Abstract
Background
Reactive oxygen species (ROS) and inflammation both contribute to the progression of aldosterone-induced renal injury. To better understand the underlying mechanisms, we examined mitochondrial dysfunction and NLRP3 inflammasome activation in aldosterone-infused rats, and explored the role of rotenone in attenuating these injuries.
Material/Methods
Sprague-Dawley rats were divided into 3 groups: vehicle-treated, aldosterone-infused, and aldosterone plus rotenone. Renal damage was evaluated using PAS staining and electron microscopy. Levels of ROS were measured from renal tissue and serum; immunohistochemistry analysis examined the inflammation pathway; Western blot and real-time PCR assessed NLRP3 inflammasome activity.
Results
Glomerular segmental sclerosis, foot process effacement, and proteinuria were demonstrated in the aldosterone-infused rats. Specifically, the thiobarbituric acid-reactive substances (TBARS) oxidative stress marker, MDA, was significantly increased; ATP content and mtDNA copy number were markedly decreased; inflammatory mediators NF-κB p65 and CTGF were upregulated; and NLRP3 inflammasome and its related target proteins, IL-1β and IL-18, were also increased. Treatment with rotenone, an inhibitor of mitochondrial complex I, significantly attenuated oxidative stress, mitochondrial dysfunction, and inflammasome response in aldosterone-infused rats.
Conclusions
Rotenone ameliorated aldosterone-infused renal injury, possibly by inhibiting oxidative stress, mitochondrial dysfunction, and NLRP3 inflammasome activity. These results provide novel evidence for the role of rotenone in aldosterone-induced renal injury or other chronic kidney disease.
MeSH Keywords: Aldosterone; Inflammasomes; Reactive Oxygen Species; Renal Insufficiency, Chronic
Background
The mineralocorticoid hormone aldosterone (Aldo) is primarily synthesized in the adrenal gland and regulates blood pressure by maintaining electrolyte and fluid homeostasis [1]. Mineralocorticoid receptors (MR) are present in tubular epithelial cells, mesangial cells, and renal fibroblasts, and Aldo is known to function through classical MR-dependent and genomic effects in the distal nephron of the kidney [2]. In a recent study, patients with hyperaldosteronism were shown to exhibit proteinuria [3]. Likewise, stroke-prone, spontaneously hypertensive rats also demonstrated significantly elevated Aldo levels. In contrast, inhibiting Aldo has been shown to markedly attenuate renal injury in hypertensive rats [4]. These observations indicate that Aldo plays a key role in the progression of kidney disease via MR-independent hemodynamic effects. However, the exact mechanisms that regulate the process in Aldo-induced renal injuries are not fully understood.
Recent results have suggested that nucleotide-binding domain and leucine-rich repeat-containing (NLR family) cytosolic pattern recognition receptors promote sterile inflammation and act in the host innate immune response to infectious stimuli [5]. Among the inflammasomes, NLRP3 inflammasome is the best characterized in many mammalian cells. NLRP3 interacts with adaptor protein apoptosis-associated speck-like protein (ASC) to activate caspase-1. Activated NLRP3 inflammasome then promotes tissue injury by upregulating pro-inflammatory cytokines IL-18 and IL-1β [6]. Previous studies have reported markedly elevated IL-18 and IL-1β levels in Aldo-induced renal inflammation and fibrosis in vivo [7,8]. Our previous work also suggests that reactive oxygen species (ROS) participate in Aldo-induced renal injury [9]. However, further investigation is needed to define the exact molecular mechanisms.
Mitochondrial dysfunction (MtD) has been implicated in the development of CKD [10]. Mitochondria are exquisitely complex regulators of cytosolic homeostasis, sensing and responding to changes in intracellular ROS and K+ [11]. Mitochondria are also producers and targets of ROS, especially mitochondrial DNA (mtDNA), which encodes 13 essential protein components of the oxidative phosphorylation complexes and is localized to the inner mitochondrial membrane [12]. Rotenone (ROT), an inhibitor of mitochondrial complex I, has recently been shown to protect renal injury against oxidative stress and inflammation in chronic kidney disease [13,14]. In this study, we investigated whether NLRP3 inflammasome is involved in Aldo-infused renal injury. Furthermore, we explored whether ROT protects against Aldo-infused kidney disease by improving oxidative stress, mitochondrial dysfunction, and inflammasome response.
Material and Methods
Animals
Sprague-Dawley rats (male, 220–250 g, n=19) were obtained from Shanghai SLAC Laboratory Animals (Shanghai, China) and underwent right uninephrectomy under anesthesia with sodium pentobarbital (50 mg/kg, IP). After 2 weeks of recovery, all rats were given high-salt drinking water containing 1% NaCl and then were randomly treated with 1 of the following for 4 weeks: group 1, control (0.5% ethanol, subcutaneously, n=6); group 2, Aldo (0.75% μg/h, subcutaneously, n=7); and group 3, Aldo + ROT (0.75% μg/h, subcutaneously, n=6, 600 ppm of ROT in a gelled diet) [13]. An osmotic mini-pump (Alzet model 2004) was implanted subcutaneously to infuse either Aldo or vehicle. At the end of the 4 weeks, the rats were sacrificed, blood and 24-h urinary samples were collected, and kidney sections were fixed in 10% formalin and embedded in paraffin for histological evaluation. The remainder of the kidney was used for mRNA and protein analyses. All experiments were performed according to the guidelines for the care and use of animals established by Jiao Tong University.
Histological examination
Renal tissues were sectioned into 3-μm slices and stained with periodic acid Schiff (PAS). According to the method of Huang et al., we assessed the severity of glomerular injury from each rat kidney section using light microscopy, before rating glomerular proliferative lesions on a scale from 0 to 4, as follows: 0 indicated no proliferation, whereas 1+, 2+, 3+, 4+ indicated 1–25%, 26–50%, 51–75%, and 76–100% of segmental lesion per glomeruli, respectively [15].
Electron microscopy analysis
To assess mitochondrial ultrastructure morphology and podocyte foot processes, we cut kidney tissue into 1-mm3 pieces using a scalpel, before fixing samples with 2.5% glutaraldehyde at room temperature. We cut ultrathin sections (60-nm) on a microtome, placed them on copper grids, and stained them with uranyl acetate and lead citrate, before detection in an electron microscope.
Detection of ROS and ATP
We detected renal thiobarbituric acid-reactive substances (TBARS) using commercial kits (Cayman Chemical Company). Moreover, we measured serum malondialdehyde (MDA) and T-SOD using commercial kits (Jiancheng Bioengineering Research Institute). According to our previous study, we used 2′7′-dichlorofluorescin diacetate (DCFDA) to detect renal ROS production [16]. We incubated each 3-μm tissue cryosection with 10 μM DCFDA in the dark at 37°C for 30 min, before imaging using fluorescence microscopy. We determined ATP levels using a luciferase-based bioluminescence assay kit (Sigma, St. Louis, MO, USA) in a FLUOstar Optima reader according to the manufacturer’s instructions.
Real-time PCR
We extracted total RNA from kidney tissue using Trizol (Invitrogen) reagent and performed reverse transcription using the first-strand cDNA synthesis kit (Fermentas, Glen Bernie, MD, USA). The following primers were used: for detection of rat mtDNA, forward 5′-TCCTCCGTGAAATCAACAACC-3′/reverse 5′-GGGAACGTATGGACGATGAAC-3′; 18s rRNA, forward 5′-GCGGTTCTATTTTGTTGGTTTT-3′/reverse 5′-ACCTCCGACTTT CGTTCTTG-3′; IL-1β, forward 5′-AGCCTTTGTCCTCTGCCAAGT-3′/reverse 5′-CCAGAATGTGCCACGGTTTT-3′; IL-18, forward 5′-GGGA TGGGAGGAACGCTACTA-3′/reverse 5′-ACAGGT TGTACTGGAAAAGCC-3′. We normalized relative amounts of mRNA to GAPDH or 18s rRNA and calculated expression levels using the ΔΔ CT (cycle threshold) method.
Immunohistochemistry
We embedded the kidneys with paraffin and cut 3-μm sections from the embedded blocks. After a 30-min wash with 3% H2O2, the slides were placed in contact with 5% fetal calf serum for 30 min. Then the slides were incubated with antibodies were diluted in phosphate-buffered saline (PBS) with 4% serum. Rabbit anti-CTGF (1:200; Santa Cruz Biotechnology, USA) and mouse anti-NF-κB P65 (1:200; Santa Cruz Biotechnology, USA) were incubated for 2 h at room temperature. The slides were washed in PBS and incubated with secondary antibodies for 1 h before treatment with streptavidin-HRP for 30 min. We added DAB to each section and stained them for 1 min, before counterstaining with hematoxylin for 5 min. We evaluated inflammatory factors by counting the number of positive cells in 20 randomly chosen kidney sections.
Western blot
We lysed and Western blotted rat renal tissue as described previously [17]. We separated lysates on 10% polyacrylamide gels, before immunoblotting using anti- NLRP3 (adipoGen company, San Diego, CA), anti-ASC (adipoGen company, San Diego, CA) antibody, anti-IL-1β (Affinity Biosciences, USA) and anti-IL-18 (Affinity Biosciences, USA) antibody at a dilution of 1:500. We used a semi-quantitative analysis using densitometry, and used β-actin to standardize the amount of protein loaded on the blots.
Statistical analysis
Data are expressed as means ± the standard error of the mean (SEM). One-way ANOVA was used to compare mean values, and a value of P<0.05 was determined as statistically significant.
Results
ROT ameliorates renal injury in Aldo-infused rats
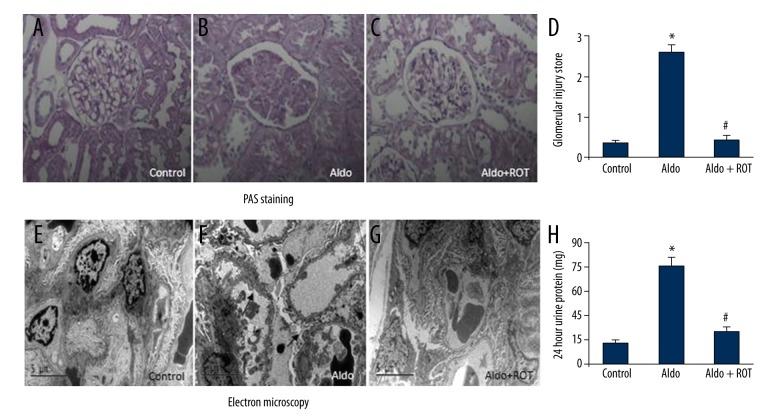
We evaluated renal damage using PAS staining, electron microscopy, and 24-h urine protein. As shown in Figure 1, compared with the control group, Aldo-infused rats exhibited thickened capillary loops, expanded mesangial regions, and glomerulosclerosis. However, treatment with ROT significantly improved kidney damage and decreased the glomerular injury score (Figures 1A–1D). Furthermore, compared with the control group, Aldo-infused rats demonstrated disappearance of the slit diaphragm gap and foot process effacement. Strikingly, ROT treatment significantly attenuated kidney ultrastructure morphology in the Aldo-infused rats (Figure 1E–1G). Similarly, the 24-h urine protein test revealed increased protein levels in the Aldo-infused rats relative to the vehicle-treated rats, but decreased levels in the Aldo + ROT group (Figure 1H).
Figure 1.
ROT attenuated kidney damage in Aldo-infused rats. Representative photomicrographs (magnification ×400) of PAS-stained kidney sections (A–D). Morphology changes of podocyte foot processes by electron microscopy (E–G; magnification ×5800). Arrow indicates podocyte foot process effacement. Twenty-four hour urine protein was measured at week 4 (H). Data are expressed as means ±SEM (n=6). * P<0.05, control versus Aldo-infused rats; # P<0.05, Aldo-infused rats versus Aldo + ROT treatment rats.
ROT decreases ROS in Aldo-infused rats
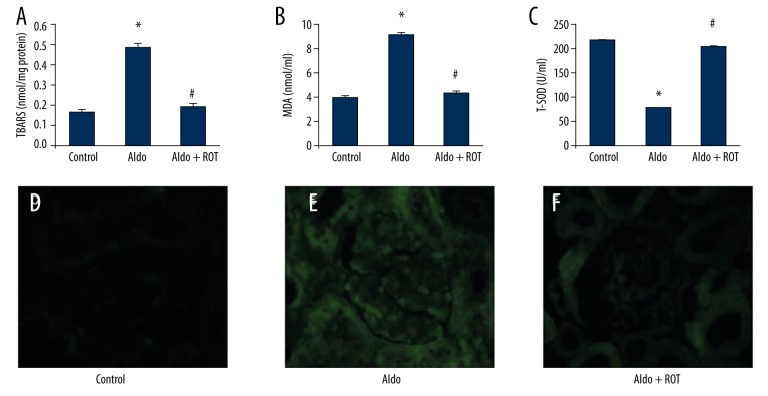
ROS is considered a major detrimental factor leading to the progression of various types of kidney disease. We therefore measured ROS production in our Aldo-infused rats. As shown in Figure 2, renal TBARS and serum MDA levels were significantly increased in the Aldo-infused rats compared with the vehicle-treated rats (Figure 2A, 2B, respectively). Serum T-SOD levels decreased in the Aldo-infused group relative to the vehicle-treated group (Figure 2C), while treatment with ROT significantly ameliorated oxidative stress in the Aldo-infused group. Moreover, DCFDA fluorescence of kidney tissue sections also demonstrated higher ROS levels in the Aldo-infused rats compared with the control group (Figure 2D–2F).
Figure 2.
ROS production was assessed by measuring renal TBARS levels (A), serum MDA levels (B), serum T-SOD levels (C) and 2′,7′-dichlorofluorescein staining of kidney sections (D–F; magnification ×400). Data are presented as mean ±SEM; (n=6); * P<0.05, control versus Aldo-infused rats; # P<0.05, Aldo-infused rats versus Aldo + ROT treatment rats.
ROT attenuates MtD in Aldo-infused rats
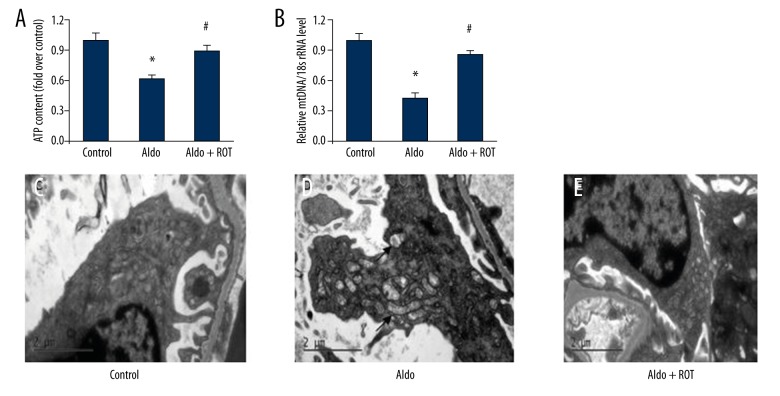
MtD is characterized by the breakdown of membrane potential, disturbance of intracellular ATP synthesis, and mtDNA damage. There is a growing body of evidence suggesting MtD is involved in the pathogenesis of various kidney diseases. We directly demonstrate evidence of MtD in the Aldo-infused rats. We show decreased ATP and mtDNA/18S rRNA levels in renal tissues from the Aldo-treated rats compared with the vehicle control group. Importantly, treatment with ROT markedly reversed these changes (Figure 3A, 3B). Looking at the mitochondrial ultrastructure morphology, rats treated with Aldo had swollen mitochondria with disorganized and fragmented cristae present in the tubular cells, while ROT treatment significantly ameliorated mitochondrial dysfunction in the Aldo-treated rats (Figure 3C–3E).
Figure 3.
ROT attenuated Aldo-induced mitochondrial dysfunction (MtD). MtD was measured by ATP content (A), mtDNA/18S rRNA (B), and mitochondrial ultrastructure morphology (C–E), (magnification ×12000). Arrow indicates swollen mitochondria. Data are presented as mean ±SEM; (n=6); * P<0.05, control versus Aldo-infused rats; # P<0.05, Aldo-infused rats versus Aldo + ROT treatment rats.
ROT reduces NF-κB activation and CTGF expression in rat kidney
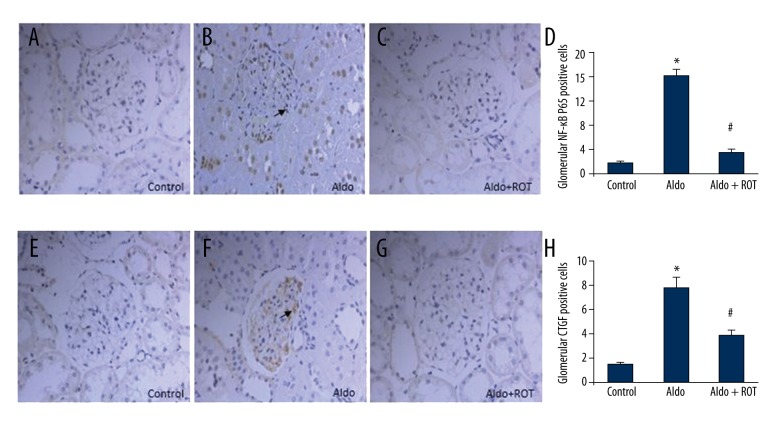
NF-κB is a ubiquitous eukaryotic transcription factor that regulates gene expression cytokines in the inflammatory response. As shown in Figure 4A–4D, we demonstrated significantly elevated NF-κB P65 positive cells in renal tissue from the Aldo-infused rats compared with the vehicle control group. Next, we demonstrated the anti-inflammatory properties of ROT through the reduction of NF-κB P65-positive cells in the ROT-treated group compared with the Aldo-infused group; however, the levels of NF-κB P65-positive cells were still higher than in the vehicle control group. Similarly, compared with vehicle-treated rats, the number of glomerular mesangial cells positive for CTGF increased in the Aldo-infused group. Notably, ROT treatment significantly reduced the number of CTGF-positive cells relative to the Aldo-infused rats (Figure 4E–4H).
Figure 4.
Renal NF-κB activation and related inflammatory cytokine, CTGF, in Aldo-infused rats. Immunohistochemistry study of NF-κB (A–D) and CTGF (E–H) are shown. Arrows indicate NF-κB positive cells (A–C) and CTGF positive cells (E–G). Original magnification ×400. Data are presented as mean ±SEM; (n=6); * P<0.05, control versus Aldo-infused rats; # P<0.05, Aldo-infused rats versus Aldo + ROT treatment rats.
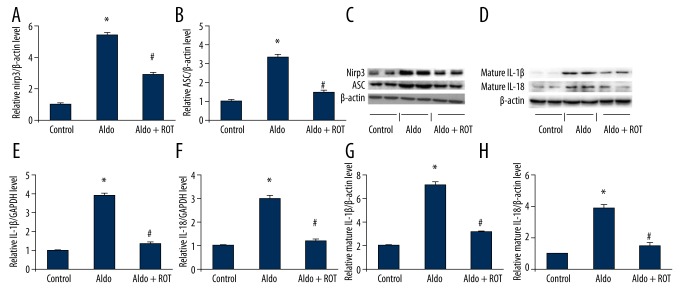
ROT decreases NLRP3 inflammasome activation in Aldo-infused rats
We determined renal NLRP3 inflammasome activation on the basis of expression of NLRP3, ASC, IL-1β, and IL-18. As shown in Figure 5A–5C, compared with the control group, NLRP3 and ASC protein expression increased significantly in renal tissue from Aldo-infused rats, while ROT treatment markedly decreased renal NLRP3 and ASC protein levels. Likewise, we observed elevated renal IL-1β and IL-18 levels in the Aldo-infused rats relative to the vehicle-treated rats (Figure 5D–5H). Accordingly, ROT treatment resulted in a decrease in IL-1β and IL-18 expression levels.
Figure 5.
Evaluation of NLRP3 inflammasome activation in Aldo-infused rats. (A) Relative expression of NLRP3 protein to β-actin expression. (B) Relative expression of ASC protein to β-actin expression. (C) Western blot analysis of NLRP3 and ASC. (D) Western blot analysis of mature IL-1β and mature IL-18. (E) mRNA expression of IL-1β normalized to GAPDH level. (F) mRNA expression of IL-18 normalized to GAPDH level. (G) Relative expression of mature IL-1β protein to β-actin expression. (H) Relative expression of mature IL-18 protein to β-actin expression. Data are presented as mean ±SEM; (n=6); * P<0.05, control versus Aldo-infused rats; # P<0.05, Aldo-infused rats versus Aldo + ROT treatment rats.
Discussion
In present study, we show Aldo increases ROS production and induces MtD. Importantly, we show that ROT, an inhibitor of mitochondrial complex I, decreases ROS levels and improves renal function, suggesting that Aldo-induced kidney damage in rats occurs via ROS of mitochondrial origin. We also demonstrate an increase in nuclear transcription factor, NF-κB, and inflammatory cytokine, CTGF, in Aldo-infused rats, in addition to activation of the NLRP3 inflammasome pathway, including NLRP3, ASC, IL-1β, and IL-18. Imperatively, treatment with ROT significantly reduced these inflammatory responses. These results indicate that Aldo-induced NLRP3 inflammasome signaling might be mediated through ROS of mitochondrial origin. This notion is further supported by an earlier study in which NLRP3 inflammasome mediated renal injury through impaired mitochondrial function in an albumin-induced kidney damage model [18].
ROS are key mediators in regulating signal transduction in various types of cells. However, excessive ROS production has been shown to increase extracellular matrix and inflammation in the progression of renal injury. Recently, there has been a focus on the relationship between ROS and MtD, and the role of mitochondria as a special target in ameliorating the progression of kidney disease [19]. Mitochondria are important cellular organelles for oxidative phosphorylation and the energy production pathway. MtD is characterized by a decrease in ATP production, loss of mitochondrial membrane potential, and decreased mtDNA [20]. Recently, MtD has been implicated in a range of kidney diseases, including Aldo-infused renal injury [10,14]. In this study, we reveal a decrease in ATP content and mtDNA/18S rRNA in the Aldo-treated rats compared with the vehicle-control group. Our Aldo-induced rats had swollen mitochondria with disorganized and fragmented cristae. According to a study by Zhang et al., mitochondria are the primary cellular source of reactive oxygen species and the use of ROT in vitro decreases ROS production and prevents epithelial mesenchymal transition [14]. Sun et al. also demonstrated that ROT protected kidneys against obstructive injury, possibly via inhibition of inflammation, mitochondrial oxidative stress, and fibrosis, suggesting an important role of ROT in the treatment of obstructive kidney disease [21]. However, the role of ROT in Aldo-induced renal injury in vivo remains unclear. Here, we found that ROT significantly decreased ROS production, which subsequently prevented MtD and improved renal function in vivo. These results suggest that targeting mitochondrial dysfunction might serve as a novel therapeutic strategy for the treatment of CKD.
Inflammation is an important response to tissue damage. Various mediators, such as cytokines, chemokines, and eicosanoids, play key roles in this response. However, long-term consequences of inflammation are responsible for the development of CKD. NF-κB is a ubiquitous eukaryotic transcription factor that regulates many inflammatory cytokines. Under normal conditions, NF-κB is silenced by its inhibitor, IκB. Upon activation, following phosphorylation and degradation of IκB, NF-κB is released and translocated into the nucleus. Persistent NF-κB activation initiates an increase in inflammatory genes that regulate cell proliferation, invasion, apoptosis, and metastasis [22]. In this study, we report increased levels of NF-κB P65 and inflammatory cytokine, CTGF, in Aldo-treated rats compared with the vehicle-treated control group. Ichikawa et al. previously showed that ROT inhibits various pro-inflammatory genes, including TNF-α and cyclooxyoxygenase-2 (COX-2) [23]. Zmijewski et al. also demonstrated the anti-inflammatory role of ROT in an acute lung injury animal model [24]. In agreement with this result, the present study found that ROT markedly inhibits Aldo-induced inflammation and ameliorates renal injury, indicating its anti-inflammatory potential in CKD.
Recently, the role of the NLRP3 inflammasome pathway in immunity and inflammation has attracted increasing attention. Within the cytoplasm, NLRP3 forms a complex with ASC and procaspase-1, forming the NLRP3 inflammasome. The NLRP3 inflammasome is a multiprotein complex that controls caspase-1 activation required for the maturation of IL-1β and IL-18 [6]. Many reports show that NLRP3 inflammasome, as a mediator, is involved in the development and progression of CKD. Vilaysane et al. reported that various kinds of non-diabetic kidney diseases exhibited increased expression of NLRP3 mRNA, which correlated with renal function, suggesting that NLRP3 inflammasome promotes renal inflammation and contributes to CKD [25]. Shahzad et al. demonstrated that NLRP3 inflammasome activation in non-myeloid-derived cells aggravated diabetic nephropathy [26]. Zhuang et al. showed that albumin-induced NLRP3 inflammasome activation resulted in MtD, which in turn led to cellular apoptosis and injury [18]. Although the NLRP3 inflammasome appears to be an important pathogenic mediator of CKD, the possible role of NLRP3 inflammasome in Aldo-induced renal injury warrants further investigation. According to the study by Cruz et al., excessive production of ROS is considered to be critical for NLRP3 inflammasome activation. Several chemical scavengers of ROS suppress NLRP3 inflammasome activation in response to a wide range of stimuli [27]. Our previous study showed significantly increased levels of ROS in Aldo-treated rats [16]. Here, we demonstrated significantly elevated NLRP3 and ASC protein levels in Aldo-infused rats. Similarly, we observed markedly increased levels of mature IL-1β and mature IL-18 in the Aldo-infused rats. Therefore, increased activation of NLRP3 inflammasome is closely associated with ROS production. Consistent with our findings, Doi et al. reported that caspase-1 and NLRP3 protein were activated in Aldo/salt-infused rats [28]. These results indicate that NLRP3 inflammasome contributes to the pathogenesis of Aldo-induced kidney damage, while also suggesting a potential treatment target. We found that ROT significantly decreased NLRP3 inflammasome-associated component proteins, including NLRP3 and ASC. ROT also inhibited IL-1β and IL-18 production in Aldo-infused rats. These results suggest that ROT may serve as an important pharmaceutical target for the treatment of Aldo-induced kidney disease.
Conclusions
Oxidative stress and inflammation are undoubtedly important detrimental responses in CKD. Our observations suggest that NLRP3 inflammasome activation contributes to inflammation in Aldo-treated rats. ROT not only decreased oxidative stress and related NF-κB activity, but also inhibited inflammasome activation and downstream effectors. Altogether, our data provide novel evidence for the role of ROT treatment in Aldo-induced renal injury and a potential role in other chronic kidney diseases.
Footnotes
Source of support: This work was supported by a grant from the National Natural Science Foundation of China (81270822, 81270009, and 81300590)
Disclosure statement
The authors declare that there is no conflict of interest.
References
- 1.Beesley AH, Hornby D, White SJ. Regulation of distal nephron K+ channels (ROMK) mRNA expression by aldosterone in rat kidney. J Physiol. 1998;509:629–34. doi: 10.1111/j.1469-7793.1998.629bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connell JM, Davies E. The new biology of aldosterone. J Endocrinol. 2005;186:1–20. doi: 10.1677/joe.1.06017. [DOI] [PubMed] [Google Scholar]
- 3.Sechi LA, Novello M, Lapenna R, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295:2638–45. doi: 10.1001/jama.295.22.2638. [DOI] [PubMed] [Google Scholar]
- 4.Rocha R, Chander PN, Zuckerman A, Stier CT., Jr Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension. 1999;33:232–37. doi: 10.1161/01.hyp.33.1.232. [DOI] [PubMed] [Google Scholar]
- 5.Bracey NA, Beck PL, Muruve DA, et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1β. Exp Physiol. 2013;98:462–72. doi: 10.1113/expphysiol.2012.068338. [DOI] [PubMed] [Google Scholar]
- 6.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 7.Blasi ER, Rocha R, Rudolph AE, et al. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 8.Li JY, Zhang SL, Ren M, et al. High-sodium intake aggravates myocardial injuries induced by aldosterone via oxidative stress in Sprague-Dawley rats. Acta Pharmacol Sin. 2012;33:393–400. doi: 10.1038/aps.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding W, Yang L, Zhang M, Gu Y. Reactive oxygen species-mediated endoplasmic reticulum stress contributes to aldosterone-induced apoptosis in tubular epithelial cells. Biochem Biophys Res Commun. 2012;418:451–56. doi: 10.1016/j.bbrc.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 10.Zhu C, Huang S, Yuan Y, et al. Mitochondrial dysfunction mediates aldosterone-induced podocyte damage: a therapeutic target of PPARγ. Am J Pathol. 2011;178:2020–31. doi: 10.1016/j.ajpath.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–14. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamagata K, Muro K, Usui J, et al. Mitochondrial DNA mutations in focal segmental glomerulosclerosis lesions. J Am Soc Nephrol. 2002;13:1816–23. doi: 10.1097/01.asn.0000019772.17954.f8. [DOI] [PubMed] [Google Scholar]
- 13.Zhang A, Jia Z, Wang N, et al. Relative contributions of mitochondria and NADPH oxidase to deoxycorticosterone acetate-salt hypertension in mice. Kidney Int. 2011;80:51–60. doi: 10.1038/ki.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang A, Jia Z, Guo X, Yang T. Aldosterone induces epithelial mesenchymal transition via ROS of mitochondrial origin. Am J Physiol Renal Physiol. 2007;293:F723–31. doi: 10.1152/ajprenal.00480.2006. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Border WA, Yu L, et al. A PAI-1 mutant, PAI-1 R, slows progression of diabetic nephrology. J Am Soc Nephrol. 2008;19:329–38. doi: 10.1681/ASN.2007040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu C, Ding W, Yang L, et al. Contributions of endoplasmic reticulum stress and reactive oxygen species to renal injury in aldosterone/salt-induced rats. Nephron Exp Nephrol. 2014;126:25–32. doi: 10.1159/000357777. [DOI] [PubMed] [Google Scholar]
- 17.Ding W, Yang L, Zhang M, Gu Y. Chronic inhibition of nuclear factor kappa B attenuates aldosterone/salt-induced renal injury. Life Sci. 2012;90:600–6. doi: 10.1016/j.lfs.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang Y, Ding G, Zhao M, et al. NLRP3 inflammasome mediates albumin-induced renal tubular injury through impaired mitochondrial function. J Biol Chem. 2014;289:25101–11. doi: 10.1074/jbc.M114.578260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casalena G, Daehn I, Bottinger Transforming growth factor-β, bioenergetics, and mitochondria in renal disease. Semin Nephrol. 2012;32:295–303. doi: 10.1016/j.semnephrol.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Che R, Yuan Y, Huang S, Zhang A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol Renal Physiol. 2014;306:F367–78. doi: 10.1152/ajprenal.00571.2013. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Zhang Y, Zhao DQ, et al. Rotenone remarkably attenuates oxidative stress, inflammation, and fibrosis in chronic obstructive uropathy. Mediators Inflamm. 2014;2014:670106. doi: 10.1155/2014/670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sethi G, Sung B, Aggarwal BB. Nuclear factor-KappaB activation: from bench to bedside. Exp Biol Med (Maywood) 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 23.Ichikawa H, Takagi T, Uchiyama K, et al. Rotenone, a mitochondrial electron transport inhibitor, ameliorates ischemia reperfusion-induced intestinal mucosal damage in rats. Redox Rep. 2004;9:313–16. doi: 10.1179/135100004225006795. [DOI] [PubMed] [Google Scholar]
- 24.Zmijewski JW, Lorne E, Zhao X, et al. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med. 2008;178:168–79. doi: 10.1164/rccm.200710-1602OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilaysane A, Chun J, Seamone E, et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–44. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahzad K, Bock F, Dong W, et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephrology. Kidney Int. 2015;87:74–84. doi: 10.1038/ki.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz CM, Rinna A, Forman HJ, et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–79. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doi T, Doi S, Nakashima A, et al. Mizoribine ameliorates renal injury and hypertension along with the attenuation of renal caspase-1 expression in aldosterone-salt-treated rats. PLoS One. 2014;9:e93513. doi: 10.1371/journal.pone.0093513. [DOI] [PMC free article] [PubMed] [Google Scholar]