Abstract
Animal cell division ends with the cutting of the microtubule and membrane intercellular bridge connecting the 2 daughter cells. This process, known as cytokinetic abscission (abscission), is widely regarded as the last step of cytokinesis, i.e., the last step of the cell cycle. Major breakthroughs have been recently achieved, illuminating mechanistic aspects of abscission; however, the timing of abscission with respect to the mammalian cell cycle remains unclear. In this study, we carefully measured the onset and progression of abscission in dividing cells expressing a G1 reporter. We conclude that abscission commences long after cells enter the G1 phase. Affiliating abscission with G1 is beyond semantics since it essentially postulates that the last step of the cell cycle is regulated in, and probably by, the following cycle.
Keywords: Abscission, Aurora, APC/C, Cell cycle, Cell division, Cytokinesis, Constriction site, CHMP, Cep55, ESCRT III, Gas2l3, Mitosis, Plk1, Spastin
Introduction
Cell division ends with the cutting of the microtubule and membrane intercellular bridge connecting the 2 daughter cells at specific locations called constriction sites. This process is known as cytokinetic abscission (abscission).1-4 A Major breakthrough has been recently achieved with the finding that components of the ESCRT (endosomal sorting complex required for transport) membrane remodeling system are recruited to the constriction site shortly before abscission. Acting in concert with Spastin and other components of the microtubule-severing machinery, this protein complex induces the membrane constriction and scission events that execute abscission and terminate cell division.5-8
The mechanism by which abscission is coordinated with the cell cycle is unclear. In fact, for most cell types, it is as yet unknown when, or even if, abscission occurs in the cell's life cycle. Traditionally, abscission is regarded as the last step of cytokinesis, i.e., the last mitotic event. However, while much of mitosis progresses rapidly (less than 20 min from metaphase to telophase), the intercellular bridge usually remains intact for a longer time. For instance, the cutting of the intercellular bridge in canine MDCK cells occurs over one hour after anaphase. At this point, the daughter cells decondense their chromatin, adhere back to the surface, and, overall, seem to be post-mitotic.5 The critical question, however, is when abscission begins relative to the cell cycle? If we were to follow the traditional model, the answer is mitosis – more specifically, anaphase. However, if the initiation of abscission, as defined by the constriction of the microtubules in the intercellular bridge,5,6 commences at G1, then abscission is essentially a G1 event.
Here, we make an attempt to differentiate between these 2 possibilities by quantifying constriction and fission of the intercellular bridge during cytokinesis in live mammalian cells expressing a biochemical marker of G1 progression. We show that cells are already in an advanced stage of G1 when abscission begins. Thus, abscission is, by definition, a G1 event. Considering these findings, it is tempting to speculate that the last step of the cell cycle is regulated by the G1 phase of the following cell cycle.
Results and Discussion
In order to relate abscission with cell cycle progression, we carefully monitored the constriction of the intercellular bridge in live mammalian cells expressing a biochemical marker of G1 progression.
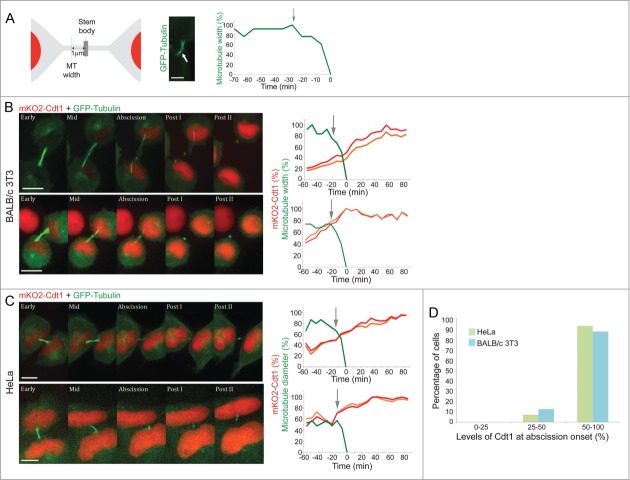
Constitutively expressed fluorescent proteins attached to degrons of canonical cell cycle proteins, can mirror molecular milestones across the mammalian cell cycle (see, for example, Refs9-11). Specifically, fluorescently tagged Cdt1 N-terminus is widely regarded as a G1 progression marker.10,11 This Cdt1 degron starts accumulating and peaks at G1, decreases in G1/S transition and early S, and remains low until the following G1 phase (see illustration in Fig. 1A). This signature dynamics is particularly optimal for monitoring G1 entry and progression numerically.11 We generated human HeLa and mouse BALB/c 3T3 cells stably expressing the human Cdt1 degron fused to monomeric KusabiraOrange 2 (mKO2). The signature oscillation of this reporter (described above) was validated in both cell types (Fig. 1B; Fig. S1, and Movie S1). We then co-expressed GFP-tubulin in these cells in order to track cytokinesis progression and abscission. A noticeable increase in mKO2-Cdt1 levels was observed in both HeLa and BALB/c 3T3 cells prior to the cutting of the intercellular bridge (Fig. 1C).
Figure 1.
Recording abscission in G1. (A) Schematic representation of Cdt1 oscillation in proliferating cells. Cdt1 degron fused to a fluorescent tag is a well-established marker of G1. The fusion protein accumulates and peaks at G1. Reduction in Cdt1 levels, as mediated by the SCFSkp2 E3 ligase, is a hallmark of S-phase entry. (B) Live-cell imaging of BALB/c 3T3 cells stably expressing mKO2-Cdt1 undergoing a complete cell cycle. Plot indicates relative Cdt1 levels [percent of maximum (max) signal] as a function of time. Measurements were taken every 15 min. Time 0 was set at anaphase. Quantification and representative images of the movie sequence of Movie S1 are shown. Dashed line marks 50% of maximum Cdt1 levels. Scale bar = 10 μm. (C) HeLa and BALB/c 3T3 cells stably expressing mKO2-Cdt1 and transfected with GFP-tubulin, were imaged during cell division. Time 0 was set at abscission completion, i.e., intercellular bridge cutting. Images were taken every 7 min. Scale bar = 10 μm.
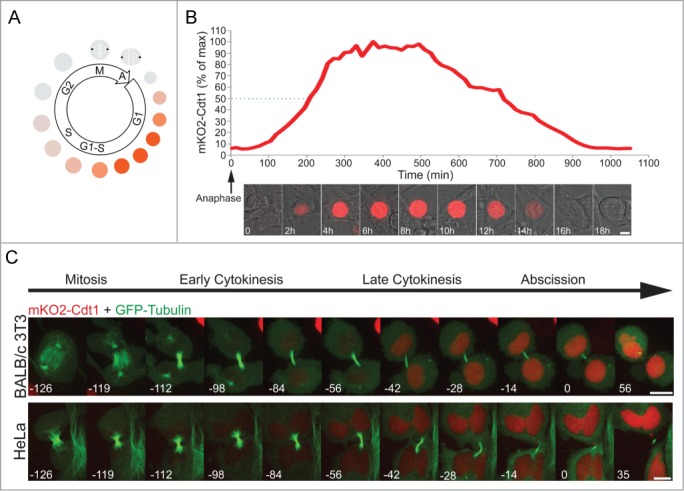
Abscission onset and progression can be accurately determined in living cells by measuring the width of the microtubule stalk accommodating the intercellular bridge (as visualized by GFP-tubulin), ∼1 μm away from the center of the bridge (see Fig. 2A and Refs1,5). mKO2-Cdt1 progressively accumulates in G1, peaking to maximum levels in mid G1.10,11 Thus, as long as cell abscission commences while mKO2 fluorescent intensity increases (positive slope), let alone if it reaches a plateau, it should be regarded as a G1 event. We recorded the daughter cells from late cytokinesis to post-abscission. In order to minimize phototoxic-induced interference to the cell cycle, we used an electron-multiplying (EM) CCD camera, which enables accurate quantification of the cell cycle and abscission progression in cells with low intensity signals.
Figure 2.
Cell abscission commences in G1. (A) Measuring abscission onset in live cells. Microtubule width was measured based on the intensity profile of a line crossing the intercellular bridge at the narrowest point along the bridge. This point (indicated by a white arrow in a representative HeLa cell expressing GFP-Tubulin; scale bar = 5 μm) is about 1 μm away from the center of the bridge (see illustration). The relative microtubule width (percentage of maximum width [%]) at this point is plotted over time (see plot). Abscission onset was set at the time of acute decrease in microtubule width at the first abscission site (depicted in the plot by an arrow). (B and C) BALB/c 3T3 cells (B) or HeLa cells (C) stably expressing mKO2-Cdt1 were transfected with GFP-tubulin and imaged during late cytokinesis at 7 min intervals. Time 0 was set at intercellular bridge cutting. Representative images of different stages during late cytokinesis (early, mid, abscission) and post-abscission (posts I and II) are shown on the left (movie series are available in Movies S2–5). Graphs on the right indicate the mKO2-Cdt1 levels in the 2 newly formed daughter cells (red and orange lines) and the diameter of the microtubules that accommodate the intercellular bridge connecting the cells. mKO2-Cdt1 levels are provided as percentages of maximum intensity (%), i.e., relative to mKO2-Cdt1 level at plateau. Arrows indicate the beginning of abscission. Scale bar = 10 μm. (D) HeLa (green) and BALB/c 3T3 (blue) cells were categorized according to the relative level (% of maximum) of mKO2-Cdt1 at the time of abscission onset (n = 16). The fraction of cells whose relative mKO2-Cdt1 level exceeded 50% at abscission onset was significantly larger (χ22 test; p = 0.005 for HeLa cells and p = 0.03 for BALB/c 3T3 cells).
The average duration of abscission was 16 min (HeLa) and 15 min (BALB/c 3T3). Thus, consistent with previous findings,5,6 abscission in our cell systems is a fairly acute and uniform process. More importantly, in 93.75% of the HeLa cells and 87.5% of the BALB/c 3T3 cells, abscission commenced when relative mKO2-Cdt1 levels exceeded 50% (Fig. 2), indicating that these cells were at an advanced G1 phase. It is noteworthy that similar mKO2-Cdt1 accumulation profiles were obtained for both newly formed daughter cells (Fig. 2B and C; see also Movies S2–5). Only 6.25% of the HeLa cells and 12.5% of the BALB/c 3T3 cells had less than 50% mKO2-Cdt1 levels at the time of abscission. We could not find cells that had less than 25% mKO2-Cdt1 levels at abscission (Fig. 2D).
The data summarized in Figure 2D was generated only from HeLa and BALB/c 3T3 cells whose mKO2-Cdt1 level reached a plateau (without reaching saturation) during the time course of the experiment. In addition, only cells whose microtubule stalk at the intercellular bridge maintained a suitable orientation and intensity throughout the experiment, were included in the analysis. This strict protocol reduced the number of cells quantified, however allowed us to define the "molecular timing" of abscission within G1 with maximum accuracy. It is important to note that overall abscission dynamics was monitored in 60 HeLa and BALB/c 3T3 cells. In 94% of the HeLa cells and 74% of the BALB/c 3T3 cells, abscission commenced when mKO2-Cdt1 levels were substantial, and more importantly, accumulating (positive slope), i.e., in G1 (see representative cells in Figure S2).
Several abscission regulators, including Spastin and late ESCRT proteins (ESCRT III and Vps4), are positioned at the forthcoming constriction sites right before abscission onset.12 We conducted immunofluorescence analysis of Spastin and the Vps4 binding protein Vta113 in mKO2-Cdt1-expressing HeLa cells. Although this analysis lacked the dimension of time, the results shown in Figure 3 demonstrate that whenever these abscission mediators are located at the stembody periphery (Stage 1) or later, in closer proximity to the constriction sites (Stage 2), mKO2-Cdt1 signal is unambiguously clear. Taken together, our findings demonstrate that cell abscission starts at the G1 phase of the cell cycle.
Figure 3.
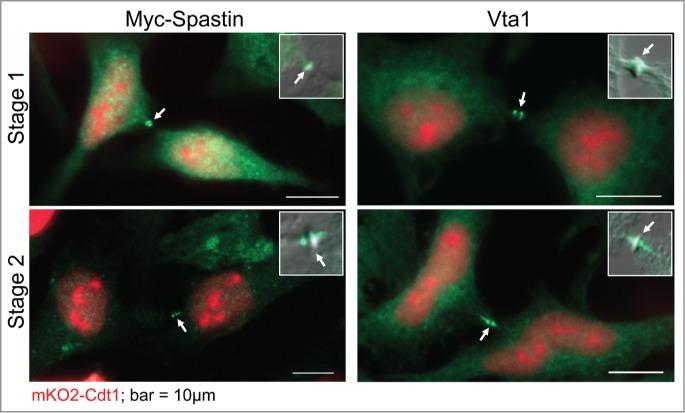
Abscission regulators at the midbody of G1 cells. HeLa cells expressing mKO2-Cdt1 were either transfected with a Myc-Spastin- expressing vector, fixed and labeled with anti-Myc tag antibodies (left), or fixed and labeled with anti Vta1 antibodies (right). Representative cells, showing abscission regulators flanking the stembody (Stage 1) or in proximity to constriction sites (Stage 2), are shown.
Cytokinesis begins with cleavage furrow ingression, and is followed by a series of well-defined events taking place sequentially. All these events can be seen as a culmination of something that started at anaphase. Following this traditional conviction, abscission, although ending at G1, is established in the previous mitosis and “delayed” until the beginning of the following cell cycle (Fig. 4A).
Figure 4.
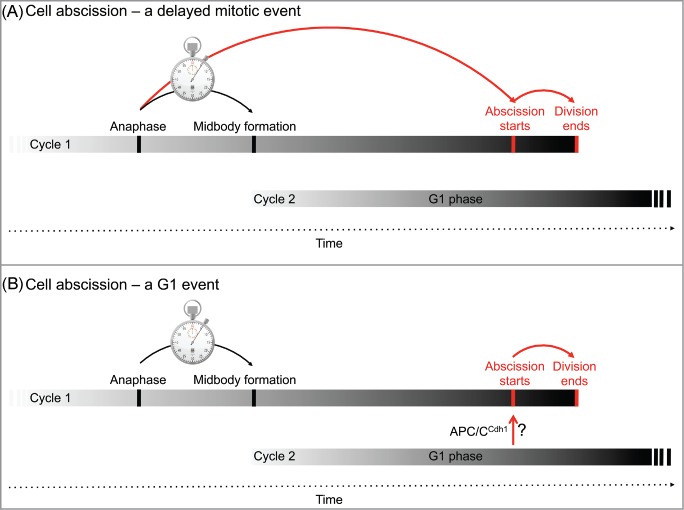
Cell cycle perspective of abscission. Cell abscission can be referred to as a “delayed” cytokinesis event that commences at mitosis and concludes at the G1 phase of the following cycle (A). Our results are in favor of an alternative model postulating that cell abscission is a bona fide G1 event that terminates the cell division of the previous cycle (B). The mechanisms underlying abscission onset in G1 may involve protein degradation, as mediated by APC/CCdh1 activity, or any other biochemical circuit in G1.
The narrowing of the intercellular bridge at the constriction sites, i.e., abscission, is not a slowly progressing process spanning from cycle 1 to cycle 2, but rather an acute ∼ 15-min event that starts long after G1 entry (Fig. 2). Moreover, cytokinesis progression is rapid and uniform up until midbody formation, while the remaining duration of cytokinesis is heterogeneous and can exceed one hour. These observations made us wonder about the tantalizing possibility that abscission is not a ‘delayed evidence’ of the previous cycle, but rather a bona fide G1 event regulated by G1 mechanisms (Fig. 4B). This alternative perception of abscission is beyond semantics; G1-regulated abscission can form a ‘post-mitotic checkpoint’ that can potentially stall or prevent a malignant cell from proliferating further, even if this cell has evaded all previous mitotic checkpoints. Indeed, abscission failure can lead to cell death.14,15 In addition, G1 mechanisms can couple or uncouple abscission onset to the cell cycle by a molecular switch unrelated to cell division. In fruit flies, for example, the number of germ cells in each Drosophila egg chamber is regulated by abscission inhibition during differentiation.16 Finally, G1-coupled abscission essentially postulates that the last step of the cell cycle, as we traditionally see it, is in fact regulated by the following cycle (Fig. 4B).
Of the list of proteins that seem to participate in cell abscission, only a few can be considered canonical cell cycle regulators: Cep55,17 Aurora B,18 Plk1,19 and the newly identified constriction site-associated protein, Gas2l3.20 All other proteins, e.g., ESCRT proteins, Rab proteins, and Spastin, generally regulate membrane remodeling and microtubule severing in the cell. Following proper signaling, these complexes are recruited to the constriction sites for executing orderly abscission.
Plk1, Aurora B, and Gas2l3 are degraded by the proteasome at the end of M phase. This degradation is mediated by the E3 ligase anaphase-promoting complex/cyclosome-Cdh1 (APC/CCdh1).20-22 APC/CCdh1 activity commences in late telophase and governs the degradation of AuroraB and Plk1, which is required for cytokinetic progression. APC/CCdh1 activity, however, does not end with the onset of G1. On the contrary, the complex remains fully active until the G1/S transition.23 Therefore, at least one potential abscission-promoting activity is available in G1, raising the possibility that further degradation of AuroraB, Plk1, and perhaps Gas2l3 and other APC/CCdh1 targets, is required for abscission onset. In support of this model, it was recently shown that the APC/CCdh1–mediated degradation of AuroraB controls cell adhesion and spreading occurring ∼45 min after anaphase onset.24 Interference with this process causes severe abscission delays.25 It is, therefore, possible that continuous AuroraB degradation in G1 is critical for abscission onset through the regulation of cell spreading. A similar scenario can apply to Plk1 degradation, although a role for Plk1 degradation at temporal proximity to abscission onset has not yet been demonstrated.
Considering the recent literature, the model in Figure 4B suggests that APC/CCdh1 activity in late mitosis is enough to push the cells into late cytokinesis, but is insufficient for executing abscission. APC/CCdh1–mediated degradation must then proceed into G1, lowering the local concentration of abscission regulators at the packed midbody below the threshold that would permit abscission.
The potential role of the APC/C, and protein degradation in general, in timing abscission is logical. That said, the significant accumulation of Cdt1 in G1 prior to abscission (Fig. 2) reminds us that orderly abscission can, in principle, be regulated by the accumulation of an ‘activator' rather than the degradation of an ‘inhibitor’ in G1.
Future perspectives
Carefully monitoring abscission in living cells has so far been done in only a few types of cells. It seems that the timing of abscission relative to anaphase may vary dramatically between different cell types (∼60 min in Hela cells, ∼75 min in NRK cells, ∼110 min in MDCK cells, and ∼ 125 min in RPE1 cells5,6,26), indicating a complex relationship between cell abscission and cell division. In-depth research of abscission in a variety of adherent and unattached culture cells and in solid tissues in vivo, would be extremely valuable for our understanding of how abscission is regulated under normal and malignant conditions, and how uniform and essential this process is in higher eukaryotes. Mechanistically, having the spotlight pointed at mitosis is obviously justified, but perhaps, in this context, we should pay more attention to G1.
Materials and Methods
Plasmids and cell lines
Monomeric KusabiraOrange 2(mKO2)hCdt1 construct was cloned into lentiviral vectors carrying a neomycin-resistance gene. Virus particles were generated in HEK293T cells following standard virus purification. HeLa and BALB/c 3T3 cells were infected (using 8 μg/ml polybrene) with mKO2hCdt1-carrying viruses and subjected to selection by geneticin. Cells used in this study originated from a single cell selected by single-cell sorting. Both cell types were maintained in DMEM (Gibco) supplemented with 10% fetal bovine serum (Gibco) and penicillin/streptomycin (Gibco) at 37°C and 5% CO2. Cells growing under selection were cultured in media containing 400 μg/ml of geneticin. The plasmids, GFP-tagged Tubulin and Myc-tagged Spastin, were previously described.5,18
Live-cell imaging
Cells were plated in low density on a 4-well chamber slide (Nunc), transfected (Lipofectamine 2000, Invitrogen), and imaged 24–72 h post-transfection. Series of Z sections were captured at the specified intervals using a spinning-disk confocal microscope (Marianas, Intelligent Imaging) and were video-recorded on an EM-CCD camera (Evolve; Photometrics) using a 40× oil objective (NA 1.3) or a 63× oil objective (NA 1.4). All experiments and video recordings were conducted at 37°C. Image processing and analysis were done using Slidebook 5 (Intelligent Imaging). Measurements of total nuclear mKO2-Cdt1 intensity levels were done on sum projection images of the 3D movie series. During mitosis (no nucleus), mKO2-Cdt1 levels were measured for the entire cell. Microtubule width was measured based on the microtubule fluorescence intensity profile along a line that was positioned perpendicular to the bridge at about 1 μm distal to the stembody at the narrowest point along the bridge (see illustration in Figure 2A) 5, 12. Graphs in Figure 2 refer to measurements of the first cleavage site. DIC images (shown in Figures 1 and S1) were subjected to a flat-field illumination correction filter (ImageJ).
Immunofluorescence
HeLa cells expressing mKO2-Cdt1 were cultured on coverslips. Cells were i) fixed (4% paraformaldehyde; 20 min at room temperature [RT]) pre-transfection or 30 h post-transfection (Lipofectamine 2000, Invitrogen); ii) blocked and permeabilized (20% fetal bovine serum, 0.1% Triton X-100 in phosphate-buffered saline; 1 h; RT); and iii) incubated (1 h; RT) with one of the 2 following primary antibodies: anti-Vta1 (PA5-21831, Thermo-Fisher Scientific) or anti-Myc tag (9E10, DSHB). Cy5-coupled secondary antibodies were used for labeling (A31571 and A31573, Molecular Probes). Coverslips were mounted on slides with Immu-Mount mounting solution (Thermo-Fisher Scientific), and sealed. AxioImager.Z1 upright microscope (Carl Zeiss, Inc.), equipped with 100× oil immersion lens (NA 1.4), was used for imaging.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Special thanks to Dr. Gad Miller, whose encouragement helped transform an unripened thought into a paper. We also thank Tal Yoskovitch Mashriki for technical assistance, Dr. Marc Kirschner for reagents, and Ms. Sharon Victor for copyediting.
Funding
Funding by the following foundations is gratefully acknowledged: Israeli Centers of Research Excellence (I-CORE), Gene Regulation in Complex Human Disease, Center no. 41/11 (AT), Israel Cancer Association grant no. 20120067 (AT), Israeli Science Foundation (ISF) grant no. 455/13 (NE), United States-Israel Binational Science Foundation (BSF) grant no. 2011309 (NE), and Marie Curie Integration grant (CIG) (NE).
References
- 1. Elia N, Ott C, Lippincott-Schwartz J. Incisive imaging and computation for cellular mysteries: lessons from abscission. Cell 2013; 155:1220-31; PMID:24315094; http://dx.doi.org/ 10.1016/j.cell.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agromayor M, Martin-Serrano J. Knowing when to cut and run: mechanisms that control cytokinetic abscission. Trends Cell Biol 2013; 23:433-41; PMID:23706391; http://dx.doi.org/ 10.1016/j.tcb.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 3. Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem 2006; 75:543-66; PMID:16756502; http://dx.doi.org/ 10.1146/annurev.biochem.74.082803.133425 [DOI] [PubMed] [Google Scholar]
- 4. Fededa JP, Gerlich DW. Molecular control of animal cell cytokinesis. Nat Cell Biol 2012; 14:440-7; PMID:22552143; http://dx.doi.org/ 10.1038/ncb2482 [DOI] [PubMed] [Google Scholar]
- 5. Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci U S A 2011; 108:4846-51; PMID:21383202; http://dx.doi.org/ 10.1073/pnas.1102714108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guizetti J, Schermelleh L, Mantler J, Maar S, Poser I, Leonhardt H, et al. . Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 2011; 331:1616-20; PMID:21310966; http://dx.doi.org/ 10.1126/science.1201847 [DOI] [PubMed] [Google Scholar]
- 7. Lumb JH, Connell JW, Allison R, Reid E. The AAA ATPase spastin links microtubule severing to membrane modelling. Biochim Bioph Acta 2012; 1823:192-7; PMID:21888932; http://dx.doi.org/ 10.1016/j.bbamcr.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 8. Yang D, Rismanchi N, Renvoise B, Lippincott-Schwartz J, Blackstone C, Hurley JH. Structural basis for midbody targeting of spastin by the ESCRT-III protein CHMP1B. Nat Struc Mol Biol 2008; 15:1278-86; PMID:18997780; http://dx.doi.org/ 10.1038/nsmb.1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zur A, Brandeis M. Timing of APC/C substrate degradation is determined by fzy/fzr specificity of destruction boxes. EMBO J 2002; 21:4500-10; PMID:12198152; http://dx.doi.org/ 10.1093/emboj/cdf452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, et al. . Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 2008; 132:487-98; PMID:18267078; http://dx.doi.org/ 10.1016/j.cell.2007.12.033 [DOI] [PubMed] [Google Scholar]
- 11. Son S, Tzur A, Weng Y, Jorgensen P, Kim J, Kirschner MW, et al. . Direct observation of mammalian cell growth and size regulation. Nat Methods 2012; 9:910-2; PMID:22863882; http://dx.doi.org/ 10.1038/nmeth.2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raiborg C, Stenmark H. Cell biology. A helix for the final cut. Science 2011; 331:1533-4; PMID:21436431; http://dx.doi.org/ 10.1126/science.1204208 [DOI] [PubMed] [Google Scholar]
- 13. Norgan AP, Davies BA, Azmi IF, Schroeder AS, Payne JA, Lynch GM, et al. . Relief of autoinhibition enhances Vta1 activation of Vps4 via the Vps4 stimulatory element. J Biolo Chem 2013; 288:26147-56; PMID:23880759; http://dx.doi.org/ 10.1074/jbc.M113.494112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chircop M, Perera S, Mariana A, Lau H, Ma MP, Gilbert J, et al. . Inhibition of dynamin by dynole 34-2 induces cell death following cytokinesis failure in cancer cells. Mol Cancer Therap 2011; 10:1553-62; PMID:21750222; http://dx.doi.org/ 10.1158/1535-7163.MCT-11-0067 [DOI] [PubMed] [Google Scholar]
- 15. Martz MK, Grabocka E, Beeharry N, Yen TJ, Wedegaertner PB. Leukemia-associated RhoGEF (LARG) is a novel RhoGEF in cytokinesis and required for the proper completion of abscission. Mol Biol Cell 2013; 24:2785-94; PMID:23885121; http://dx.doi.org/ 10.1091/mbc.E12-07-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mathieu J, Cauvin C, Moch C, Radford SJ, Sampaio P, Perdigoto CN, Schweisguth F, Bardin AJ, Sunkel CE, McKim K, et al. . Aurora B and cyclin B have opposite effects on the timing of cytokinesis abscission in Drosophila germ cells and in vertebrate somatic cells. Dev Cell 2013; 26:250-65; PMID:23948252; http://dx.doi.org/ 10.1016/j.devcel.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao WM, Seki A, Fang G. Cep55, a microtubule-bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis. Mol Biol Cell 2006; 17:3881-96; PMID: 16790497; http://dx.doi.org/ 10.1091/mbc.E06-01-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carlton JG, Caballe A, Agromayor M, Kloc M, Martin-Serrano J. ESCRT-III governs the Aurora B-mediated abscission checkpoint through CHMP4C. Science 2012; 336:220-5; PMID:22422861; http://dx.doi.org/ 10.1126/science.1217180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bastos RN, Barr FA. Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission. J Cell Biol 2010; 191:751-60; PMID:21079244; http://dx.doi.org/ 10.1083/jcb.201008108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pe'er T, Lahmi R, Sharaby Y, Chorni E, Noach M, Vecsler M, et al. . Gas2l3, a novel constriction site-associated protein whose regulation is mediated by the APC/C Cdh1 complex. PloS one 2013; 8:e57532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindon C, Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J Cell Biol 2004; 164:233-41; PMID: 14734534; http://dx.doi.org/ 10.1083/jcb.200309035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stewart S, Fang G. Destruction box-dependent degradation of aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1. Cancer Res 2005; 65:8730-5; PMID:16204042; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-1500 [DOI] [PubMed] [Google Scholar]
- 23. Kraft C, Gmachl M, Peters JM. Methods to measure ubiquitin-dependent proteolysis mediated by the anaphase-promoting complex. Methods 2006; 38:39-51; PMID:16343932; http://dx.doi.org/ 10.1016/j.ymeth.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 24. Floyd S, Whiffin N, Gavilan MP, Kutscheidt S, De Luca M, Marcozzi C, Min M, Watkins J, Chung K, Fackler OT, et al. . Spatiotemporal organization of Aurora-B by APC/CCdh1 after mitosis coordinates cell spreading through FHOD1. J Cell Sci 2013; 126:2845-56; PMID:23613471; http://dx.doi.org/ 10.1242/jcs.123232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Florindo C, Perdigao J, Fesquet D, Schiebel E, Pines J, Tavares AA. Human Mob1 proteins are required for cytokinesis by controlling microtubule stability. J Cell Sci 2012; 125:3085-90; PMID:22454515; http://dx.doi.org/ 10.1242/jcs.097147 [DOI] [PubMed] [Google Scholar]
- 26. Steigemann P, Wurzenberger C, Schmitz MH, Held M, Guizetti J, Maar S, Gerlich DW. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 2009; 136:473-84; PMID:19203582; http://dx.doi.org/ 10.1016/j.cell.2008.12.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.