Abstract
ONC201/TIC10 is a small molecule initially discovered by its ability to coordinately induce and activate the TRAIL pathway selectively in tumor cells and has recently entered clinical trials in adult advanced cancers. The anti-tumor activity of ONC201 has previously been demonstrated in several preclinical models of cancer, including refractory solid tumors and a transgenic lymphoma mouse model. Based on the need for new safe and effective therapies in pediatric non-Hodgkin's lymphoma (NHL) and the non-toxic preclinical profile of ONC201, we investigated the in vitro efficacy of ONC201 in non-Hodgkin's lymphoma (NHL) cell lines to evaluate its therapeutic potential for this disease. ONC201 caused a dose-dependent reduction in the cell viability of NHL cell lines that resulted from induction of apoptosis. As expected from prior observations, induction of TRAIL and its receptor DR5 was also observed in these cell lines. Furthermore, dual induction of TRAIL and DR5 appeared to drive the observed apoptosis and TRAIL expression was correlated linearly with sub-G1 DNA content, suggesting its potential role as a biomarker of tumor response to ONC201-treated lymphoma cells. We further investigated combinations of ONC201 with approved chemotherapeutic agents used to treat lymphoma. ONC201 exhibited synergy in combination with the anti-metabolic agent cytarabine in vitro, in addition to cooperating with other therapies. Together these findings indicate that ONC201 is an effective TRAIL pathway-inducer as a monoagent that can be combined with chemotherapy to enhance therapeutic responses in pediatric NHL.
Keywords: cancer, lymphoma, NHL, ONC201, TIC10, TRAIL
Abbreviations
- TRAIL
TNF-related apoptosis-inducing ligand
- DR5
death receptor 5
- TNF
tumor necrosis family
- NHL
non-Hodgkin's lymphoma.
Introduction
Lymphoma is the third most common childhood malignancy, with non-Hodgkin's Lymphoma (NHL) accounting for about 7% of new cancer cases in children, most of which are high grade.1-3 Current treatment approaches rely heavily on intensive multi-agent chemotherapy regimens such as the cyclophosphamide–doxorubicin–vincristine–prednisone (CHOP) regimen, in addition to the anti-CD20 antibody rituximab. Despite significant progress in 5-year survival rates for pediatric NHL, there is still a need for improved therapies to treat advanced-stage, relapsed, and/or refractory disease.
The TNF-related apoptosis-inducing ligand (TRAIL) is an endogenous protein expressed by several immune cells to selectively induce apoptosis in cancer cells as a critical effector mechanism in the immune surveillance of cancer.4,5 The ability of TRAIL to confer apoptosis in various malignant cells while leaving normal cells unharmed has highlighted this ligand and its receptors as exciting potential therapeutic targets.6 While early-phase clinical trials have investigated the role of recombinant TRAIL and TRAIL death receptor-agonist antibodies in several malignancies, pediatric lymphoma remains uninvestigated.7-11 Preclinical studies have found that recombinant TRAIL is active in NHL when combined chemotherapy.12-14 Interestingly, a prior study in Burkitt's lymphoma found that TRAIL is highly active by a paracrine mechanism in a xenograft model as compared to recombinant protein administration.15 Thus TRAIL-based therapies have been recognized and remain as a therapeutic intervention for further investigation in pediatric and adult lymphomas.16
The clinical development of protein-based TRAIL therapeutics has been stymied following clinical efficacy results in lead indications that were less robust than anticipated, despite a benign safety profile. The limited clinical activity of these TRAIL-based agents has been ascribed to intrinsic tumor cell attributes such as O-glycosylation enzymes,17,18 the tumor microenvironment,19,20 and limitations of the therapeutic agents themselves such as suboptimal death receptor clustering and poor chemical characteristics.21 Nonetheless, TRAIL possesses ideal therapeutic properties as an endogenous, natural, anti-cancer protein that was evolved as part of the immune system.5
The power of the immune system in general and TRAIL pathway in particular in cancer suppression motivated our effort to identify small molecules that harness the therapeutic potential of the endogenous death receptor pathway without the limitations of exogenous protein mimetics.22 Small molecule screening led to the identification of ONC201, a small molecule with a unique pharmacophore that induces TRAIL in a p53-independent manner as well as its proapoptotic receptor DR5, leading to the coordinate activation of apoptosis in tumor cells while sparing normal cells. Mechanistic studies revealed a late-stage inactivation of Akt and ERK that activates Foxo3a, which transcriptionally regulates the TRAIL gene. The involvement of these mechanisms as well as additional early-stage signaling that is currently under investigation supports the strong efficacy and safety characteristics of ONC201 that extends the scope of prior TRAIL-based therapies.
The preclinical efficacy of ONC201 has been reported in a wide range of tumor types as a monoagent and in combination with approved therapies,22,23 including extension of survival in a Eu-myc transgenic model of lymphoma. A unique feature of ONC201 is the coupling of its strong efficacy and benign safety features, which is particularly important in pediatric cancer patients where genotoxicity is germane and accompanies many of its standard-of-care therapies.24,25 Based on the aforementioned efficacy, mechanistic, and safety characteristics of ONC201 we investigated its activity in pediatric NHL cell lines to determine its therapeutic potential in this indication that is in need of novel effective and safe therapies.
Materials and Methods
Cell lines
Granta, UPN2, NCEB, Karpas299 and Daudi cell lines were a kind gift from Dr. E. Epner at Penn State Hershey Cancer Institute; Ramos and Raji cells were a kind gift from Dr. S. Dovat at Penn State Hershey Medical Center; the BJAB cell line was a kind gift from Dr. D. Tantin at University of Utah. Cells were cultured in ATCC-recommended media and maintained in a humidified incubator at 5% CO2 and 37°C.
Cell culture
Cell lines (all except Granta) were cultured in RPMI 1640, which was supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin/streptomycin (all from Life Technologies, Inc.). Raji cells in addition needed 0.05 mM 2-mercaptoethanol. Granta cells were maintained in DMEM with 10% FBS and 100 units/ml penicillin/streptomycin. All cells were incubated in a fully humidified atmosphere with 5% CO2 at 37°C. Cell media was renewed every 3–4 d per standard specifications. All experiments were conducted with a cell number of 1 × 106 cells/ml.
Cell viability assays
Cell viability was measured using the CellTiter Glo® Luminescent Cell Viability assay kit (Promega). Briefly 1–2 × 104 cells per well were incubated in fresh media in 96-well black-walled plates (Nunc) at a concentration of 2 × 106 cells per mL and a volume of 50–100 μL per well, along with the indicated concentrations of specified drug. Viability assays were performed according to the manufacturer's protocol at 72 hours post-treatment or at otherwise indicated time points. Bioluminescent imaging was recorded on an IVIS imaging system (Xenogen). GI50 values were calculated using non-linear regression curve fitting (GraphPad Prism).
Flow cytometry
For sub-G1 DNA content analyses, cells were harvested at indicated time points and fixed in 80% ethanol at 4°C. Cells were then stained with propidium iodide in the presence of RNase and analyzed on an Epics Elite flow cytometer (Beckman Coulter).
For surface TRAIL expression analyses, cells were harvested and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min, incubated with an anti-TRAIL antibody (Abcam, ab2435) at 1:250 overnight, washed and incubated with anti-rabbit Alexa Fluor 488 (Invitrogen) for 30 min, and analyzed. Cells were gated on forward and side scatter to eliminate debris and dead cells from the analysis. Surface TRAIL data are expressed as median fluorescence intensity relative to that of control samples unless indicated otherwise.
For DR5 expression analyses, cells were harvested and fixed in 4% paraformaldehyde to avoid receptor internalization. Cells were then incubated with primary antibodies at 1:250 (DR5, Imgenex Img-120A) for 1 hour, washed in PBS, incubated with Alexafluor 488 as a secondary antibody, and resuspended in PBS for analysis. Surface DR5 data are expressed as median fluorescence intensity relative to that of control samples unless indicated otherwise. RIK-2 (Santa Cruz Biotechnology, sc-56246) was used as a TRAIL sequestering antibody for testing inhibition of ONC201 mediated cytotoxicity per product specifications. Recombinant His-tagged TRAIL was produced as previously described.26
Western blot analysis
Cells were grown in 6-well plates and treated in log-phase as indicated. Cells were harvested then centrifuged and lysed on ice for 2 hours. Samples were electrophoresed under reducing conditions on NuPAGE 4–12% Bis-Tris gels (Invitrogen), transferred to PVDF, and blocked in 10% non-fat milk in TBST for 1 hour. Membranes were then incubated with the primary antibodies overnight: DR5 (Cell Signaling #3696) and Ran (BD Biosciences # 610341 at 1:1000 in TBST overnight at 4°C. Membranes were washed in TBST, incubated with the appropriate secondary antibody for 1 hour, washed in TBST, and visualized using ECL−Plus (Amersham) and X-Ray film (Thermo-Scientific) or by Odyssey Li-Cor protocol.
Statistical Analyses
Pairwise comparisons were evaluated by a Student's 2-tailed t-test using Microsoft Excel. The GI50 values and Pearson's correlation were evaluated using GraphPad Prism.
Combination indices
Combination indices were computed with Calcusyn software (Biosoft) using the Chou-Talalay method.
Results
ONC201 induces cytotoxic responses in pediatric NHL cell lines
We selected a panel of human lymphoma cell lines that were primarily derived from pediatric patients, including Burkitt's lymphoma and the Karpas299 T-cell lymphoma cell lines (Table S1) to assess in vitro sensitivity to ONC201. In parallel, we examined mantle cell lymphoma as another indication for comparison. Cell viability assays at 72 hours post-treatment with ONC201 demonstrated dose-response curves in this panel that tended to saturate beyond 5 μM (Fig. 1). The GI50 among this panel ranged from 1.3 to 5.1 μM, which is comparable to reported activity of ONC201 in other tumor types.22
Figure 1.
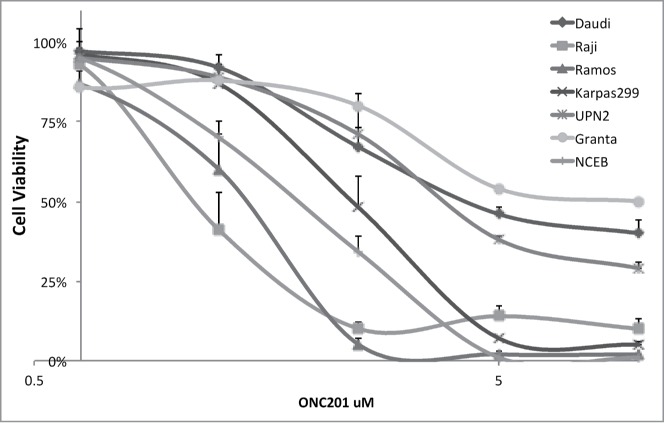
ONC201 induces a dose-dependent reduction in the cell viability of human lymphoma cell lines. Cell viability assays with ONC201 for 72 hours treatment.
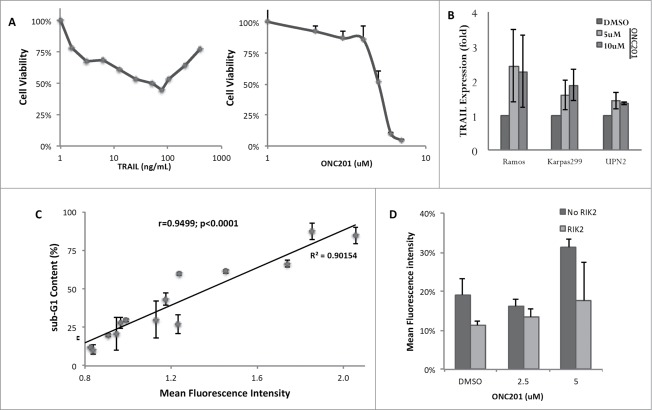
To further understand the observed activity of ONC201, we performed sub-G1 analysis by flow cytometry that revealed significant levels of apoptosis at 72 hours post-treatment (Fig. 2A). The Ramos and Karpas299 pediatric cell lines exhibited the strongest levels of apoptosis under the evaluated conditions. The other cell lines that exhibited weaker levels of cell death likely undergo a cytostatic response based on their responsiveness in cell viability assays (e.g. NCEB cells). In general, the induction of cell death was dose-dependent but saturated at 5 μM for some cell lines such as Ramos and Karpas299. Caspase-mediated apoptosis was confirmed by reduced sub-G1 DNA content that resulted from co-incubation with the pan-caspase inhibitor zVAD-fmk (Fig. 2B).
Figure 2.
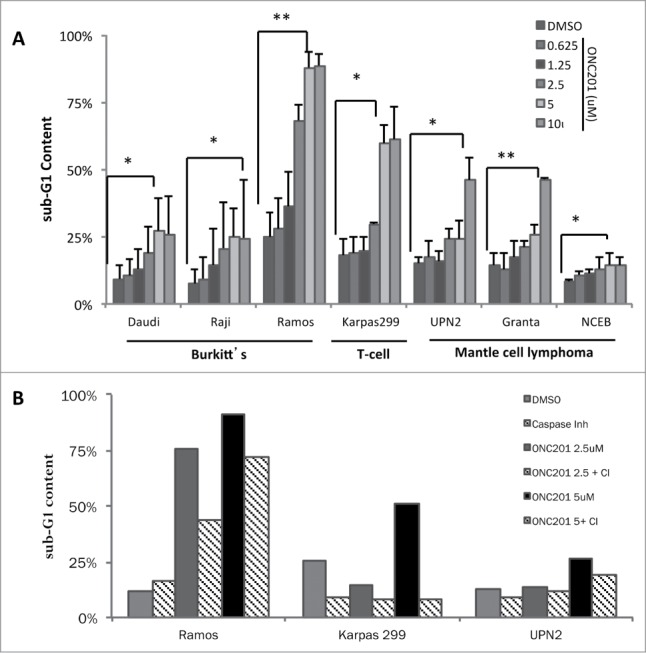
ONC201 induces caspase-dependent apoptosis in human lymphoma cell lines. (A) Sub-G1 DNA content analysis lymphoma cell lines treated with ONC201 at 0.625, 1.25, 2.5, 5 and 10 μM for 72 hours (n = 3). *P < 0.05; **P < 0.005. (B) Sub-G1 DNA content analysis of lymphoma cell lines in the presence or absence of the pan-caspase inhibitor zVAD-fmk (CI) and/or ONC201 for 72 hours.
The TRAIL pathway is induced by ONC201
Due to the prior demonstration of the TRAIL pathway as a critical component of the cytotoxic response to ONC201, we compared the activity of recombinant TRAIL to that of ONC201. Interestingly, BJAB cells were moderately responsive to recombinant TRAIL but were strongly responsive to ONC201 (Fig. 3A). Flow cytometry analysis revealed that pediatric lymphoma cell lines upregulate TRAIL expression on their cell surface in response to ONC201 (Fig. 3B). We noticed that the saturation of TRAIL induction in these experiments occurred at the same doses where efficacy was saturated in cell viability and cell death assays. Further investigation of this relationship revealed that the induction of TRAIL protein correlates linearly with induction of cell death across the various evaluated lymphoma cell lines and doses (Fig. 3C).
Figure 3.
ONC201 induces the TRAIL pathway in human lymphoma cell lines. (A) Dose-response curve of BJAB cells to recombinant TRAIL or ONC201 at 72 hours post-treatment (n = 3). (B) Fold TRAIL expression of lymphoma cell lines at 60 hours post treatment with ONC201 (n = 3). (C) Correlation of surface TRAIL expression by flow cytometry with sub-G1 DNA content of Ramos, Karpas299, and UPN2 lymphoma cells treated with several doses of ONC201 (0.625, 1.25, 2.5, 5, and 10 μM, n = 3). The Pearson correlation coefficient “r” was 0.9499 with 95% confidence interval of 0.8526 to 0.9836 and a P value of <0.0001. (D) Reduction of cell death by co-incubation with RIK2, a TRAIL-sequestering antibody in Karpas299 cells with 72 hour treatment with ONC201 (n = 2).
To further investigate activation of the TRAIL pathway by ONC201 and potentially explain its strong cytotoxicity, we examined expression levels of DR5 that is a proapoptotic receptor for TRAIL previously reported to be co-induced by ONC201 along with its ligand.22 In agreement with the prior findings, an increase in surface DR5 expression in response to ONC201 was noted in a dose-dependent manner (Fig. S1A, B). Co-administration of a TRAIL-sequestering antibody reduced induction of cell death, which is indicative of at least a partial role for activation of the TRAIL pathway in ONC201-induced cell death of these pediatric cancer cells (Fig. 3D).
ONC201 cooperates with several pediatric lymphoma chemotherapies
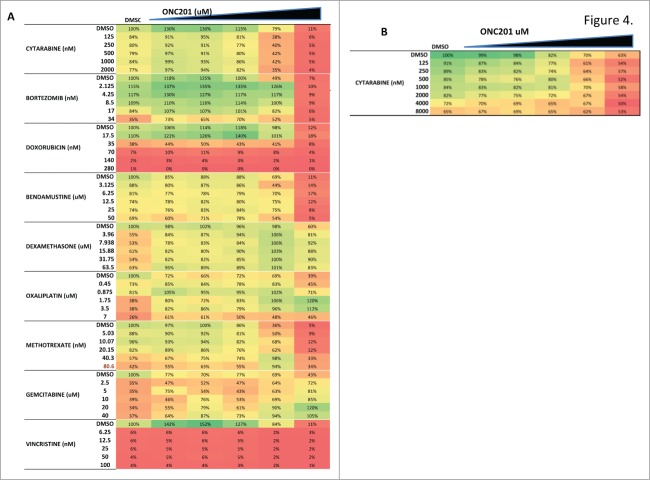
Preclinical studies in adult cancer models support combining TRAIL-based agents with various chemotherapeutic agents. We recently reported that ONC201 exhibits synergy with several approved targeted agents and chemotherapies that range from multikinase inhibitors, alkylating agents and inhibitors of DNA/nucleoside synthesis.27 We specifically examined the combinatorial efficacy of ONC201 with chemotherapies used to treat pediatric lymphoma (Fig. 4A). While enhanced efficacy was noted with bortezomib, doxorubicin, and bendamustine, ONC201 combined with cytarabine was found to synergistically reduce cell viability as confirmed objectively by combination indices (Table 1).
Figure 4.
ONC201-induced cytotoxicity is enhanced in combination with pediatric NHL chemotherapy. (A) Cell viability assays of Ramos cells following 72 hour treatment with various doses of ONC201 in combination with increasing doses of NHL chemotherapies. 100% cell viability (100%) is represented as darkest green and lowest cell viability (approaching zero) is represented as darkest red. ONC201 doses used were 0.093, 0.1875, 0.375, 0.75, 1.5 μM and n = 2. (B) Cell viability assays of Karpas299 cells following 72 hour treatment with various doses of ONC201 (0.156, 0.3125, 0.625,1.25, 2.5 μM) in combination with increasing doses of cytarabine (n = 2).
Table 1.
Combination indices for ONC201 in combination with cytarabine
| Cytarabine(nM) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Karpas299 | 31.25 | 62.5 | 125 | 250 | 500 | 1000 | 2000 | ||
| ONC201 (uM) | 0.3125 | 0.55 | 0.76 | 0.41 | 0.38 | 0.40 | 1.03 | 1.01 | |
| 0.625 | 0.54 | 0.64 | 0.46 | 0.50 | 0.46 | 1.12 | 1.01 | ||
| 1.25 | 0.58 | 0.79 | 0.57 | 0.57 | 0.92 | 1.25 | 1.03 | ||
| 2.5 | 0.70 | 0.64 | 0.66 | 0.72 | 0.83 | 1.10 | 1.13 | ||
Combination indices obtained for Karpas299 cells at 72H post combination treatment with increasing doses of ONC201 combined with increasing doses of cytarabine confirm synergistic cytotoxicity.
Discussion
In this study, we show that ONC201 is an effective anti-cancer agent against NHL cell lines in vitro as a monoagent and in combination with chemotherapies. The significant levels of reduction in cell viability and induction of apoptosis were observed at doses that are achievable in mice22 and in other species in GLP toxicology studies. The fact that ONC201 was effective in both B-cell and T-cell NHL cell lines is in accordance with its broad-spectrum activity that bodes well for its scalability into several indications in the clinic, including pediatric lymphoma. The Ramos pediatric Burkitt's lymphoma cell line exhibited the highest degree of sensitivity to ONC201 in vitro, which is interesting given that Burkitt's lymphoma is myc-driven28 and that c-myc has been linked to multiple aspects of the mechanism of ONC201 such as TRAIL29,30 and Ras signaling.31
Pediatric lymphomas remain in need of improved therapies for children and adolescents with advanced-stage disease and for those who relapse after conventional therapy. In pediatric lymphomas, the need for safer therapies is particularly underscored by the incidence of secondary neoplasms caused by the available chemotherapies and radiation that are genotoxic.24,25 Thus the lack of toxicity, which includes lack of genotoxicity,32 that accompanies the efficacy of ONC201 is particularly important for pediatric populations that have a longer post-therapy life spans where secondary neoplasms can occur.
The observation that the TRAIL pathway mediates ONC201-induced apoptosis in Karpas 299 cells supports the previously reported contribution of TRAIL to the apoptotic activity of ONC201.22,23 Accordingly, surface TRAIL expression was correlated linearly with the apoptotic activity of ONC201. Since surface TRAIL protein is cleaved to form a soluble ligand, assaying serum levels in addition to tumor cell expression of TRAIL is suggested for pharmacodynamic studies in ongoing clinical trials of ONC201.
ONC201 is a first-in-class compound with a unique pharmacophore33 and associated novel mechanism of action. The mechanism of ONC201 remains an area of active study, particularly the upstream signaling mechanisms that trigger its late anti-cancer effects that appear to involve both cytostatic and cytotoxic phenotypes in a cell-type dependent manner. The apparent involvement of cytostatic mechanisms is evident in cells that respond strongly in cell viability assays to ONC201, but do not undergo apoptosis, such as the NCEB cell line evaluated in this study. Thus, the robust anti-cancer activity of ONC201 appears to be a function of both TRAIL-dependent and independent anti-cancer mechanisms that differentially manifest in a cancer cell type-dependent manner but appear to be well tolerated by normal cells.
A recent preclinical study evaluated a panel of FDA-approved anti-cancer small molecules in solid tumors to reveal that ONC201 exhibits a broad synergistic profile.27 This study investigated a subset of approved small molecules that were curated for NHL relevance. Our limited combinatorial study identified cytarabine as a synergistic combination with ONC201 in pediatric NHL. Synergy between cytarabine and TRAIL, thought to be driven by cytarabine-mediated DR5 induction, has been previously documented in preclinical hematological malignancies.34 While the combination of ONC201 with cytarabine should be further studied mechanistically and in vivo, the combination could be translated in the clinic.
In summary, this preclinical study demonstrates that ONC201 induces dose- and caspase-dependent apoptosis and activates the TRAIL pathway at low micromolar concentrations in pediatric NHL cell lines. While TRAIL-mediated apoptosis appears to play a part in the cytotoxic activity of ONC201, further studies are needed to explain its full spectrum of activity that includes non-apoptotic effects. These preclinical studies bode well for the monoagent activity of ONC201 in clinical trials in lymphoma. The support for TRAIL as a response-dependent biomarker of ONC201 as well as the identification of cytarabine as a synergistic combination provide additional approaches to realize the therapeutic potential of this first-in-class compound. Furthermore, the demonstration of preclinical efficacy with ONC201 in pediatric NHL provides the rationale for clinical investigation of this novel agent in a patient population where its pronounced safety is highly pertinent.
Disclosure of Potential Conflicts of Interest
Joshua Allen is an employee and stockholder of Oncoceutics, Inc. and Wafik El-Deiry is a Founder and stockholder of Oncoceutics, Inc.
Funding
This work was supported by grants to WSE-D. WSE-D. is an American Cancer Society Research Professor.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Vardiman JW. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: an overview with emphasis on the myeloid neoplasms. Chem Biol Interact 2010; 184:16-20; PMID:19857474; http://dx.doi.org/ 10.1016/j.cbi.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 2.Ries LA, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, Vernon SW, Cronin K, Edwards BK. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer 2000; 88:2398-424; PMID:10820364; http://dx.doi.org/ 10.1002/(SICI)1097-0142(20000515)88:10%3c2398::AID-CNCR26%3e3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- 3.Sandlund JT, Downing JR, Crist WM. Non-Hodgkin's lymphoma in childhood. N Engl J Med 1996; 334:1238-48; PMID:8606720; http://dx.doi.org/ 10.1056/NEJM199605093341906 [DOI] [PubMed] [Google Scholar]
- 4.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol 2002; 168:1356-61; http://dx.doi.org/ 10.4049/jimmunol.168.3.1356 [DOI] [PubMed] [Google Scholar]
- 5.Falschlehner C, Schaefer U, Walczak H. Following TRAIL's path in the immune system. Immunology 2009; 127:145-54; PMID:19476510; http://dx.doi.org/ 10.1111/j.1365-2567.2009.03058.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowder RN, El-Deiry WS. Caspase-8 regulation of TRAIL-mediated cell death. Exp Oncol 2012; 34:160-4; PMID:23070000 [PubMed] [Google Scholar]
- 7.Georgakis GV, Li Y, Humphreys R, Andreeff M, O'Brien S, Younes M, Carbone A, Albert V, Younes A. Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death. Br J Haematol 2005; 130:501-10; PMID:16098063; http://dx.doi.org/ 10.1111/j.1365-2141.2005.05656.x [DOI] [PubMed] [Google Scholar]
- 8.Smith MA, Morton CL, Kolb EA, Gorlick R, Keir ST, Carol H, Lock R, Kang MH, Reynolds CP, Maris JM, et al.. Initial testing (stage 1) of mapatumumab (HGS-ETR1) by the pediatric preclinical testing program. Pediatr Blood Cancer 2010; 54:307-10; PMID:19856388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003; 22:8628-33; PMID:14634624; http://dx.doi.org/ 10.1038/sj.onc.1207232 [DOI] [PubMed] [Google Scholar]
- 10.Fox NL, Humphreys R, Luster TA, Klein J, Gallant G. Tumor Necrosis Factor-related apoptosis-inducing ligand (TRAIL) Receptor-1 and Receptor-2 agonists for cancer therapy. Expert Opin Biol Ther 2010; 10:1-18; PMID:19857186; http://dx.doi.org/ 10.1517/14712590903319656 [DOI] [PubMed] [Google Scholar]
- 11.Abdulghani J, El-Deiry WS. TRAIL receptor signaling and therapeutics. Exp Opin Ther Targets 2010; 14:1091-108; PMID:20819019; http://dx.doi.org/ 10.1517/14728222.2010.519701 [DOI] [PubMed] [Google Scholar]
- 12.Daniel D, Yang B, Lawrence DA, Totpal K, Balter I, Lee WP, Gogineni A, Cole MJ, Yee SF, Ross S, et al.. Cooperation of the proapoptotic receptor agonist rhApo2L/TRAIL with the CD20 antibody rituximab against non-Hodgkin lymphoma xenografts. Blood 2007; 110:4037-46; PMID:17724141; http://dx.doi.org/ 10.1182/blood-2007-02-076075 [DOI] [PubMed] [Google Scholar]
- 13.Mouzakiti A, Packham G. Regulation of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Burkitt's lymphoma cell lines. Br J Haematol 2003; 122:61-9; PMID:12823346; http://dx.doi.org/ 10.1046/j.1365-2141.2003.04424.x [DOI] [PubMed] [Google Scholar]
- 14.Hussain A, Doucet JP, Gutierrez M, Ahmad M, Al-Hussein K, Capello D, Gaidano G, Bhatia K. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas apoptosis in Burkitt's lymphomas with loss of multiple pro-apoptotic proteins. Haematologica 2003; 88:167-75; PMID:12604406 [PubMed] [Google Scholar]
- 15.Ucur E, Mattern J, Wenger T, Okouoyo S, Schroth A, Debatin KM, Herr I. Induction of apoptosis in experimental human B cell lymphomas by conditional TRAIL-expressing T cells. Br J Cancer 2003; 89:2155-62; PMID:14647152; http://dx.doi.org/ 10.1038/sj.bjc.6601407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasparini C, Vecchi Brumatti L, Monasta L, Zauli G. TRAIL-based therapeutic approaches for the treatment of pediatric malignancies. Curr Med Chem 2013; 20:2254-71; PMID:23458616; http://dx.doi.org/ 10.2174/0929867311320170009 [DOI] [PubMed] [Google Scholar]
- 17.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, et al.. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med 2007; 13:1070-7; PMID:17767167; http://dx.doi.org/ 10.1038/nm1627 [DOI] [PubMed] [Google Scholar]
- 18.Stern HM, Padilla M, Wagner K, Amler L, Ashkenazi A. Development of immunohistochemistry assays to assess GALNT14 and FUT3/6 in clinical trials of dulanermin and drozitumab. Clin Cancer Res 2010; 16:1587-96; PMID:20179215; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-3108 [DOI] [PubMed] [Google Scholar]
- 19.Travert M, Ame-Thomas P, Pangault C, Morizot A, Micheau O, Semana G, Lamy T, Fest T, Tarte K, Guillaudeux T. CD40 ligand protects from TRAIL-induced apoptosis in follicular lymphomas through NF-kappaB activation and up-regulation of c-FLIP and Bcl−xL. J Immunol 2008; 181:1001-11; http://dx.doi.org/ 10.4049/jimmunol.181.2.1001 [DOI] [PubMed] [Google Scholar]
- 20.Gallouet AS, Travert M, Bresson-Bepoldin L, Guilloton F, Pangault C, Caulet-Maugendre S, Lamy T, Tarte K, Guillaudeux T. COX-2-independent effects of celecoxib sensitize lymphoma B cells to TRAIL-mediated apoptosis. Clin Cancer Res 2014; 20:2663-73; PMID:24637636; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2305 [DOI] [PubMed] [Google Scholar]
- 21.Allen JE, Ferrini R, Dicker DT, Batzer G, Chen E, Oltean DI, Lin B, Renshaw MW, Kretz-Rommel A, El-Deiry WS. Targeting TRAIL death receptor 4 with trivalent DR4 Atrimer complexes. Mol Cancer Ther 2012; 11:2087-95; PMID:22802267; http://dx.doi.org/ 10.1158/1535-7163.MCT-12-0366 [DOI] [PubMed] [Google Scholar]
- 22.Allen JE, Krigsfeld G, Mayes PA, Patel L, Dicker DT, Patel AS, Dolloff NG, Messaris E, Scata KA, Wang W, et al.. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med 2013; 5:171ra17; PMID:23390247; http://dx.doi.org/ 10.1126/scitranslmed.3004828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prabhu VV, Allen JE, Dicker DT, El-Deiry WS. Small molecule ONC201/TIC10 targets chemotherapy-resistant colorectal cancer stem-like cells in an Akt/Foxo3a/TRAIL-dependent manner. Cancer Res 2015; 75(7):1423-32; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chargari C, Cosset JM. [The issue of low doses in radiation therapy and impact on radiation-induced secondary malignancies]. Bull Cancer 2013; 100:1333-42; PMID:24257106 [DOI] [PubMed] [Google Scholar]
- 25.Henry M, Savasan S. Controversies in the role of radiotherapy in the treatment of pediatric Hodgkin lymphoma. Ind J Pediatr 2013; 80:863-9; PMID:23975267; http://dx.doi.org/ 10.1007/s12098-013-1106-8 [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Kim K, Kwagh JG, Dicker DT, Herlyn M, Rustgi AK, Chen Y, El-Deiry WS. Death induction by recombinant native TRAIL and its prevention by a caspase 9 inhibitor in primary human esophageal epithelial cells. J Biol Chem 2004; 279:40044-52; PMID:15226295; http://dx.doi.org/ 10.1074/jbc.M404541200 [DOI] [PubMed] [Google Scholar]
- 27.Allen JE, Prabhu VV, Talekar M, van den Heuvel P, Lim B, Dicker DT, Fritz JL, Beck A, El-Deiry WS. Genetic and pharmacological screens converge in identifying FLIP, BCL2 and IAP proteins as key regulators of sensitivity to the TRAIL-inducing anti-cancer agent ONC201/TIC10. Cancer Res 2015; 75(8):1668-74; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boxer LM, Dang CV. Translocations involving c-myc and c-myc function. Oncogene 2001; 20:5595-610; PMID:11607812; http://dx.doi.org/ 10.1038/sj.onc.1204595 [DOI] [PubMed] [Google Scholar]
- 29.Klefstrom J, Verschuren EW, Evan G. c-Myc augments the apoptotic activity of cytosolic death receptor signaling proteins by engaging the mitochondrial apoptotic pathway. J Biol Chem 2002; 277:43224-32; PMID:12202489; http://dx.doi.org/ 10.1074/jbc.M206967200 [DOI] [PubMed] [Google Scholar]
- 30.Ricci MS, Jin Z, Dews M, Yu D, Thomas-Tikhonenko A, Dicker DT, El-Deiry WS. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol 2004; 24:8541-55; PMID:15367674; http://dx.doi.org/ 10.1128/MCB.24.19.8541-8555.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Devel 2000; 14:2501-14; PMID:11018017; http://dx.doi.org/ 10.1101/gad.836800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishizawa J, Kojima K, Chachad D, Ruvolo PP, Ruvolo VR, Jacamo R, Dilip A, Mu H, Zeng Z, Matre P, et al.. ONC201 Induces p53-Independent Apoptosis and Cell Cycle Arrest in Hematological Malignancies and Leukemic Stem/Progenitor Cells By Inducing ER Stress and mTOR Inhibition. 2014 [Google Scholar]
- 33.Wagner J, Kline CL, Pottorf RS, Nallaganchu BR, Olson GL, Dicker DT, Allen JE, El-Deiry WS. The angular structure of ONC201, a TRAIL pathway-inducing compound, determines its potent anti-cancer activity. Oncotarget 2014; 5:12728-37; PMID:25587031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao J, Zhang RY, Chen Y, Chen GA, Xiao CJ. [Combined effect of TNF-related apoptosis induced ligand and Ara-C in inducing apoptosis of HL-60 cells and its mechanism]. Zhongguo Shi Yan Xue Ye Xue Za Xhi 2008; 16:510-5 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.