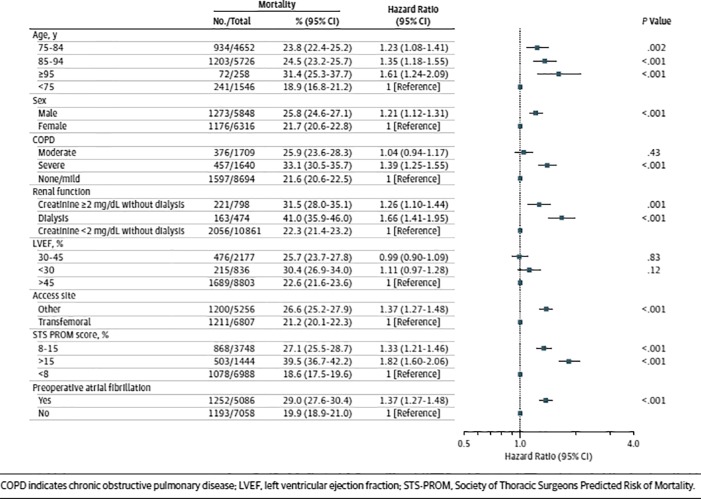
Following the initial report of the in-hospital and 30-day outcome data from the US Transcatheter Valve Therapy (TVT) registry for patients who underwent TAVR from November 2011 to May 2013,6 longer-term outcomes of these patients have recently been published. Dr. David Holmes and colleagues looked at 1-year clinical outcomes for 12,182 patients (median age 84 years; 52% female) who underwent TAVR using the Sapien valve (Edwards Lifesciences) between November 2011 and June 2013 at 299 US hospitals. The majority of cases were performed via femoral access (56.4%). The median STS Predicted Risk of Operative Mortality (STS PROM) score was 7.1%, with 30.8% of patients falling in the 8–15% range and 11.9% with a risk greater than 15%. The rate of comorbidities was high, and approximately 40% of patients had slow gait indicative of frailty. At 30 days, 7% of patients had died, and stroke had occurred in 2.5%. Mortality and stroke increased at 1 year to 23.7% and 4.1%, respectively. The 1-year composite outcome of incidence of death or stroke was 26% (Figure 5). About one-quarter of patients were re-hospitalized once within 1 year, 12.5% twice, and 11.6% 3 or more times. The most common reason for readmission was a composite of stroke, heart failure, or repeat aortic valve intervention (18.6%), primarily driven by heart failure (14.3%). On multivariate analysis, advanced age, male sex, renal failure, severe lung disease, preoperative atrial fibrillation and STS PROM score >15 and non-transfemoral access were among the factors associated with greater 1-year mortality (Figure 6). In exploratory analysis of 198 patients aged 75 years or older who were undergoing dialysis and had an STS PROM score greater than 15%, the 1-year mortality rate was 48.8%. Interestingly, analysis of baseline variables identified only female sex as being associated with increased stroke risk at 1 year.
Figure 5.
Cumulative incidence of outcomes over time.
Figure 6.
Multivariate risk-adjusted outcome of mortality.
Discussion
Over the past 4 years, the landscape of the therapeutic options for valvular heart disease have changed dramatically after the publication of the PARTNER4 and CoreValve trials,5 which represented the most important breakthrough in the field of transcatheter treatment of aortic stenosis.
The PARTNER cohort A trial showed for the first time that in high risk patients with severe aortic stenosis, both surgical and transcatheter valve replacement were associated with similar rates of survival at 1 year.
Similarly, the US CoreValve trial showed for the first time the superiority of transcatheter approach in respect to surgery with an absolute 1-year survival advantage of 4.8% in high risk patients affected by severe aortic stenosis. These conclusions immediately translated into new guidelines issued by the European Society of Cardiology and ACC/AHA/SCAI. In both these trials, patients were highly selected and surgical risks were estimated based on the opinion of a cardiologist and cardiac surgeon reviewed by a panel appointed by the trial coordinators. Therefore, the results may not represent the “real world” transcatheter and surgical aortic valve replacement experience, particularly in Europe.
The recently published UK TAVI registry represents the largest long-term experience of an entire country to date with up to 6 years follow up. This offered not only the opportunity to analyze the trends and predictors of outcome in all-comers registry that has captured an entire country's experience with TAVR, but also to better understand how TAVR is used in “real life” and how early and late outcomes compare. The authors show that although the baseline characteristics of the patients did not change dramatically in later years except for a trend towards treating patients with lower cardiac function, there was a survival benefit in the later cohort. This can be interpreted by the increasing experience of the multidisciplinary teams over the years in selecting patients who are most likely to benefit from TAVR.
In an effort to analyze the learning curve of individuals and institutions in respect to outcomes, the authors explored the issue by looking at early versus later cases, as well as centers that started TAVR program early compared to late adopters. Against the background of an overall gradual reduction in 30-day mortality with time, there was only a trend towards higher 30-day mortality in cases treated early in a center's experience compared with subsequent cases. This observation is in keeping with the rigorous proctoring program that has formalized the learning process in the UK. Interestingly, there was a reduction in the rate of peri-procedural stroke and vascular complications, which can partly be explained by the use of smaller delivery sheaths in the recent years.
The recently published 1-year clinical outcome of US TAVR registry data represent the largest cohort of patients treated to date following the FDA approval and commercialization of the SAPIEN valve. The 1-year mortality observed in the TVT registry is similar to that seen in other registries including the UK TAVI and FRANCE 2.7 Specific baseline characteristics were associated with worse outcome, including advanced age, male sex and increased STS PROM score. In line with the UK TAVI data, patients with severe comorbidities including severe lung disease and renal failure as well as history of atrial fibrillation had higher 1-year mortality. In addition, non-transfemoral access was associated with increased mortality in both registries.
As noted in the US study, the median STS PROM score was lower compared to that in the PARTNER A and B cohorts (7.1% vs 11.8% and 11.2%, respectively). Whether that represents a broadening in selection criteria to include lower risk patients or whether that specific score may actually underestimate surgical risk as determined by experienced cardiovascular surgeons is unknown. The higher mortality seen in the small subset of patients with advanced age and severe comorbidities suggests that it may be possible to identify patients who may not benefit from this procedure and who should be counseled accordingly. However, caution should be used in applying this conclusion in decision-making regarding patient selection, as patients who though extremely frail may have the most to gain, as did Cohort B in the PARTNER trial. Attempts to evaluate for risk factors associated with risk of stroke at 1 year showed only female sex to be statistically significant. Whether this relates to other unmeasured comorbidities in women, such as the presence and distribution of ascending aortic atheroma or the use of large delivery sheaths is unknown, but may be important for patient and family counseling.
What have we learned?
Although three randomized trials and multiple single-center and multicenter registry studies have been published,3–5,7–13 the profile and longer-term outcome of patients undergoing TAVR have been limited. The UK TAVI registry report represents the largest long-term follow up analysis of its kind to date. Along with the US TAVR registry report which includes the largest cohort of patients studied, both these reports provide an invaluable model that should be helpful in guiding patient selection as well as counseling patients undergoing TAVR. Unlike clinical trials were exclusion criteria apply, these registries are reflective of “real world” clinical experience where practitioners are frequently encountered with making difficult clinical decisions in managing very sick patients with severe comorbidities. The survival benefit in the later years of the UK experience, without significant changes in the patients' characteristics, can be interpreted as a result of better patient selection in identifying patients who would do poorly after TAVR. This finding reinforces the importance of following a multidisciplinary “Heart” team model to better select patients who would gain the most from this procedure based on their overall risks. Similarly, the higher mortality rate seen in the subset of patients with advanced age and comorbidities in the US experience, suggests that it may be possible to identify patients who may not benefit from this procedure and who should be counseled accordingly. Nevertheless, despite the high mortality risk in this patient population, it is important to take quality of life and the potential to reduce rehospitalizations for heart failure into consideration during decision-making. Another important observation of these recent reports is the fact that there was no emergence of any structural valve failure over the years, providing reassurance of the long-term durability of these valves and strengthening our confidence that these devices will last at least for several years.
References
- 1.Ludman PF, Moat N, de Belder MA, Blackman DJ, Duncan A, Banya W, MacCarthy PA, Cunningham D, Wendler O, Marlee D, Hildick-Smith D, Young CP, Kovac J, Uren NG, Spyt T, Trivedi U, Howell J, Gray H. Transcatheter Aortic Valve Implantation in the UK: Temporal Trends, Predictors of Outcome and 6 Year Follow Up: A Report from the UK TAVI Registry 2007 to 2012. Circ. 2015 doi: 10.1161/CIRCULATIONAHA.114.013947. Epub 2015/02/01. [DOI] [PubMed] [Google Scholar]
- 2.Holmes DR, Jr, Brennan JM, Rumsfeld JS, Dai D, O'Brien SM, Vemulapalli S, Edwards FH, Carroll J, Shahian D, Grover F, Tuzcu EM, Peterson ED, Brindis RG, Mack MJ. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313(10):1019–28. doi: 10.1001/jama.2015.1474. Epub 2015/03/11. [DOI] [PubMed] [Google Scholar]
- 3.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. Epub 2010/10/22. [DOI] [PubMed] [Google Scholar]
- 4.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. Epub 2011/06/07. [DOI] [PubMed] [Google Scholar]
- 5.Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;371(10):967–968. doi: 10.1056/NEJMc1408396. Epub 2014/09/04. [DOI] [PubMed] [Google Scholar]
- 6.Mack MJ, Brennan JM, Brindis R, Carroll J, Edwards F, Grover F, Shahian D, Tuzcu EM, Peterson ED, Rumsfeld JS, Hewitt K, Shewan C, Michaels J, Christensen B, Christian A, O'Brien S, Holmes D. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310(19):2069–2077. doi: 10.1001/jama.2013.282043. Epub 2013/11/19. [DOI] [PubMed] [Google Scholar]
- 7.Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, Teiger E, Lefevre T, Himbert D, Tchetche D, Carrié D, Albat B, Cribier A, Rioufol G, Sudre A, Blanchard D, Collet F. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366(18):1705–1715. doi: 10.1056/NEJMoa1114705. Epub 2012/05/04 Houel R, Delpine S, Souteyrand G, Favereau X, Ohlmann P, Doisy V, Grollier G, Gommeaux A, Claudel JP, Bourlon F, Bertrand B, VanBelle E, Laskar M. [DOI] [PubMed] [Google Scholar]
- 8.Hamm CW, Mollmann H, Holzhey D, Beckmann A, Veit C, Figulla HR, Cremer J, Kuck KH, Lange R, Zahn R, Sack S, Schuler G, Walther T, Beyersdorf F, Böhm M, Heusch G, Funkat AK, Meinertz T, Neumann T, Papoutsis K, Schneider S. The German Aortic Valve Registry (GARY): in-hospital outcome. Eur heart j. 2014;35(24):1588–1598. doi: 10.1093/eurheartj/eht381. Epub 2013/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moat NE, Ludman P, deBelder MA, Bridgewater B, Cunningham AD, Young CP, Thomas M, Kovac J, Spyt T, MacCarthy PA, Wendler O, Hildick-Smith D, Davies SW, Trivedi U, Blackman DJ, Levy RD, Brecker SJ, Baumbach A, Daniel T, Gray H, Mullen MJ. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Amer Coll Cardiol. 2011;58(20):2130–2138. doi: 10.1016/j.jacc.2011.08.050. Epub 2011/10/25. [DOI] [PubMed] [Google Scholar]
- 10.Nombela-Franco L, Ruel M, Radhakrishnan S, Webb JG, Hansen M, Labinaz M, Thompson C, Fremes S, Dumont E, DeLarochellière R, Doyle D, Urena M, Mok M, Ribeiro HB, Roifman I, Watkins S, Dumesnil JG, Pibarot P, Rodés-Cabau J. Comparison of hemodynamic performance of self-expandable CoreValve versus balloon-expandable Edwards SAPIEN aortic valves inserted by catheter for aortic stenosis. Amer j cardiol. 2013;111(7):1026–1033. doi: 10.1016/j.amjcard.2012.11.063. Epub 2013/01/29. [DOI] [PubMed] [Google Scholar]
- 11.Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, Klugmann S, Bedogni F, Maisano F, Marzocchi A, Poli A, Antoniucci D, Napodano M, DeCarlo M, Fiorina C, Ussia GP. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circ. 2011;123(3):299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. Epub 2011/01/12. [DOI] [PubMed] [Google Scholar]
- 12.Thomas M, Schymik G, Walther T, Himbert D, Lefevre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, Anderson WN, Wendler O. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circ. 2010;122(1):62–69. doi: 10.1161/CIRCULATIONAHA.109.907402. Epub 2010/06/23. [DOI] [PubMed] [Google Scholar]
- 13.Unbehaun A, Pasic M, Dreysse S, Drews T, Kukucka M, Mladenow A, Ivanitskaja-Kühn E, Hetzer R, Buz S. Transapical aortic valve implantation: incidence and predictors of paravalvular leakage and transvalvular regurgitation in a series of 358 patients. J Amer Coll Cardiol. 2012;59(3):211–221. doi: 10.1016/j.jacc.2011.10.857. Epub 2012/01/14. [DOI] [PubMed] [Google Scholar]