Introduction
Acute coronary syndrome (ACS) represents a life-threatening manifestation of atherosclerosis, which usually occurs in the setting of sudden plaque erosion or rupture with intracoronary thrombosis and partial to complete cessation of the downstream myocardial perfusion.1 The diagnosis, management, and treatment of the various forms of ACS, which include persistent ST-segment elevation myocardial infarction (MI), non-ST-segment elevation MI, and unstable angina (UA), have rapidly been evolving in recent years with subsequently a significant decrease in early and late mortality.2–4 Nevertheless, large multicenter studies have shown despite early and successful reperfusion and optimized medication, the actual hospital mortality remains approximately 7%, not mentioning the evolution towards ischemic heart failure in a considerable number of surviving patients.5
The current diagnostic tools - i.e., blood biomarkers (e.g. troponin), electrocardiography, and ultrasonography - are key tools in the diagnosis of ACS and fast patient triage, but these markers provide only a partial insight in the complex, evolving processes occurring in the jeopardized myocardium.6 Moreover, patients with ACS encompasses a broad and heterogeneous population and many patients with acute chest pain turn out not to have ACS. The accurate diagnosis and differentiation of ACS from other acute cardiac diseases (e.g. aortic dissection, pulmonary embolism, myocarditis, Takotsubo cardiomyopathy) which is essential for therapeutic decision making and specific therapies (e.g. timely reperfusion) may be challenging or even impossible with the above diagnostic tools.
Cardiovascular magnetic resonance (CMR) imaging offers the cardiologist a comprehensive view on ACS at the tissue level (e.g. edema, necrosis, microvascular injury, hemorrhage) and may add important information for the diagnosis and differential diagnosis of ACS. As recent studies have demonstrated CMR-derived parameters yield independent prognostic value in addition to traditional risk factors, CMR may be also important for patient risk stratification. Despite the diagnostic and prognostic utility of CMR in ACS many cardiologists are not yet familiar with this fascinating and clinical helpful imaging modality. The aim of this review is therefore to elucidate the role of CMR in this group of cardiac high-risk patients.
Cardiovascular magnetic resonance: Technical features
Magnetic resonance images are created using the relaxation behavior of hydrogen protons in a high magnetic field – typically 1.5T or 3.0T – following excitation by selective radiofrequency pulses.7 The magnetic resonance technique, which was first applied for medical purposes more than three decades ago, has been characterized by a tremendous technical evolution over the years. Generally speaking it has evolved from a static imaging technique requiring long acquisition times towards a highly versatile, fast and organ-dedicated imaging modality. Also for cardiovascular applications, this technique has a lot to offer but on the other hand faces many specific difficulties, likely explaining why it took much longer than for most other organs to get truly integrated in clinical practice.
Intrinsic strengths are the high spatial and contrast image resolution, the free choice of image plane and size of field of view allowing an excellent view on the heart and great vessels. However, specific sequence modifications are needed to get rid of image blurring due to motion incurred by the heart, respiration and flowing blood.7
Cardiac motion and blood flow are frozen by synchronizing data acquisition to the electrical activity of the heart (electrocardiographic gating). Respiratory motion is halted by performing the acquisition during (repeated) breath-holds or by using a respiratory navigator monitoring the diaphragmatic excursion.
Similar to MR imaging in other parts of the body, cardiovascular magnetic resonance (CMR) sequences are either spin-echo or gradient-(recalled)-echo based. Spin-echo images offer a morphologic view on the heart and pericardium. These sequences are designed that blood has a dark or black-blood appearance, thus providing a natural contrast with the myocardium and vessel walls.8 Using a different weighting (e.g., tbl1- and tbl2-weighting) information can be achieved about tissue characteristics.
The gradient-(recalled)-echo sequence should be regarded as a versatile, multi-purpose sequence which can be used for functional, perfusion, and angiographic imaging, and similar to spin-echo imaging is often used for cardiac tissue characterization. For functional cardiac imaging, balanced steady-state free-precession (SSFP) imaging has become the gradient-echo sequence of choice.9 The high signal of blood at SSFP provides an excellent contrast with the myocardium. Images can be obtained with a high temporal resolution (e.g. every 30 ms) over the cardiac cycle and played in a cine loop, allowing to assess cardiac motion and deformation. Paramagnetic gadolinium-based contrast agents alter tissue magnetization and relaxation, and are often used in CMR for a variety of purposes. Firstly, ultrafast gradient-echo imaging during the passage of intravenously administrated gadolinium-chelates through blood vessels and myocardium can be used for three-dimensional MR angiography, and evaluation of myocardial perfusion, respectively. Secondly, differences in wash-in and wash-out kinetics, and distribution volume of the administered contrast agents can be exploited for cardiac tissue characterization.
CMR is rapidly evolving from a pure imaging modality toward a modality offering quantitative data with regard to tissue markers (using tbl1, tbl2, tbl2* mapping techniques), myocardial perfusion, blood flow and myocardial function.10 It should be noted that administration of gadolinium compounds is contra-indicated in patients with severe renal insufficiency.11 Moreover, permanent pacemakers and ICD's are considered a contra-indication for CMR, and although MR-conditional devices have become available, the benefits versus potential risks of performing a CMR should be carefully weighted in each individual patient.12
How to apply CMR in practice
In patients with a typical presentation of ACS, there is currently no role for CMR in the diagnostic work-up and patient triage. These patients need urgent (STEMI) or a timely (NSTEMI) reperfusion of the infarcted vessel. However, it may be of great value in patients with a doubtful clinical presentation, and/or equivocal routine diagnostic tests. After the treatment/reperfusion of acute MI CMR is able to visualize the exact tissue damage and provides important prognostic information.
CMR in patients with acute infarction
In acute MI patients, CMR is typically performed in the first two to five days following the acute event, which in experienced hands is finished in about 30 minutes. Patient monitoring during the exam is important. However, it is of note that the ECG signal is disturbed – and therefore not clinically useful – once the patient is inside the magnet. Visual and verbal patient contact, blood pressure measurements and pulse oximetry allow close patient monitoring. Moreover, in case of adverse events the personal should be trained for rapid evacuation and resuscitation in an MR safe environment. Once patient preparation and positioning in the magnet is completed, imaging is started with a set of preparatory scans including scout views which are used for determining cardiac imaging planes.
Next, a series of different sequences are applied to comprehensively study the heart, and to answer the clinical question (Figure 1) Cardiac function is studied using SSFP cine imaging along the different cardiac axes, allowing to evaluate myocardial wall motion and contraction patterns, and to visualize valve leaflet motion as well as abnormal – regurgitant/stenotic – flow patterns. At least one set of contiguous cine images completely encompassing the ventricles - usually in short-axis direction – is acquired to calculate ventricular volumes, myocardial mass and ejection fraction.
Figure 1.
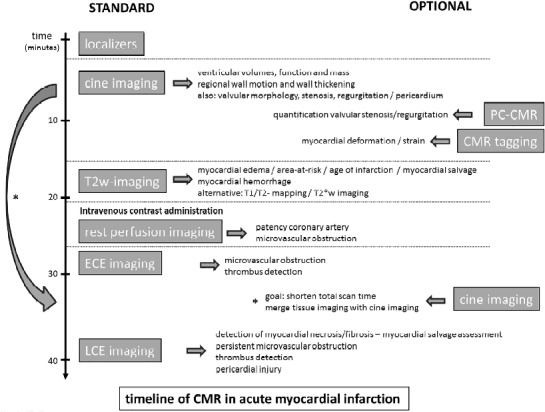
Timeline of comprehensive CMR in a patient with established diagnosis of acute myocardial infarction. The standard sequences are shown on the left, the optional sequences on the right. To shorten total imaging time, cine imaging can be performed following contrast administration. Abbreviations: PC-CMR, phase-contrast CMR. Adapted from Ischemic Heart Disease by Bogaert J and Dymarkowski S, in Clinical Cardiac MRI Second Edition, Bogaert J, Dymarkowski S, Taylor AM, Muthurangu V (eds). Springer Heidelberg, Germany (ISBN 978-642-23034-9).
In ACS patients, the ischemically injured - jeopardized - myocardium can be reliably depicted by tbl2-weighted imaging. Alternatively, sequences such as pre-contrast tbl1 mapping and tbl2 mapping, have shown appealing initial results for this purpose.13–14 Myocardial perfusion patterns – reflecting the adequacy of myocardial reperfusion – are studied using the first-pass of intravenously injected gadolinium contrast agents through the heart.15 Similar information with regard to the presence of microvascular obstruction can be obtained using the so-called early contrast enhanced (ECE) imaging.16 Areas of incomplete myocardial reperfusion are depicted as dark, non-enhancing areas. Late contrast enhanced (LCE) imaging – performed 10–15 minutes post-contrast administration – is used for myocardial infarct imaging. Since the injured myocardium enhances, it may of be interest to perform at least a part of cine imaging (e.g. in cardiac short-axis) after contrast administration, thus providing a kind of merged viability-functional imaging (Figure 2).17–18
Figure 2.
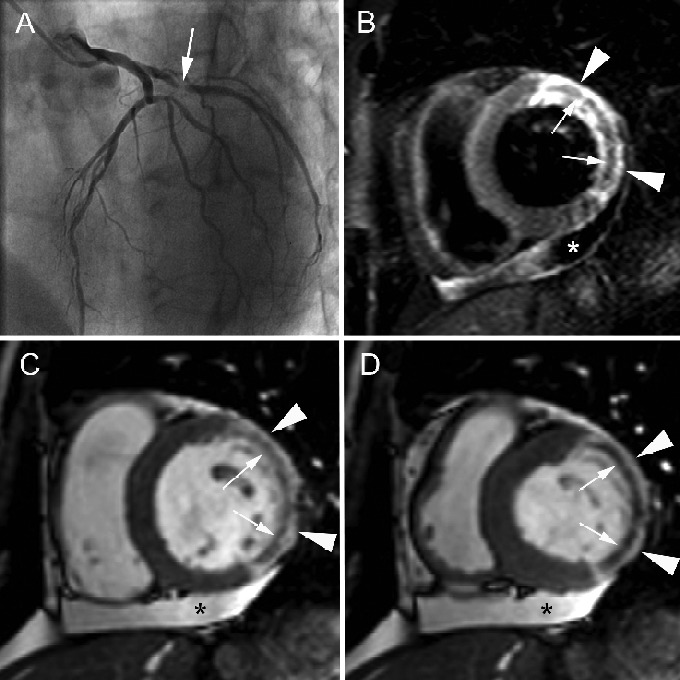
Extensive hemorrhagic myocardial infarction in 60-year-old woman presenting with non-STEMI. Cardiac catheterization shows 95% stenosis in proximal left circumflex coronary artery (arrow, A) and 80% stenosis in mid LAD. Both lesions treated with PCI. CMR, performed 5 days after the acute event, shows extensive myocardial edema in the entire lateral LV wall (arrowheads, B) with presence of a central dark zone (arrows, B) reflecting intramural hemorrhage. Cine CMR imaging in cardiac short-axis at end diastole (C), and end systole (D) shows transmural enhancement of the lateral LV wall (arrowheads, C, D) with presence of a microvascular obstruction (arrows, C,D). Note significant impairment of the contractility in the infarcted myocardium while systolic wall thickening is well preserved in the viable – dark-gray – myocardium. LV function at CMR is mildly to moderately reduced (EF 40%). Presence of a mild pericardial effusion(*, B, C, D).
In case of concomitant valve pathology, phase-contrast or velocity-encoded cine imaging can be applied to quantify regurgitant flow volumes and/or measure peak velocities/transvalvular gradients.19 Moreover, a series of CMR sequences are available for additional measurements, but these are mostly applied in a scientific setting. Amongst them, CMR myocardial tagging has been of great use to unravel the myocardial deformation patterns in infarct patients, providing similar information as can be obtained by tissue Doppler imaging.20
Finally, image analysis and interpretation are performed on off-line dedicated workstation. To quantify ventricular volumes and myocardial mass, endo- and epicardial borders are contoured manually or using (semi)-automated approaches. A similar approach is used to assess the extent of quantify the extent of the jeopardized and/or infarcted myocardium. Studies have shown an excellent reproducibility of CMR post-processing with low inter- and intra-observer variability for LV volumes, ejection fraction, infarct size and myocardial salvage assessment.21–22
CMR in patients with clinical suspicion of ACS
In patients with clinical suspicion of ACS but equivocal diagnostic testing, a modified CMR protocol is used including morphological and/or functional depiction of significant coronary stenoses, and exclusion of extracardiac causes of acute chest pain such as aortic dissection or pulmonary embolism (Figure 3) Stress perfusion CMR imaging alone, or in combination with rest perfusion CMR imaging is an accurate technique to depicting hemodynamically significant stenosis.23–24 Intravenous administration of vasodilator (e.g. adenosine, dipyridamole) causes a preferential flow towards normally perfused myocardium at the expense of blood flow to areas perfused by hemodynamically significant stenoses (Figure 4). Hypo-perfused myocardium appears as dark, non-enhancing myocardium at first-pass myocardial perfusion CMR. Combining rest-stress perfusion allows calculation of myocardial perfusion reserve.25 At present, a variety of three-dimensional (3D) free-breathing and breath-hold CMR techniques are available for coronary artery imaging allowing to visualize large portions of the coronary artery tree.26–27 Imaging can be targeted on the course of the coronary arteries or contain the whole-heart. Finally, bright blood (SSFP) imaging and contrast-enhanced 3D mono-phasic or time-resolved CMR angiography can be applied to diagnose aortic dissection or pulmonary embolism, as cause of acute chest pain.
Figure 3.
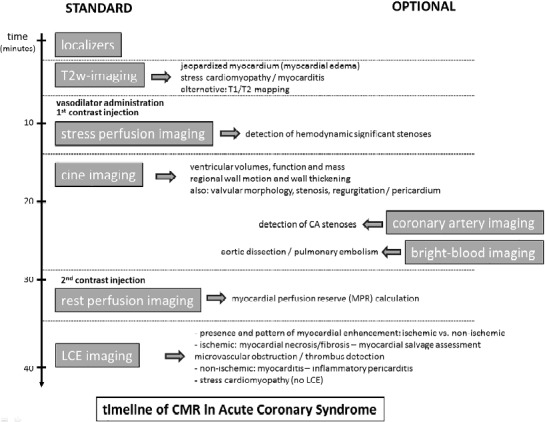
Timeline of comprehensive CMR in a patient with clinical presentation of ACS. The standard sequences are shown on the left, the optional sequences on the right. Adapted from Ischemic Heart Disease by Bogaert J and Dymarkowski S, in Clinical Cardiac MRI Second Edition, Bogaert J, Dymarkowski S, Taylor AM, Muthurangu V (eds). Springer Heidelberg, Germany (ISBN 978-642-23034-9).
Figure 4.
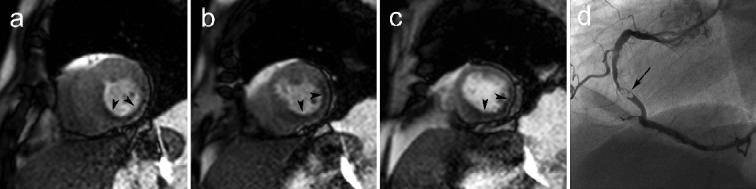
Example of an extensive perfusion defect in the inferolateral LV wall during stress perfusion CMR (a-c) in a patient with a high-grade stenosis of the mid right coronary artery (arrow, d). First-pass perfusion CMR during adenosine stress at 3 short-axis levels (basal, a; mid, b; apical, c) shows presence of an extensive hypo-enhancement of the entire inferior and inferolateral LV wall. Note the concomitant lack of systolic wall thickening (most remarkable in the basal slice) caused by myocardial ischemia. Adapted from Myocardial Perfusion by Bogaert J and Goetschalckx K, in Clinical Cardiac MRI Second Edition, Bogaert J, Dymarkowski S, Taylor AM, Muthurangu V (eds). Springer Heidelberg, Germany (ISBN 978-642-23034-9).
Visualization and characterization of the jeopardized myocardium
The jeopardized myocardium – i.e., the myocardium devoid of blood supply distally to the coronary artery occlusion – is at risk of becoming necrotic if not timely reperfused. Restoring reperfusion aims to halt the irreversible cell necrosis and to salvage the ischemically injured, still viable myocardium. The extent of jeopardized myocardium is mainly determined by the location of the culprit lesion along the coronary artery. In contrast, myocardial cell death is multifactorial determined including time of symptom onset to reperfusion, imbalance between oxygen need and supply, severity of coronary artery occlusion, presence of prodromal angina as well as presence of collaterals, success of revascularization, and reperfusion injury.28–30 Assessment of the extent of the jeopardized myocardium or area at risk is important, but remains challenging. It can be estimated using ECG criteria such as the total amount of ST-segment deviation on admission (Aldrich method) and angiographic criteria such as BARI, APPROACH risk score.31 Moreover, this area can be visualized by injection of a tracer (SPECT) or contrast material (cardiac computed tomography) prior to PCI but this approach is rarely used in clinical practice because most patients are immediately transferred to cardiac catheterization for PCI.32
CMR, in contrast, offers an appealing alternative to visualize the area at risk. Moreover, this information can combined with infarct imaging, thus allowing calculation of a myocardial salvage ratio.33–36 Back in the 1980s, it was reported that in ischemically damaged myocardium, tbl1 and tbl2 relaxation times increase due an augmentation of free water (caused by disturbances in cellular membrane function and presence of interstitial edema).37–38 This phenomenon can be exploited to noninvasively discriminate normal from ischemic myocardium.
Over the years, in particular, tbl2-weighted imaging has increasingly been used for this purpose. Jeopardized myocardium appears bright (“hyperintense”) compared to normal myocardium (Figure 5).14 Abnormalities on tbl2-weighting imaging occur in an experimental setting about 30 minutes after onset of ischemia and are closely matched to the area of myocardial dysfunction.39 The abnormalities remain visible at least one week after the acute event, thus providing a method to retrospectively obtain evidence of a recent ischemic myocardial event.32 In patients with a clinical suspicion of ACS, detection of myocardial edema can allow the identification of the infarct-related artery (IRA) whereas lack of increased myocardial signal intensity on tbl2-weighted imaging practically excludes an acute ischemic event.40 However, since myocardial edema may occur in other ‘acute’ cardiac conditions, such as myocarditis or stress cardiomyopathy, as well, abnormal findings on tbl2-weighted imaging should be interpreted correctly, using information obtained by LCE and cine imaging. Although well-validated in animal models, some concerns remain with regard to the accuracy of tbl2-weighted imaging in depicting the myocardium at risk.41–45 Moreover, several tbl2-imaging CMR methods have become available for myocardial edema depiction. The different sequences, however, are not interchangeable and their reproducibility is variable.46
Figure 5.
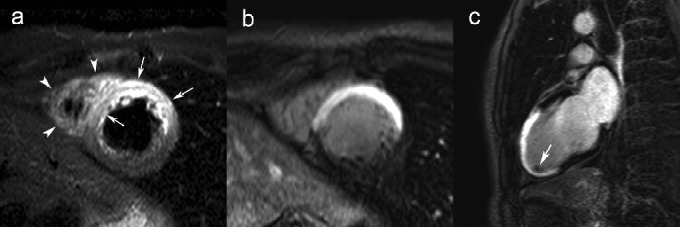
Combined tbl2-weighted and LCE imaging in a patient with a reperfused anteroseptal myocardial infarction. Short-axis tbl2-weighted imaging (a), and LCE imaging in short-axis (b) and vertrical long-axis plane (c). While the extent of edema can be well appreciated on tbl2-weighted imaging (arrows, a), there is a strong enhancement transmural enhancement on LCE imaging (b) of the anteroseptal LV wall, corresponding to the area the edema. The involvement of the LV apex can be appreciated on the vertical long-axis view (c). Note the presence of a small thrombus in the LV apex (arrow, c). Extension of myocardial edema to the RV free wall (arrowheads, a). Adapted from Ischemic Heart Disease by Bogaert J and Dymarkowski S, in Clinical Cardiac MRI Second Edition, Bogaert J, Dymarkowski S, Taylor AM, Muthurangu V (eds). Springer Heidelberg, Germany (ISBN 978-642-23034-9).
While tbl2-imaging provides information with regard to the area at risk, contrast-enhanced imaging is essential to visualize the infarcted myocardium.47–48 The contrast kinetics as well as the distribution volume of an extracellular gadolinium-based contrast agent are altered in the infarcted myocardium which results in an increased accumulation of gadolinium molecules and subsequently a greater shortening of tbl1-relaxation time in the irreversibly damaged myocardium compared to normal myocardium. Use of an inversion-recovery pre-pulse has a major impact on improving infarct visualization.49 In brief, longitudinal relaxation is tissue specific and this phenomenon can be exploited to enhance contrast between normal and pathological myocardium. This pre-pulse in combination with an appropriate inversion time enables to nullify signal of normal myocardium which results in an excellent depiction of infarcted myocardium – with infarct volumes as small as or even less than 1g of myocardial tissue. Infarct images are typically acquired relative late – i.e. 10 to 15 minutes – post-contrast administration, yielding the most accurate appraisal of the extent of myocardial necrosis)(Figure 5). As mentioned above, this sequence is called LCE imaging in contrast to ECE imaging acquired immediately following contrast administration which is used for other purposes (e.g, microvascular obstruction). Using a multi-slice two-dimensional or three-dimensional approach encompassing the ventricles, the presence and extent of infarcted myocardium can be visualized and quantified – expressed as a volume or as percentage of LV mass. A myocardial infarct is typically located in one of the coronary perfusion territories, is always subendocardial located and has a variable transmural extent.
A myocardial salvage ratio can be calculated relating infarct size measured by LCE imaging to area at risk measured by tbl2-weighted imaging (Figure 6).33,35,36 This ratio provides valuable information with regard to the extent of irreversible myocardial damage in the jeopardized myocardium.50 On one end of the spectrum is the aborted MI with presence of myocardial edema but with no evidence of infarction on LCE,51 while infarcts involving the entire area at risk have a ratio of 0. The myocardial salvage ratio is directly related to post-reperfusion ST-segment resolution, and inversely to adverse LV remodeling and it bears independent prognostic value.35,52–53
Figure 6.
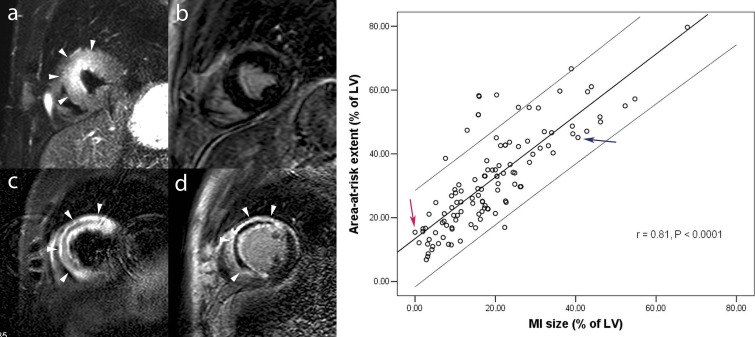
Complementary value of tbl2-weighted and LCE imaging in acute myocardial infarction. Short-axis tbl2-weighted imaging (a, c) and LCE imaging (b, d). Patient one shows a large area of myocardial edema in the anteroseptal LV wall (arrowheads, a) but not late enhancement (b), corresponding to an aborted MI. Patient two shows an extensive area of myocardial in the anteroseptal LV wall (arrowheads, c). Following contrast administration strong enhancement occurs in the anteroseptal LV wall (arrowheads, D). The extent of enhancement nearly equals the extent of myocardial edema, leaving no or minimal amount myocardial salvage. In addition, this patient shows a large central dark zone within the edematous myocardium corresponding to myocardial hemorrhage (c), and an extensive area of MVO (d). The relationship between extent of myocardial edema – reflecting the area-at-risk – and extent of late enhancement – reflecting infarct size – obtained in 137 patients is shown in the graph (adapted from Masci et al. 2010). On the graph, patient one is shown by the red arrow, patient two by the blue arrow.
Tissue markers of myocardial infarction severity
Infarct size and transmurality
A key characteristic of myocardial tissue is its low regenerative capacity since myocytes are terminally differentiated having lost the capacity to renew damaged myocardium. As a consequence, irreversibly damaged myocardium is replaced by an afunctional fibrotic scar. Thus, the amount of contractile tissue lost – or the infarct size – reflects infarct severity, is a crucial determinant of adverse LV remodeling and patient outcome, and therefore often used as surrogate end point to assess the efficacy of novel treatments.54–56 LCE imaging is a well-validated, accurate and reproducible tool for infarct sizing independent of the time post-infarction.57–59 Moreover, it is key to depict small-sized infarcts, as little as 1g of tissue, that drop below the radar of ECG other imaging modalities such as SPECT.60–61 Since the presence of unrecognized MI's portends unfavorable outcome, CMR has become a preferred modality for infarct detection and sizing.62 Moreover, since timely reperfusion may stop the transmural progress of necrosis and salvage the jeopardized myocardium in the viable subepicardial layers, assessment of infarct transmurality is another tissue marker of infarct severity which because its high spatial and contrast resolution can be well assessed by CMR.63 Several studies have shown that increased infarct transmurality is related with lack of inotropic reserve and impaired recovery of contractile function, and is associated with more pronounced post-infarct wall thinning, aneurysm formation and adverse ventricular remodeling.64–67
Microvascular obstruction
Although endothelial cells are more resistant to prolonged ischemia than myocytes, successful coronary reperfusion is often associated with failure to achieve uniform myocardial reperfusion, i.e., the so-called “no-reflow” or “microvascular obstruction” (MVO) phenomenon.68 It is believed that other mechanisms such as reperfusion injury, distal embolization, and likely also an individual susceptibility contribute to the occurrence of MVO.69 For example, Rochitte and colleagues showed a significant increase in the extent of MVO and infarct size as well as the ratio of MVO to infarct size during the first 48 hours after MI, supporting the concept that microvascular and myocardial injury continue beyond reperfusion.68 MVO occurs in both ST-segment and non-ST-segment elevation MI with a prevalence ranging from 5% to more than 50%.69–72 Presence and extent of MVO is related to the duration of ischemia time, coronary collaterals, more severe myocardial damage, and importantly is independently associated with lack of functional recovery, adverse remodeling, and worse patient outcome.72–78 Information with regard to the presence of MVO comes at CMR as a free bonus when intravenous contrast material is injected.78–82 In case of MVO, this part of the myocardium does not enhance (i.e. remains dark) after contrast administration. The presence and maximal extent of MVO can be best evaluated with first-pass myocardial perfusion imaging, or at ECE imaging.16,82 Use a long inversion time is advantageous to visualize MVO at ECE.83 As gadolinium-molecules diffuse in the MVO, the extent as well as the prevalence of MVO decrease following contrast administration (Figure 7).16,72 Several studies have shown that persistent MVO at LCE imaging is prognostically more relevant than MVO at ECE, and it may be the best prognosticator of hard clinical events.78,84–85 Limiting microvascular damage and improving microvascular circulation in acute MI patients has become an area of intensive research, often using CMR-based endpoints.86–90
Figure 7.
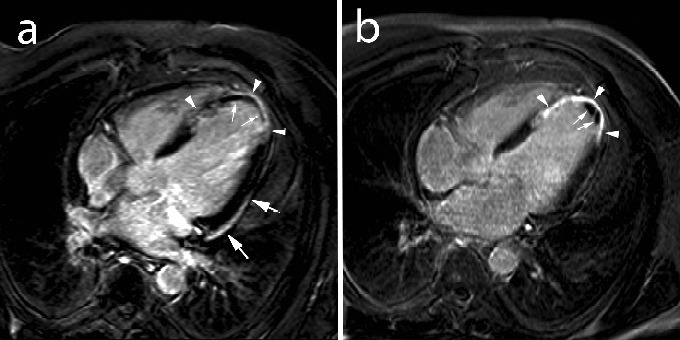
Post myocardial infarction LV remodeling in a 52-year-old male with anteroapical myocardial infarction. LCE imaging in the horizontal long-axis image plane at 1 week (a) and 4 months (b) post-infarction. At 1 week, presence of diffuse enhancement of the apical part of the ventricular septum and LV apex (arrowheads, a), with a large area of microvascular obstruction (small arrows, a). Note the presence of pericardial enhancement over the laterobasal part of the LV (arrows, a). At 4 month follow-up, the infarct has thinned and strongly enhances (arrowheads, b). Note the presence of a small mural thrombus in LV apex (arrows, b). Adapted from Ischemic Heart Disease by Bogaert J and Dymarkowski S, in Clinical Cardiac MRI Second Edition, Bogaert J, Dymarkowski S, Taylor AM, Muthurangu V (eds). Springer Heidelberg, Germany (ISBN 978-642-23034-9).
Intramyocardial hemorrhage
Despite successful recanalization of the culprit lesion, studies in animal models and patients have frequently shown the presence of myocardial hemorrhage in the infarct core.91–93 It is believed that reperfusion of ischemic myocardium with irreversible microvascular damage may cause intramyocardial hemorrhage (IMH) with massive red blood extravasation into the extracellular space. This phenomenon is associated with longer ischemia times, severity of flow depression before reperfusion and extent of necrosis, and has been observed after pharmacological and mechanical reperfusion. Exploiting the paramagnetic properties of the hemoglobin breakdown product - deoxyhemoglobin - causing shortening of tbl2-relaxation times, tbl2-weighted and tbl2*-weighted CMR can be used to noninvasively demonstrate IMH.94–98 Whereas the jeopardized myocardium in non-hemorrhagic infarcts is homogenously bright, hemorrhagic infarct presents with a central dark appearance (Figure 2). CMR studies have invariably shown that IMH is related to more extensive infarcts with higher infarct transmurality and lower baseline EF. IMH is independently related to adverse regional and global LV remodeling, lack of functional recovery, markers of late arrhythmic risk, and worse patient outcome.99–102 Since IMH is consistently associated with (large) MVO, most likely the presence of IMH reflects greater cellular damage resulting in greater adverse remodeling and poorer outcomes.70 Thus, future strategies aimed at preserving microvascular integrity may improve patient outcome by reducing IMH.96
Myocardial infarct heterogeneity
Patients with a recent history of MI are at increased risk for arrhythmias. Whereas the center of the infarcted myocardium evolves towards a dense fibrous scar incapable of depolarization, the infarct border zone in contrast - containing a mixture of non-viable and viable myocardium - may be an arrhythmogenic substrate potentially causing ventricular arrhythmias and sudden cardiac death. CMR has potential to depict infarct tissue heterogeneity, and thus to estimate increased risk for sudden cardiac death.103–104 Dense fibrotic myocardium at LCE imaging has typically a high signal intensity (SI) - defined as ≥ 5 SD above the SI of normal myocardium. Lower SI - i.e. between 2SD and 5SD - the so-called ‘gray’ myocardium corresponds to infarct areas with variable amounts of fibrosis and myocytes. Of note, the gray myocardium is not necessarily confined to the infarct border, but can also be found in more central locations, and also in the papillary muscles.105 High spatial resolution imaging may improve visualization of infarct heterogeneity and may be an important predictor of arrhythmogenic clinical events.105
Right ventricular infarction
Isolated right ventricular (RV) infarction is rare ( < 3% of all infarcts), and therefore, infarction of the RV is usually an expression of a biventricular MI.105 In most cases, the LV is predominantly involved with a variable degree of infarct extension towards the anterior or inferior RV wall, causing RV dysfunction. Lack of functional recovery, however, is associated with high mortality rate. Current techniques, such as ECG with right precordial leads and echocardiography, underestimate the true incidence of RV ischemic injury. Imaging of the thin, trabeculated RV wall is challenging, even with high spatial and contrast resolution techniques such as CMR. Nevertheless, this technique has been of great value to better appreciate reversible and irreversible RV damage using a comprehensive approach including tbl2-weighted, LCE and cine imaging.106–108 Grothoff and colleagues showed that RV injury evidenced by CMR yields strong, independent predictive value of clinical outcome after acute reperfused STEMI.109
Role of CMR in patients with acute chest pain suspected of ACS
Growing evidence has been nurturing the role of CMR in evaluating patients with acute pain. Although evolving ECG abnormalities and increased cardiac biomarkers remain key diagnostic tools in decision making in ACS patients, many “ACS” patients do not have ACS. Moreover, cardiac troponin is a highly sensitive but poorly specific biomarker of ischemic myocardial damage, since any condition provoking myocyte necrosis will result in a rise in cardiac troponin. Also, the increase in cardiac troponin is limited to a narrow time window reducing the sensitivity to depict subacute MI. Furthermore, ECG abnormalities may overlap between ischemic and non-ischemic cardiac etiology, and small infarcts may not necessarily cause typical ECG abnormalities. Comprehensive CMR provides valuable information in the majority of the above patients (Figure 3). Several studies have shown that CMR can be applied in the emergency department to confirm or to exclude ACS in patients with chest pain but absent ST-segment elevation.110–113 Stress perfusion CMR is accurate to depict hemodynamic significant stenoses,23–24 can be easily integrated in a CMR protocol but patients are not allowed to have caffeine-containing beverages consumed 24 hours prior to a CMR perfusion study. Moreover, although much progress has been achieved in the field of CMR coronary imaging, results are not always consistent,114–115 and multidetector computed tomography (MDCT) is a faster and more appealing alternative for non-invasive coronary artery imaging.116 Additionally, coronary artery imaging can be combined with imaging of the thoracic aorta and pulmonary arteries using a so-called triple rule-out MDCT approach.117 The current value of CMR, at present, in ACS patients is primarily in the diagnostic work-up of patients with acute chest pain showing normal or non-obstructed coronaries at cardiac catheterization.118 The diagnostic problem to solve is whether these patients have a spontaneous recanalization of the culprit lesion, obstruction of one of the smaller side branches, another cardiac disease causing ECG changes and release of cardiac enzymes, or an extracardiac origin of their complaints. In case of spontaneous clot resolution, CMR is often able to depict signs of myocardial ischemic damage, such as myocardial edema, LCE and functional impairment in a coronary artery perfusion territory. CMR findings in occlusion of one of the side branches are similar but the abnormalities are much more limited in extent and do not correspond to the perfusion territory found in typical MI's. Instead these present as focal involvement of the myocardium corresponding to the perfusion territory of the affected side branch. Retrospective analysis of the ‘normal’ coronary angiograms may reveal a missed proximal occlusion of one of the side branches, although often no abnormalities can be depicted.
A substantial number of patients presenting acute chest pain, troponin raise but unobstructed coronary angiograms turn out to have acute myocarditis.118 Comprehensive CMR has evolved as a valuable clinical tool for the diagnosis of suspected acute myocarditis.119–121 Abnormalities include focal to generalized myocardial edema, hyperemia on ECE, area of focal LCE, functional abnormalities and concomitant pericardial pathology.122 Similar to infarct patients, myocardial edema in acute myocarditis can be depicted by tbl2-weighted CMR techniques, while newer tbl2-mapping techniques are promising to better depict the extent of abnormalities.121 Myocardial contrast enhancement, using LCE, is a frequent finding in the clinical setting of suspected myocarditis and is associated with active inflammation at histopathology. The pattern of enhancement, however, is different from the enhancement pattern in acute MI, providing a powerful tool to differentiate both entities. Two patterns are typical for myocarditis, a) subepicardial (patchy) distribution in the free LV lateral wall, b) an intramural, rim-like pattern in the septal wall (Figure 8).123 In myocarditis patients, presence of LCE is the strongest, independent predictor of adverse outcome.124 In a recent study, Francone et al. showed that the CMR sensitivity is dependent on the clinical pattern of presentation, yielding the highest sensitivity in patients with an infarct-like clinical presentation.125 Finally, native and post-contrast tbl1 mapping with calculation of myocardial extracellular volume (ECV) hold promise in further improving the accuracy of CMR to diagnose acute myocarditis.126–127 Stress cardiomyopathy or takotsubo cardiomyopathy represents a reversible LV dysfunction with acute myocardial infarction-like ST-segment elevation without coronary artery lesions and with minimal myocardial enzymatic release. The typical presentation is apical – less frequently midventricular – ballooning with preserved contractility in the other LV segments. Although the diagnosis is usually established at left ventriculography during cardiac catheterization, CMR is of particular interest to differentiate this entity from an acute myocarditis or acute MI.128 CMR typically shows a pattern of LV dysfunction, myocardial edema, and absence of necrosis/fibrosis on LCE (Figure 9).129
Figure 8.
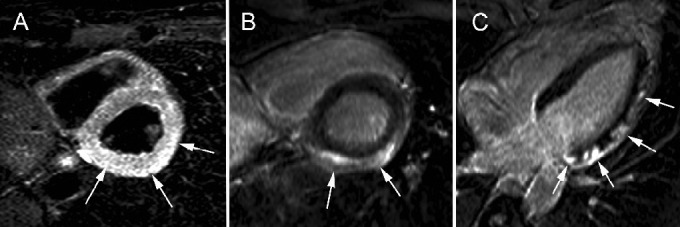
Acute myocarditis in 49-year-old male patient presenting with acute chest pain, cardiac enzymes, ST-elevation (lateral leads) but normal coronary arteries at cardiac catheterization. Short-axis tbl2-weighted imaging shows diffuse myocardial edema in the lateral LV wall (arrows, A). At LCE imaging (short-axis, B; horizontal long-axis, C) multifocal subepicardial enhancement is present (arrows, B, C). This pattern of CMR abnormalities is strongly suggestive of acute myocarditis.
Figure 9.
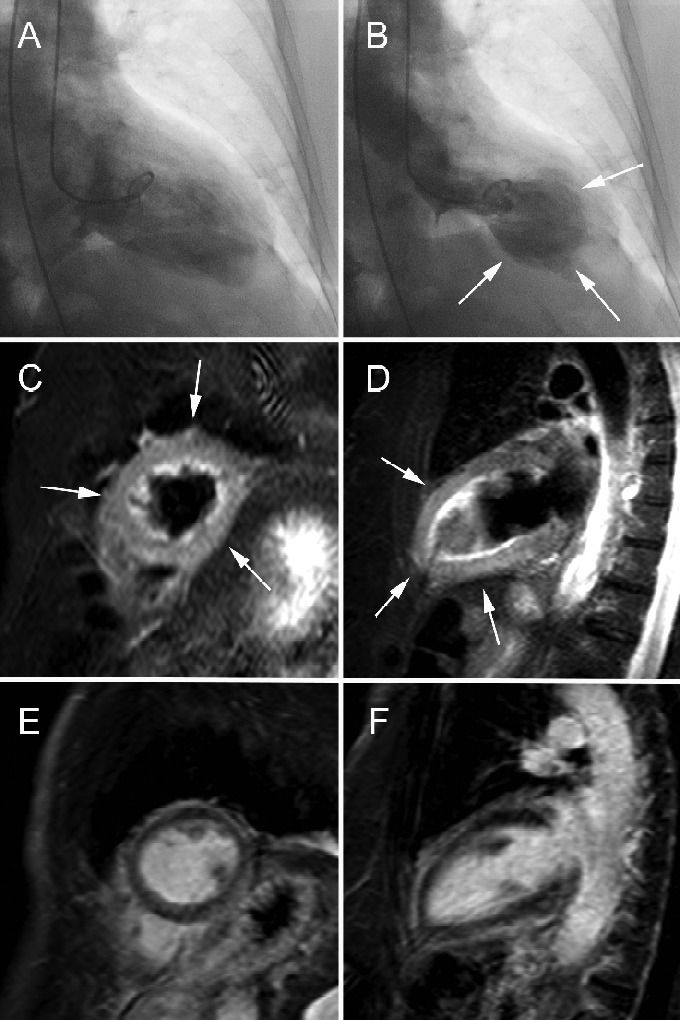
Takotsubo cardiomyopathy in 78-year-old woman presenting with retrosternal chest pain irradiating to shoulders, ST-elevation V6-I and mildly increased cardiac enzymes. Findings of apical ballooning (arrows, B) at cardiac catheterization (end-diastolic A and end-systolic (B) time frame). tbl2-weighted CMR in short-axis (C) and vertical long-axis (D) and LCE CMR in short-axis (E) and vertical long-axis (F). Presence of diffuse myocardial edema in apical half of LV (arrows, C, D). No evidence of increased myocardial enhancement at LCE CMR (E, F). Cine CMR imaging (not shown) shows moderate to severe hypokinesia in apical part of LV with global LV EF of 40%.
CMR in infarct remodeling
Myocardial necrosis triggers inflammatory and reparative processes in the affected myocardium. Whereas in an initial phase infarct swelling/expansion occurs due to myocardial edema and inflammatory reaction, the necrotic myocardium is consecutively replaced by granulation tissue ultimately evolving towards scarred tissue, a process deemed to take several weeks. This process of infarct healing is coupled with adaptive ventricular remodeling. Since CMR fuses in vivo tissue characterization with function imaging, this technique has extensively been used the last two decades to study infarct and ventricular remodeling (Figure 7).62 Moreover, its noninvasive approach allows long-term follow up to better understand the processes contributing to evolution towards ischemic heart failure.59,99
Imaging of infarct-related complications
Acute MI may be associated by potentially harmful, even lethal complications such as aneurysm and thrombus formation, valve leakage and post-infarction pericardial injury. Using a comprehensive CMR approach even rare complications, such as a dissecting myocardial hematoma, can reliably be depicted. Although CMR has no primary role in detecting mitral valve leakage post-infarction, it enables to depict papillary muscle infarction as potential cause of valve regurgitation.105,130–132 CMR is definitely the best imaging modality to depict ventricular thrombi (Figure 7). In particular contrast-enhanced imaging allows to depict or to exclude even small thrombi, typically appearing as dark structures surrounded by the contrast-enhanced blood.132–133 CMR may of help in differentiating true from false aneurysms.134 Finally, since the damage inflicted by the coronary artery occlusion is not halted at the myocardial border, but may affect in the pericardium as well, acute MI patients frequently show pericardial effusion and/or inflammation, which can be very well depicted at CMR (Figure 7).135–137
CMR and prognosis assessment in ACS
Prognosis assessment and predicting of future major adverse cardiac events (MACE) in patients with ACS are crucial. CMR, because of its versatility, may be of great value. Firstly, in patients with suspected CAD but no evidence of prior myocardial infarction, stress imaging (either perfusion or dobutamine) help in patient risk stratification by adding important complementary information to recognized risk factors such as impairment in LV ejection fraction.138–141 Other CMR findings adding incremental value for prediction of future events are presence of LV hypertrophy (defined as LV wall thickness ≥ 12 mm or LV mass > 96 g/m2 in men and >77 g/m2 in women), and evidence of ischemia-related myocardial scarring – reflecting prior MI – at LCE imaging.142–144 In ACS patients in whom MI is excluded by cardiac biomarkers and non-diagnostic electrocardiograms, stress perfusion CMR is a more accurate predictor of future events than traditional risk factors. In acute MI, the multi-parametric approach by CMR has shed new light on risk stratification. This technique allows accurate assessment of ventricular volumes and function, infarct sizing, visualization of infarct transmurality and extension and tissue characterization of the jeopardized and infarcted myocardium. Many of the CMR parameters bear independent predictive value.52,53,73,78,101,109,131 However, since some of the parameters are interrelated (e.g. MVO and IMH) further studies are necessary to determine whether there are the expression of one phenomenon (e.g. microvascular injury) or are independent parameters.72
Conclusions
CMR has revolutionized our look on ACS and myocardial infarction imaging, offering an in-vivo tool for characterization of the reversible and irreversible myocardial damage, assessment of the functional consequences, depicting infarct-related complications, and evaluating of ventricular remodeling. CMR is key in differentiating ACS from non-ACS related causes of chest pain. Several CMR biomarkers provide independent predictive value of patient outcome. Finally, CMR is increasingly used to evaluate efficacy of novel treatment strategies in ACS patients.
References
- 1.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists' view. Eur Heart J. 2013;34:719–728. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. Myocardial reperfusion, limitation of infarct size, reduction of left ventricular dysfunction, and improved survival. Should the paradigm be extended? Circulation. 1989;79:441–444. doi: 10.1161/01.cir.79.2.441. [DOI] [PubMed] [Google Scholar]
- 3.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA Guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 4.2012 Writing Committee Members. Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126:875–890. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]
- 5.Steg PG, López-Sendón J, Lopez de Sa E, Goodman SG, Gore JM, Anderson FA, Jr, Himbert D, Allegrone J, Van de Werf F. GRACE Investigators. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007;167:68–73. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]
- 6.Dall'Armellina E, Karamitsos TD, Neubauer S, Choudbury RP. CMR for characterization of the myocardium in acute coronary syndromes. Nat Rev Cardiol. 2010;7:624–636. doi: 10.1038/nrcardio.2010.140. [DOI] [PubMed] [Google Scholar]
- 7.Muthurangu V, Dymarkowski S. Cardiac MRI physics in Clinical Cardiac MRI. 2nd Edition. Germany: Springer Heidelberg; 2012. pp. 1–29. ISBN 978-3-642-23034-9. [Google Scholar]
- 8.Simonetti OP, Finn P, White RD, Laub G, Henry DA. “Black blood” tbl2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199:49–57. doi: 10.1148/radiology.199.1.8633172. [DOI] [PubMed] [Google Scholar]
- 9.Carr JC, Simonetti O, Bundy J, Li D, Pereles S, Finn JP. Cine MR angiography of the heart with segmented true fast imaging with steady-state precession. Radiology. 2001;219:828–834. doi: 10.1148/radiology.219.3.r01jn44828. [DOI] [PubMed] [Google Scholar]
- 10.Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging. 2013;6:806–822. doi: 10.1016/j.jcmg.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dymarkowski S. Practical set-up in Clinical Cardiac MRI. 2nd Edition. Germany: Springer Heidelberg; 2012. pp. 53–67. ISBN 978-3-642-23034-9. [Google Scholar]
- 12.Nazarian S, Beinart R, Halperin HR. Magnetic resonance imaging and implantable devices. Circ. Arrhythm. Electrophysiol. 2013;6:419–428. doi: 10.1161/CIRCEP.113.000116. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S. Non-contrast tbl1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to tbl2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:42. doi: 10.1186/1532-429X-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ugander M, Bagi PS, Oki AJ, Chen B, Hsu LY, Aletras AH, Shah S, Greiser A, Kellman P, Arai AE. Myocardial edema as detected by pre-contrast tbl1 and tbl2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. 2012;5:596–603. doi: 10.1016/j.jcmg.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor AJ, Al-Saadi N, Abdel-Aty H, Schulz-Menger J, Messroghli DR, Friedrich MG. Detection of acutely impaired microvascular reperfusion after infarct angioplasty with magnetic resonance imaging. Circulation. 2004;109:2080–2085. doi: 10.1161/01.CIR.0000127812.62277.50. [DOI] [PubMed] [Google Scholar]
- 16.Bogaert J, Kalantzi M, Rademakers FE, Dymarkowski S, Janssens S. Determinants and impact of microvascular obstruction in successfully reperfused ST-segment elevation myocardial infarction. Assessment by magnetic resonance imaging. Eur Radiol. 2007;17:2572–2580. doi: 10.1007/s00330-007-0627-9. [DOI] [PubMed] [Google Scholar]
- 17.Masci PG, Dymarkowski S, Rademakers FE, Bogaert J. Determination of regional ejection fraction in patients with myocardial infarction by using merged late gadolinium enhancement and cine MR: feasibility study. Radiology. 2009;250(1):50–60. doi: 10.1148/radiol.2493080340. [DOI] [PubMed] [Google Scholar]
- 18.Setser RM, Kim JK, Chung YC, Chen K, Stillman AE, Loeffler R, Simonetti OP, Weaver JA, Lieber ML, White RD. Cine delayed-enhancement MR imaging of the heart: initial experience. Radiology. 2006;239(3):856–862. doi: 10.1148/radiol.2393050228. [DOI] [PubMed] [Google Scholar]
- 19.Masci PG, Dymarkowski S, Bogaert J. Valvular heart disease: what does cardiovascular MRI add? Eur Radiol. 2008;18:197–208. doi: 10.1007/s00330-007-0731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rademakers F, Van de Werf F, Mortelmans L, Marchal G, Bogaert J. Evolution of regional performance after an acute anterior myocardial infarction in humans using magnetic resonance tagging. J Physiol. 2003;546:777–787. doi: 10.1113/jphysiol.2002.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desch S, Engelhardt H, Meissner J, Eitel I, Sareban M, Fuernau G, de Waha S, Grothoff M, Gutberlet M, Schuler G, Thiele H. Reliability of myocardial salvage assessment by cardiac magnetic resonance imaging in acute reperfused myocardial infarction. Int J Cardiovasc Imaging. 2012;28:263–272. doi: 10.1007/s10554-011-9802-9. [DOI] [PubMed] [Google Scholar]
- 22.Thiele H, Kappl MJ, Conradi S, Niebauer J, Hambrecht R, Schuler G. Reproducibility of chronic and acute infarct measurement by delayed enhancement-magnetic resonance imaging. J Am Coll Cardiol. 2006;47:1641–1645. doi: 10.1016/j.jacc.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 23.Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, Schönberg SO, Luchner A, Strohm O, Ahlstrom H, Dill T, Hoebel N, Simor T. MR-IMPACT Investigators. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicenter, multivendor trial. Eur Heart J. 2013;34:775–781. doi: 10.1093/eurheartj/ehs022. [DOI] [PubMed] [Google Scholar]
- 25.Al-Saadi N, Nagel E, Gross M, Bornstedt A, Schnackenburg B, Klein C, Klimek W, Oswald H, Fleck E. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. 2000;101:1379–1383. doi: 10.1161/01.cir.101.12.1379. [DOI] [PubMed] [Google Scholar]
- 26.Jahnke C, Paetsch I, Schnackenburg B, Bornstedt A, Gebker R, Fleck E, Nagel E, Coronary MR. angiography with steady-state free precession: individually adapted breath-hold technique versus free-breathing technique. Radiology. 2004;232:669–676. doi: 10.1148/radiol.2323031225. [DOI] [PubMed] [Google Scholar]
- 27.Ishida M, Sakuma H. Coronary MR angiography revealed: how to optimize image quality. Magn Reson Imaging Clin N Am. 2015;23:117–125. doi: 10.1016/j.mric.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Lonborg J, Kelbæk H, Vejlstrup N, Bøtker HE, Kim WY, Holmvang L, Jørgensen E, Helqvist S, Saunamäki K, Thuesen L, Krusell LR, Clemmensen P, Engstrøm T. Influence of pre-infarction angina, collateral flow, and pre-procedural TIMI flow on myocardial salvage index by cardiac magnetic resonance in patients with ST-segment elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2012;13:433–443. doi: 10.1093/ejechocard/jer296. [DOI] [PubMed] [Google Scholar]
- 29.Masci PG, Andreini D, Francone M, Bertella E, De Luca L, Coceani M, Mushtaq S, Mariani M, Carbone I, Pontone G, Agati L, Bogaert J, Lombardi M. Prodromal angina is associated with myocardial salvage in acute ST-segment elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2013;14:1041–1048. doi: 10.1093/ehjci/jet063. [DOI] [PubMed] [Google Scholar]
- 30.Bainey KR, Armstrong PW. Clinical perspectives on reperfusion injury in acute myocardial infarction. Am Heart J. 2014;167:637–645. doi: 10.1016/j.ahj.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz-Perez JT, Meyers SN, Lee DC, Kansal P, Klocke FJ, Holly TA, Davidson CJ, Bonow RO, Wu E. Angiographic estimates of myocardium at risk during acute myocardial infarction: validation study using cardiac magnetic resonance imaging. Eur Heart J. 2007;28:1750–1758. doi: 10.1093/eurheartj/ehm212. [DOI] [PubMed] [Google Scholar]
- 32.Wright J, Adriaenssens T, Dymarkowski S, Desmet W, Bogaert J. Quantification of myocardial area at risk with tbl2-weighted CMR: comparison with contrast-enhanced CMR and coronary angiography. JACC Cardiovasc Imaging. 2009;2:825–831. doi: 10.1016/j.jcmg.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581–1587. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Fuernau G, Eitel I, Franke V, Hildebrandt L, Meissner J, de Waha S, Lurz P, Gutberlet M, Desch S, Schuler G, Thiele H. Myocardium at risk in ST-segment elevation myocardial infarction comparison of tbl2-weighted edema imaging with the MR-assessed endocardial surface area and validation against angiographic scoring. JACC Cardiovasc Imaging. 2011;4:967–976. doi: 10.1016/j.jcmg.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Masci PG, Ganame J, Strata E, Desmet W, Aquaro GD, Dymarkowski S, Valenti V, Janssens S, Lombardi M, Van de Werf F, L'Abbate A, Bogaert J. Myocardial salvage by CMR correlates with LV remodeling and early ST-segment resolution in acute myocardial infarction. JACC Cardiovasc Imaging. 2010;3:45–51. doi: 10.1016/j.jcmg.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz-Pérez JT, Lee DC, Meyers SN, Davidson CJ, Bonow RO, Wu E. Determinants of myocardial salvage during acute myocardial infarction: evalution with a combined angiographic and CMR myocardial salvage index. JACC Cardiovasc Imaging. 2010;3:491–500. doi: 10.1016/j.jcmg.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Canby RC, Reeves RC, Evanochko WT, Elgavish GA, Pohost GM. Proton nuclear magnetic resonance relaxation times in severe myocardial ischemia. J Am Coll Cardiol. 1987;10:412–420. doi: 10.1016/s0735-1097(87)80026-8. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Dorado D, Oliveras J, Gili J, Sanz E, Pérez-Villa F, Barrabés J, Carreras MJ, Solares J, Soler-Soler J. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27:1462–1469. doi: 10.1093/cvr/27.8.1462. [DOI] [PubMed] [Google Scholar]
- 39. Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study J Am Coll Cardiol. 2009. 53:1194 1201 [DOI] [PubMed] [Google Scholar]
- 40.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and tbl2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 41.Croisille P, Kim HW, Kim RJ. Controversies in cardiovascular MR imaging: tbl2-weighted imaging should not be used to delineate the area at risk in ischemic myocardial injury. Radiology. 2012;265:12–22. doi: 10.1148/radiol.12111769. [DOI] [PubMed] [Google Scholar]
- 42.Arai AE, Leung S, Kellman P. Controversies in cardiovascular MR imaging: reasons why imaging myocardial tbl2 has clinical and pathophysiologic value in acute myocardial infarction. Radiology. 2012;265:23–32. doi: 10.1148/radiol.12112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ubachs JF, Engblom H, Erlinge D, Jovinge S, Hedström E, Carlsson M, Arheden H. Cardiovascular magnetic resonance of the myocardium at risk in acute reperfused myocardial infarction: comparison of tbl2-weighted imaging versus the circumferential endocardial extent of late gadolinium enhancement with transmural projection. J Cardiovasc Magn Reson. 2010;12:18. doi: 10.1186/1532-429X-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF, Jr, Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with tbl2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 45.Dymarkowski S, Ni Y, Miao Y, Bogaert J, Rademakers F, Bosmans H, Marchal G. Value of tbl2-weighted magnetic resonance imaging early after myocardial infarction in dogs: comparison with bis-gadolinium-mesoporphyrin enhanced tbl1-weighted magnetic resonance imaging and functional data from cine magnetic resonance imaging. Invest Radiol. 2002;37:77–85. doi: 10.1097/00004424-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 46.McAlindon EJ, Pufulete M, Harris JM, Lawton CB, Moon JC, Manghat N, Hamilton MC, Weale PJ, Bucciarelli-Ducci C. Measurement of myocardium at risk with cardiovascular MR: comparison of techniques for edema imaging. Radiology. 2015 doi: 10.1148/radiol.14131980. ahead of press. [DOI] [PubMed] [Google Scholar]
- 47.Judd RM, Lugo-Olivieri CH, Arai M, Kondo T, Croisille P, Lima JA, Mohan V, Becker LC, Zerhouni EA. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995;92:1902–1910. doi: 10.1161/01.cir.92.7.1902. [DOI] [PubMed] [Google Scholar]
- 48.Ni Y, Pislaru C, Bosmans H, Pislaru S, Miao Y, Bogaert J, Dymarkowski S, Yu J, Semmler W, Van de Werf F, Baert AL, Marchal G. Intracoronary delivery of Gd-DTPA and gadophrin-2 for determination of myocardial viability with MR imaging. Eur Radiol. 2001;11:876–883. doi: 10.1007/s003300000791. [DOI] [PubMed] [Google Scholar]
- 49.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 50.Masci PG, Ganame J, Francone M, Desmet W, Lorenzoni V, Iacucci I, Barison A, Carbone I, Lombardi M, Agati L, Janssens S, Bogaert J. Relationship between location and size of myocardial infarction and their reciprocal influences on post-infarction left ventricular remodelling. Eur Heart J. 2011;32:1640–1648. doi: 10.1093/eurheartj/ehr064. [DOI] [PubMed] [Google Scholar]
- 51.Eitel I, Desch S, Sareban M, Fuernau G, Gutberlet M, Schuler G, Thiele H. Prognostic significance and magnetic resonance imaging findings in aborted myocardial infarction after primary angioplasty. Am Heart J. 2009;158:806–813. doi: 10.1016/j.ahj.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 52.Eitel I, Desch S, Fuernau G, Hildebrand L, Gutberlet M, Schuler G, Thiele H. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:2470–2479. doi: 10.1016/j.jacc.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 53.Eitel I, Desch S, de Waha S, Fuernau G, Gutberlet M, Schuler G, Thiele H. Long-term prognostic value of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. Heart. 2011;97:2038–2045. doi: 10.1136/heartjnl-2011-300098. [DOI] [PubMed] [Google Scholar]
- 54.Fuster V, Sanz J, Viles-Gonzalez JF, Rajagopalan S. The utility of magnetic resonance imaging in cardiac tissue regeneration trials. Nat Clin Pract Cardiovasc Med. 2006;3:S2–S7. doi: 10.1038/ncpcardio0418. [DOI] [PubMed] [Google Scholar]
- 55.Ye Y, Bogaert J. Cell therapy in myocardial infarction: emphasis on the role of MRI. Eur Radiol. 2008;18:548–569. doi: 10.1007/s00330-007-0777-9. [DOI] [PubMed] [Google Scholar]
- 56.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone narrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 57.Mahrholdt H, Wagner A, Holly TA, Elliott MD, Bonow RO, Kim RJ, Judd RM. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation. 2002;106:2322–2327. doi: 10.1161/01.cir.0000036368.63317.1c. [DOI] [PubMed] [Google Scholar]
- 58.Ibrahim T, Nekolla SG, Hörnke M, Bülow HP, Dirschinger J, Schömig A, Schwaiger M. Quantitative measurement of infarct size by contrast-enhanced magnetic resonance imaging early after acute myocardial infarction. Comparison with single-photon emission tomography using Tc99m-sestamibi. J Am Coll Cardiol. 2005;45:544–552. doi: 10.1016/j.jacc.2004.10.058. [DOI] [PubMed] [Google Scholar]
- 59.Florian A, Slavich M, Masci PG, Janssens S, Bogaert J. Electrocardiographic Q-wave “remodeling” in reperfused ST-segment elevation myocardial infarction. Validation study with CMR. J Am Coll Cardiol Imag. 2012;5:1003–1013. doi: 10.1016/j.jcmg.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 60.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 61.Barbier CE, Bjerner T, Johansson L, Lind L, Ahlström H. Myocardial scars more frequent than expected. Magnetic resonance imaging detects potential risk group. J Am Coll Cardiol. 2006;48:765–771. doi: 10.1016/j.jacc.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 62.Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Arai AE. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890–897. doi: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thiele H, Kappl MJ, Linke A, Erbs S, Boudriot E, Lembcke A, Kivelitz D, Schuler G. Influence of time-to-treatment, TIMI-flow grades, and ST-segment resolution on infarct size and infarct transmurality as assessed by delayed enhancement magnetic resonance imaging. Eur Heart J. 2007;28:1433–1439. [DOI] [PubMed]
- 64.Bogaert J, Maes A, Van de Werf F, Bosmans H, Herregods MC, Nuyts J, Desmet W, Mortelmans L, Marchal G, Rademakers FE. Functional recovery of subepicardial myocardial tissue in transmural myocardial infarction after successful reperfusion. An important contribution to the improvement in regional and global left ventricular function. Circulation. 1999;99:36–43. doi: 10.1161/01.cir.99.1.36. [DOI] [PubMed] [Google Scholar]
- 65.Hillenbrand HB, Kim RJ, Parker MA, Fieno DS, Judd RM. Early assessment of myocardial salvage by contrast-enhanced magnetic resonance imaging. Circulation. 2000;102:1678–1683. doi: 10.1161/01.cir.102.14.1678. [DOI] [PubMed] [Google Scholar]
- 66.Tarantini G, Razzolini R, Cacciavillani L, Bilato C, Sarais C, Corbetti F, Marra MP, Napodano M, Ramondo A, Iliceto S. Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol. 2006;98:1033–1040. doi: 10.1016/j.amjcard.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 67.de Waha S, Eitel I, Desch S, Fuernau G, Lurz P, Haznedar D, Grothoff M, Gutberlet M, Schuler G, Thiele H. Time dependency, predictors and clinical impact of infarct transmurality assessed by magnetic resonance imaging in patients with ST-elevation myocardial infarction reperfused by primary coronary percutaneous intervention. Clin Res Cardiol. 2012;101:191–200. doi: 10.1007/s00392-011-0380-6. [DOI] [PubMed] [Google Scholar]
- 68.Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 70.Mewton N, Bonnefoy E, Revel D, Ovize M, Kirkorian G, Croisille P. Presence and extent of cardiac magnetic resonance microvascular obstruction in reperfused non-ST-elevated myocardial infarction and correlation with infarct size and myocardial enzyme release. Cardiology. 2009;113:50–58. doi: 10.1159/000167042. [DOI] [PubMed] [Google Scholar]
- 71.van Kranen burg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, Cottin Y, Atar D, Buser P, Wu E, Lee D, Bodi V, Klug G, Metzler B, Delewi R, Bernhardt P, Rottbauer W, Boersma E, Zijlstra F, vanGeuns RJ. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014;7:930–939. doi: 10.1016/j.jcmg.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 72.Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction. A systematic review and meta-analysis. JACC Cardiovasc Imaging. 2014;7:940–952. doi: 10.1016/j.jcmg.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 74.Gerber BL, Rochitte CE, Melin JA, McVeigh ER, Bluemke DA, Wu KC, Becker LC, Lima JA. Microvascular obstruction and left ventricular remodeling early after acute myocardial infarction. Circulation. 2000;101:2734–2741. doi: 10.1161/01.cir.101.23.2734. [DOI] [PubMed] [Google Scholar]
- 75.Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, Wöhrle J, Kestler HA. Sequela of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549–557. doi: 10.1093/eurheartj/ehi147. [DOI] [PubMed] [Google Scholar]
- 76.Francone M, Bucciarelli-Ducci C, Carbone I, Canali E, Scardala R, Calabrese FA, Sardella G, Mancone M, Catalano C, Fedele F, Passariello R, Bogaert J, Agati L. Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with ST-segment elevation myocardial infarction: insight from cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54:2145–2153. doi: 10.1016/j.jacc.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 77.Desch S, Eitel I, Schmitt J, Sareban M, Fuernau G, Schuler G, Thiele H. Effect of coronary collaterals on microvascular obstruction as assessed by magnetic resonance imaging in patients with acute ST-elevation myocardial infarction treated by primary coronary intervention. Am J Cardiol. 2009;104:1204–1209. doi: 10.1016/j.amjcard.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 78.Eitel I, de Waha S, Wöhrle J, Fuernau G, Lurz P, Pauschinger M, Desch S, Schuler G, Thiele H. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1217–1226. doi: 10.1016/j.jacc.2014.06.1194. [DOI] [PubMed] [Google Scholar]
- 79.Ito H, Tomooka T, Sakai N, Yu H, Higashino Y, Fujii K, Masuyama T, Kitabatake A, Minamino T. Lack of myocardial perfusion immediately after successful thrombolysis. A predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation. 1992;85:1699–1705. doi: 10.1161/01.cir.85.5.1699. [DOI] [PubMed] [Google Scholar]
- 80.Rochitte CE, Kim RJ, Hillenbrand HB, Chen E-L, Lima JAC. Microvascular integrity and the time course of myocardial sodium accumulation after acute infarction. Circ Res. 2000;87:648–655. doi: 10.1161/01.res.87.8.648. [DOI] [PubMed] [Google Scholar]
- 81.Porto I, Burzotta F, Brancati M, Trani C, Lombardo A, Romagnoli E, Niccoli G, Natale L, Bonomo L, Crea F. Relation of myocardial blush grade to microvascular perfusion and myocardial infarct size after primary or rescue percutaneous coronary intervention. Am J Cardiol. 2007;99:1671–1673. doi: 10.1016/j.amjcard.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 82.McGeoch R, Watkins S, Berry C, Steedman T, Davie A, Byrne J, Hillis S, Lindsay M, Robb S, Dargie H, Oldroyd K. The index of microcirculatory resistence measured acutely predicts the extent and severity of myocardial infarction in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interventions. 2010;3:715–722. doi: 10.1016/j.jcin.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 83.Bekkers SC, Backes WH, Kim RJ, Snoep G, Gorgels AP, Passos VL, Waltenberger J, Crijns HJ, Schalla S. Detection and characteristics of microvascular obstruction in reperfused acute myocardial infarction using an optimized protocol for contrast-enhanced cardiovascular magnetic resonance imaging. Eur Radiol. 2009;19:2904–2912. doi: 10.1007/s00330-009-1489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nijveldt R, Hofman MB, Hirsch A, Beek AM, Umans VA, Algra PR, Piek JJ, van Rossum AC. Assessment of microvascular obstruction and prediction of short-term remodeling after acute myocardial infarction: cardiac MR imaging study. Radiology. 2008;250:363–370. doi: 10.1148/radiol.2502080739. [DOI] [PubMed] [Google Scholar]
- 85.de Waha S, Desch S, Eitel I, Fuernau G, Lurz P, Leuschner A, Grothoff M, Gutberlet M, Schuler G, Thiele H. Relationship and prognostic value of microvascular obstruction and infarct size in ST-elevation myocardial infarction as visualized by magnetic resonance imaging. Clin Res Cardiol. 2012;101:487–495. doi: 10.1007/s00392-012-0419-3. [DOI] [PubMed] [Google Scholar]
- 86.Sardella G, Mancone M, Bucciarelli-Ducci C, Agati L, Scardala R, Carbone I, Francone M, Di Roma A, Benedetti G, Conti G, Fedele F. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduced infarct size. J Am Coll Cardiol. 2009;53:309–315. doi: 10.1016/j.jacc.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 87.Desmet W, Bogaert J, Dubois C, Sinnaeve P, Adriaenssens T, Pappas C, Ganame J, Dymarkowski S, Janssens S, Belmans A, Van de Werf F. High-dose intracoronary adenosine for myocardial salvage in patients with acute ST-segment elevation myocardial infarction. Eur Heart J. 2011;32:867–877. doi: 10.1093/eurheartj/ehq492. [DOI] [PubMed] [Google Scholar]
- 88.Liu X, Huang Y, Pokreisz P, Vermeersch P, Marsboom G, Swinnen M, Verbeken E, Santos J, Pellens M, Gillijns H, Van de Werf F, Bloch KD, Janssens S. Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischemia and reperfusion. J Am Coll Cardiol. 2007;50:808–817. doi: 10.1016/j.jacc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 89.Thiele H, de Waha S, Zeymer U, Desch S, Scheller B, Lauer B, Geisler T, Gawaz M, Gunkel O, Bruch L, Klein N, Pfeiffer D, Schuler G, Eitel I. Effect of aspiration thrombectomy on microvascular obstruciton in NSTEMI patients. The TATORT-NSTEMI trial. Am Coll Cardiol. 2014;64:1117–1124. doi: 10.1016/j.jacc.2014.05.064. [DOI] [PubMed] [Google Scholar]
- 90. Eitel I, Wöhrle J, Suenkel H, Meissner J, Kerber S, Lauer B, Pauschinger M, Birkemeyer R, Axthelm C, Zimmermann R, Neuhaus P, Brosteanu O, de Waha S, Desch S, Gutberlet M, Schuler G, Thiele H. Intracoronary compared with intravenous bolus Abciximab application during primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. Cardiac magnetic resonance substudy of the AIDA STEMI Trial Am Coll Cardiol 2013. 61:1447 1454 [DOI] [PubMed] [Google Scholar]
- 91.Bresnahan GF, Roberts R, Shell WE, Ross JJr. Deleterious effects due to hemorrhage after myocardial reperfusion. Am J Cardiol. 1974;33:82–86. doi: 10.1016/0002-9149(74)90742-5. [DOI] [PubMed] [Google Scholar]
- 92.Higginson LA, White F, Heggtveit HA, Sanders TM, Bloor CM, Covell JW. Determinants of myocardial hemorrhage after coronary reperfusion in the anesthesized dog. Circulation. 1982;65:62–69. doi: 10.1161/01.cir.65.1.62. [DOI] [PubMed] [Google Scholar]
- 93.Betgem RP, de Waard G, Nijveldt R, Beek AM, Escaned J, van Royen N. Intramyocardial haemorrhage after acute myocardial infarction. Nat Rev Cardiol. 2014 doi: 10.1038/nrcardio.2014.188. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 94.Lotan CS, Bouchard A, Cranney GB, Bishop SP, Pohost GM. Assessment of postreperfusion myocardial hemorrhage using proton NMR imaging at 1.5T. Circulation. 1992;86:1018–1025. doi: 10.1161/01.cir.86.3.1018. [DOI] [PubMed] [Google Scholar]
- 95.Basso C, Corbetti F, Silva C, Abudureheman A, Lacognata C, Cacciavillani L, Tarantini G, Marra MP, Ramondo A, Thiene G, Iliceto S. Morphologic validation of reperfused hemorrhagic infarction by cardiovascular magnetic resonance. Am J Cardiol. 2007;100:1322–1327. doi: 10.1016/j.amjcard.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 96.Robbers LF, Eerenberg ES, Teunissen PF, Jansen MF, Hollander MR, Horrevoets AJ, Knaapen P, Nijveldt R, Heymans MW, Levi MM, van Rossum AC, Niessen HW, Marcu CB, Beek AM, van Royen N. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J. 2013;34:2346–2353. doi: 10.1093/eurheartj/eht100. [DOI] [PubMed] [Google Scholar]
- 97.Zia MI, Ghugre NR, Connelly KA, Strauss BH, Sparkes JD, Dick AJ, Wright GA. Characterizing myocardial edema and hemorrhage using quantitative tbl2 and tbl2* mapping at multiple time intervals post ST-segment elevation myocardial infarction. Circ Cardiovasc Imaging. 2012;5:556–572. doi: 10.1161/CIRCIMAGING.112.973222. [DOI] [PubMed] [Google Scholar]
- 98.Kandler D, Lücke C, Grothoff M, Andres C, Lehmkuhl L, Nitzsche S, Riese F, Mende M, de Waha S, Desch S, Lurz P, Eitel I, Gutberlet M. The relation between hypointense core, microvascular obstruction and intramyocardial haemorrhage in acute reperfused myocardial infarction assessed by cardiac magnetic resonance imaging. Eur Radiol. 2014;24:3277–3288. doi: 10.1007/s00330-014-3318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ganame J, Messalli G, Dymarkowski S, Rademakers FE, Desmet W, VandeWerf F, Bogaert J. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J. 2009;30:1440–1449. doi: 10.1093/eurheartj/ehp093. [DOI] [PubMed] [Google Scholar]
- 100.Mather AN, Fairbairn TA, Bali SG, Greenwood JP, Plein S. Reperfusion haemorrhage as determined by cardiovascular MRI is a predictor of adverse left ventricular remodelling and markers of late arrhythmic risk. Heart. 2010;97:453–459. doi: 10.1136/hrt.2010.202028. [DOI] [PubMed] [Google Scholar]
- 101.Eitel I, Kubusch K, Strohm O, Desch S, Mikami Y, de Waha S, Gutberlet M, Schuler G, Friedrich MG, Thiele H. Prognostic value and determinants of a hypointense infarct core in tbl2-weighted cardiac magnetic resonance in acute reperfused ST-elevation-myocardial infarction. Circ Cardiovasc Imaging. 2011;4:354–362. doi: 10.1161/CIRCIMAGING.110.960500. [DOI] [PubMed] [Google Scholar]
- 102.Symons R, Masci PG, Goetschalckx K, Doulaptsis K, Janssens S, Bogaert J. Effect of infarct severity on regional and global left ventricular remodeling in patients with successfully reperfused ST segment elevation myocardial infarction. Radiology. 2014 doi: 10.1148/radiol.14132746. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 103.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 104.Robbers LF, Delewi R, Nijveldt R, Hirsch A, Beek AM, Kemme MJ, van Beur den Y, van der Laan AM, van der Vleuten PA, Tio RA, Zijlstra F, Piek JJ, vanRossum AC. Myocardial infarct heterogeneity assessment by late enhancement cardiovascular magnetic resonance imaging shows predictive value for ventricular arrhythmia development after acute myocardial infarction. Eur Heart J Cardiovasc Imaging. 2013;14:1150–1158. doi: 10.1093/ehjci/jet111. [DOI] [PubMed] [Google Scholar]
- 105.Peters DC, Appelbaum EA, Nezafat R, Dokhan B, Han Y, Kissinger KV, Goddu B, Manning WJ. Left ventricular infarct size, peri-infarct zone, and papillary scar measurements: a comparison of high-resolution 3D and conventional 2D late gadolinium enhancement cardiac MRI. J Magn Reson Imaging. 2009;30:794–800. doi: 10.1002/jmri.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar A, Abdel-Aty H, Kriedemann I, Schulz-Menger J, Gross CM, Dietz R, Friedrich MG. Contrast-enhanced cardiovascular magnetic resonance imaging of right ventricular infarction. J Am Coll Cardiol. 2006;48:1969–1976. doi: 10.1016/j.jacc.2006.05.078. [DOI] [PubMed] [Google Scholar]
- 107.Masci PG, Francone M, Desmet W, Ganame J, Todiere G, Donato R, Siciliano V, Carbone I, Mangia M, Strata E, Catalano C, Lombardi M, Agati L, Janssens S, Bogaert J. Right ventricular ischemic injury in patients with acute ST-segment elevation myocardial infarction. Characterization with cardiovascular magnetic resonance. Circulation. 2010;122:1405–1412. doi: 10.1161/CIRCULATIONAHA.110.940254. [DOI] [PubMed] [Google Scholar]
- 108. Bodi V, Sanchis J, Mainar L, Chorro FJ, Nunez J, Monmeneu JV, Chaustre F, Forteza MJ, Ruiz-Sauri A, Lopez-Lereu MP, Gomez C, Noguera I, Diaz A, Giner F, Llacer A. Right ventricular involvement in anterior myocardial infarction: a translational approach Cardiovasc Res. 2010. 87:601 608 [DOI] [PubMed] [Google Scholar]
- 109.Grothoff M, Elpert C, Hoffmann J, Zachrau J, Lehmkuhl L, de Waha S, Desch S, Eitel I, Mende M, Thiele H, Gutberlet M. Right ventricular injury in ST-elevation myocardial infarction: risk stratification by visualization of wall motion, edema, and delayed-enhancement cardiac magnetic resonance. Circ Cardiovasc Imaging. 2012;5:60–68. doi: 10.1161/CIRCIMAGING.111.967810. [DOI] [PubMed] [Google Scholar]
- 110.Chiu CW, So NMS, Lam WWM, Chan KY, Sanderson JE. Combined first-pass perfusion and viability study at MR imaging in patients with non-ST segment-elevation acute coronary syndromes: feasibility study. Radiology. 2003;226:717–722. doi: 10.1148/radiol.2263011902. [DOI] [PubMed] [Google Scholar]
- 111.Kwong RY, Schussheim AE, Rekhraj S, Aletras AH, Geller N, Davis J, Christian TF, Balaban RS, Arai AE. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;170:531–537. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
- 112.Plein S, Greenwood JP, Ridgway JP, Cranny G, Ball SG, Sivanathan MU. Assessment of non-ST-segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2004;44:2173–2181. doi: 10.1016/j.jacc.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 113.Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, Abbara S, Bamberg F, Ferencik M, Schmidt EJ, Brown DF, Hoffmann U, Brady TJ. Cardiac magnetic resonance with tbl2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118:837–844. doi: 10.1161/CIRCULATIONAHA.107.740597. [DOI] [PubMed] [Google Scholar]
- 114.Bogaert J, Kuzo R, Dymarkowski S, Becker R, Piessens J, Rademakers FE. Coronary artery imaging with real-time navigator three-dimensional turbo-field-echo MR coronary angiography: initial experience. Radiology. 2003;226:707–716. doi: 10.1148/radiol.2263011750. [DOI] [PubMed] [Google Scholar]
- 115.Yonezawa M, Nagata M, Kitagawa K, Kato S, Yoon Y, Nakajima H, Nakamori S, Sakuma H, Hatakenaka M, Honda H. Quantitative analysis of 1.5T whole-heart coronary MR angiograms obtained with 32-channel cardiac coils: a comparison with conventional coronary angiography. Radiology. 2014;271:356–364. doi: 10.1148/radiol.13122491. [DOI] [PubMed] [Google Scholar]
- 116.Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393–1403. doi: 10.1056/NEJMoa1201163. [DOI] [PubMed] [Google Scholar]
- 117.Vrachliotis TG, Bis KG, Haidary A, Kosuri R, Balasubramaniam M, Gallagher M, Raff G, Ross M, O'neil B, O'neill W. Atypical chest pain: coronary, aortic and pulmonary vasculature enhancement at biphasic single-injection 64-section CT angiography. Radiology. 2007;243:368–376. doi: 10.1148/radiol.2432060447. [DOI] [PubMed] [Google Scholar]
- 118.Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, Pennell DJ, Prasad SK. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28:1242–1249. doi: 10.1093/eurheartj/ehm113. [DOI] [PubMed] [Google Scholar]
- 119.Abdel-Aty H, Boyé P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis. J Am Coll Cardiol. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 120.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, Böhm M. Update on myocarditis. J Am Coll Cardiol. 2012;59:779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 122.Yelgec NC, Dymarkowski S, Ganame S, Bogaert J. Value of MRI in patients with a clinical suspicion of acute myocarditis. Eur Radiol. 2007;17:2211–2217. doi: 10.1007/s00330-007-0612-3. [DOI] [PubMed] [Google Scholar]
- 123.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis. A comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 124.Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K, Kandolf R, Sechtem U, Mahrholdt H. Long-term follow-up of biopsy-proven viral myocarditis. Predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–1615. doi: 10.1016/j.jacc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 125.Francone M, Chimenti C, Galea N, Scopelliti F, Verardo R, Galea R, Carbone I, Catalano C, Fedele F, Frustaci A. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. J Am Coll Cardiol Img. 2014;7:254–263. doi: 10.1016/j.jcmg.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 126.Ferreira V, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S. T1 mapping for the diagnosis of acute myocarditis using CMR. Comparison to tbl2-weighted and late gadolinium enhanced imaging. J Am Coll Cardiol Img. 2013;6:1048–1058. doi: 10.1016/j.jcmg.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 127.Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, Blankenberg S, Muellerleile K. CMR in patients with severe myocarditis. Diagnostic value of quantitative tissue markers including extracellular volume imaging. J Am Coll Cardiol Img. 2014;7:667–675. doi: 10.1016/j.jcmg.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 128.Eitel I, Behrendt F, Schindler K, Kivelitz D, Gutberlet M, Schuler G, Thiele H. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J. 2008;29:2651–2659. doi: 10.1093/eurheartj/ehn433. [DOI] [PubMed] [Google Scholar]
- 129.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G, Schulz-Menger J, Thiele H, Friedrich MG. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]
- 130.Tanimoto T, Imanishi T, Kitabata H, Nakamura N, Kimura K, Yamano T, Ishibashi K, Komukai K, Ino Y, Takarada S, Kubo T, Hirata K, Mizukoshi M, Tanaka A, Akasaka T. Prevalence and Clinical Significance of Papillary Muscle Infarction Detected by Late Gadolinium-Enhanced Magnetic Resonance Imaging in Patients With ST-Segment Elevation Myocardial Infarction. Circulation. 2010;122(22):2281–2287. doi: 10.1161/CIRCULATIONAHA.109.935338. doi:10.1161/CIRCULATIONAHA.109.935338 [DOI] [PubMed] [Google Scholar]
- 131.Eitel I, Gehmlich D, Amer O, Wöhrle J, Kerber S, Lauer B, Pauschinger M, Schwab J, Birkemeyer R, Zimmermann R, Mende M, de Waha S, Desch S, Gutberlet M, Schuler G, Thiele H. Prognostic relevance of papillary muscle infarction in reperfused infarction as visualized by cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2013;6:890–898. doi: 10.1161/CIRCIMAGING.113.000411. [DOI] [PubMed] [Google Scholar]
- 132.Mollet NR, Dymarkowski S, Volders W, Wathiong J, Herbots L, Rademakers FE, Bogaert J. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation. 2002;106:2873–2876. doi: 10.1161/01.cir.0000044389.51236.91. [DOI] [PubMed] [Google Scholar]
- 133.Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, James OG, Patel MR, Heitner J, Parker M, Velazquez EJ, Steenbergen C, Judd RM, Kim RJ. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance. Prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2005;52:148–157. doi: 10.1016/j.jacc.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 134.Konen E, Merchant N, Gutierrez C, Provost Y, Mickleborough L, Paul NS, Butany J. True versus false left ventricular aneurysm: differentiation with MR imaging – initial experience. Radiology. 2005;236:65–70. doi: 10.1148/radiol.2361031699. [DOI] [PubMed] [Google Scholar]
- 135.Taylor AM, Dymarkowski S, Verbeken E, Bogaert J. Detection of pericardial inflammation with late-enhancement cardiac magnetic resonance imaging: initial results. Eur Radiol. 2006;16:569–574. doi: 10.1007/s00330-005-0025-0. [DOI] [PubMed] [Google Scholar]
- 136. Doulaptsis C, Goetschalckx K, Masci PG, Florian A, Janssens S, Bogaert J. Assessment of early post-infarction pericardial injury by CMR JACC Cardiovasc Imaging. 2013. 6:411 413 [DOI] [PubMed] [Google Scholar]
- 137.Bogaert J, Francone M. Pericardial disease: value of CT and MR imaging. Radiology. 2013;267:340–356. doi: 10.1148/radiol.13121059. [DOI] [PubMed] [Google Scholar]
- 138.Hundley WG, Morgan TM, Neagle CM, Hamilton CA, Rerkpattanapipat P, Link KM. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–2333. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 139.Bodi V, Sanchis J, Lopez-Lereu MP, Nunez J, Mainar L, Monmeneu JV, Husser O, Dominguez E, Chorro FJ, Llacer A. Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;50:1174–1179. doi: 10.1016/j.jacc.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 140.Jahnke C, Nagel E, Gebker R. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall imaging. Circulation. 2007;115:1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 141.Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, Paterson DI, Syed MA, Aletras AH, Arai AE. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427–1432. doi: 10.1016/j.jacc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 142.Charoenpanichkit C, Morgan TM, Hamilton CA, Wallace EL, Robinson K, Ntim WO, Hundley WG. Left ventricular hypertrophy influences cardiac prognosis in patients undergoing dobutamine cardiac stress testing. Circ Cardiovasc Imaging. 2010;3:392–397. doi: 10.1161/CIRCIMAGING.109.912071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Steel K, Broderick R, Gandla V, Larose E, Resnic F, Jerosch-Herold M, Brown KA, Kwong RY. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation. 2009;120:1390–1400. doi: 10.1161/CIRCULATIONAHA.108.812503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bingham SE, Hachamovitch R. Incremental prognostic significance of combined cardiac magnetic resonance imaging, adenosine stress perfusion, delayed enhancement and left ventricular function over pre-imaging information for the prediction of adverse events. Circulation. 2011;123:1509–1518. doi: 10.1161/CIRCULATIONAHA.109.907659. [DOI] [PubMed] [Google Scholar]