Human immunodeficiency virus-infected women with a normal Pap result who nonetheless test positive for human papillomavirus (HPV) type 16 have a high risk of cervical precancer that may warrant immediate colposcopy, whereas those positive for other oncogenic HPV types are at moderate risk.
Keywords: HIV, human papillomavirus, cervical cancer screening, Pap test
Abstract
Background. Determining cervical precancer risk among human immunodeficiency virus (HIV)–infected women who despite a normal Pap test are positive for oncogenic human papillomavirus (oncHPV) types is important for setting screening practices.
Methods. A total of 2791 HIV-infected and 975 HIV-uninfected women in the Women's Interagency HIV Study were followed semiannually with Pap tests and colposcopy. Cumulative risks of cervical intraepithelial neoplasia grade 2 or greater (CIN-2+; threshold used for CIN treatment) and grade 3 or greater (CIN-3+; threshold to set screening practices) were measured in HIV-infected and HIV-uninfected women with normal Pap tests, stratified by baseline HPV results, and also in HIV-infected women with a low-grade squamous intraepithelial lesion (LSIL; benchmark indication for colposcopy).
Results. At baseline, 1021 HIV-infected and 518 HIV-uninfected women had normal Pap tests, of whom 154 (15%) and 27 (5%), respectively, tested oncHPV positive. The 5-year CIN-2+ cumulative risk in the HIV-infected oncHPV-positive women was 22% (95% confidence interval [CI], 9%–34%), 12% (95% CI, 0%–22%), and 14% (95% CI, 2%–25%) among those with CD4 counts <350, 350–499, and ≥500 cells/µL, respectively, whereas it was 10% (95% CI, 0%–21%) in those without HIV. For CIN-3+, the cumulative risk averaged 4% (95% CI, 1%–8%) in HIV-infected oncHPV-positive women, and 10% (95% CI, 0%–23%) among those positive for HPV type 16. In HIV-infected women with LSIL, CIN-3+ risk was 7% (95% CI, 3%–11%). In multivariate analysis, HIV-infected HPV16-positive women had 13-fold (P = .001) greater CIN-3+ risk than oncHPV-negative women (referent), and HIV-infected women with LSIL had 9-fold (P < .0001) greater risk.
Conclusions. HIV-infected women with a normal Pap result who test HPV16 positive have high precancer risk (similar to those with LSIL), possibly warranting immediate colposcopy. Repeat screening in 1 year may be appropriate if non-16 oncHPV is detected.
Clinical guidelines for cervical cancer screening in the general population have undergone significant changes in the past several years. In particular, testing for oncogenic human papillomavirus (oncHPV), the central etiologic agent in most cervical tumors, has been increasingly incorporated into routine screening practices. Current US guidelines endorse concurrent Pap and oncHPV testing (“co-testing”) for women aged 30 years and older [1], with repeat screening recommended in 5 years if both tests are negative. These guidelines reflect the strong 5-year negative predictive value of a negative co-test in screening for cervical cancer and precancer [2]. Conversely, if the Pap result is normal but the oncHPV test is positive, then screening is to be repeated in 12 months, and if genotyping specifically detects either HPV type 16 or 18 (which account for approximately 50% and 10% of cervical cancers, respectively), immediate colposcopy is recommended [1]. In April 2014, the US Food and Drug Administration additionally approved an oncHPV assay for use as an alternative to the Pap test in primary screening among women in the general population aged ≥25 years [3, 4].
Women with human immunodeficiency virus (HIV) infection are at several-fold greater risk of cervical cancer than those in the general population [5–7], as well as several-fold greater risk of high-grade cervical intraepithelial neoplasia (CIN-2+ and CIN-3+) [8, 9]. Nonetheless, a recent study in the Women's Interagency HIV Study (WIHS), a large prospective cohort of HIV-infected and HIV-uninfected women, reported that HIV-infected women with normal Pap tests who additionally co-tested negative for oncHPV have a similar low 5-year risk of CIN-2+ and CIN-3+ as those who are HIV uninfected [10]. Under the principle of “equal management for equal risk,” agreed to at a 2012 US consensus conference [11–13], these data suggested that similar screening practices could be used to co-test negative women regardless of HIV status.
However, that prior WIHS study did not examine CIN-2+ and CIN-3+ risk among the subset of HIV-infected women with normal Pap tests who co-tested positive (not negative) for oncHPV. In addition, data in HIV-infected women are needed to more specifically assess risk in those with HPV16 or HPV18 despite a normal Pap, and it is important to concomitantly study HIV-infected women with low-grade squamous intraepithelial lesions (LSILs) by Pap test. The 2012 consensus conference suggested that LSIL be considered a benchmark indication for immediate colposcopy, and the similarity in CIN-3+ risk (ie, >5%) between women with LSIL and women with a normal Pap who were positive for HPV16 or HPV18 was a major reason that detection of either HPV type was named an additional indication for immediate colposcopy in the general population [12].
To have adequate data to address these issues, the current study used results from 2 WIHS enrollment cohorts, including women recruited during 1994–1995, and those recruited during 2001–2002 (who were participants in the WIHS study mentioned above). We then contrasted the risks of CIN-2+ and CIN-3+ in HIV-infected women with a normal Pap who tested positive for oncHPV (particularly HPV16 and HPV18), those without oncHPV, and HIV-infected women with LSIL [14].
METHODS
Study Participants
The WIHS is an ongoing prospective study of HIV-infected and at-risk HIV-uninfected women enrolled through similar sources at 6 sites (Bronx and Brooklyn, New York; Chicago, Illinois; Los Angeles and San Francisco, California; Washington, District of Columbia). Enrollment was initially conducted between October 1994 and November 1995 (n = 2054 HIV infected, n = 569 HIV uninfected), and a second cohort was similarly enrolled between October 2001 and September 2002 (n = 737 HIV infected, n = 406 HIV uninfected). Details of the WIHS data collection and recruitment methods have been reported [15]. In brief, participants undergo a semiannual visit that involves a gynecologic examination with specimen collection, including a Pap test and cervicovaginal lavage for HPV DNA testing. All cervical cytology was centrally interpreted using the 1991 or 2001 Bethesda System criteria for cytologic diagnosis [16, 17]. Colposcopy was recommended for all Pap results of atypical squamous cells of undetermined significance or worse (ASC-US+). Written informed consent was obtained from all participants, and the study was approved by each local institutional review board.
Laboratory Testing
HPV DNA detection methods have been described in detail elsewhere [8, 18]. In brief, HPV DNA was detected using a well-established degenerate primer MY09/MY11/HMBO1 polymerase chain reaction (PCR) assay. Primer set PC04/GH20, which amplifies a cellular β-globin DNA fragment, was used as an internal control to assess the adequacy of amplification. The amplification products were then probed for the presence of “any HPV” DNA with a generic probe mixture, and probed for individual HPV types using filters hybridized with type-specific biotinylated oligonucleotides for >40 individual HPV DNA types. β-globin–negative specimens were excluded. Consistent with recommendations from the International Agency for Research on Cancer, HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 were considered oncogenic, and all other types were considered nononcogenic [19, 20].
Statistical Methods
The analysis included HIV-infected and HIV-uninfected women who had a normal Pap result or LSIL at their baseline WIHS enrollment visit (during either 1994–1995 or 2001–2002). Descriptive statistics were used to examine the baseline characteristics of these participants, stratified by HIV status, and compared using the t test (to assess means), Wilcoxon test (for medians), or Pearson χ2 test (for proportions). Standard life-table methods were used to estimate the cumulative risk of CIN-2+ and CIN-3+, with 95% confidence intervals (CIs) calculated based on the life-table estimator under a normal approximation assumption. Participants who had a hysterectomy or who reported having had treatment for CIN were censored at the visit before the procedure. The log-rank test was used to compare the cumulative risk of CIN-2+ and CIN-3+ in HIV-infected women with a CD4 count <350, 350–499, or ≥500 cells/μL, as well as HIV-uninfected women, according to their HPV DNA results at baseline. HIV-infected women with LSIL were studied similarly.
Multivariable Cox models were conducted to study the associations of CIN-2+ and CIN-3+ with detection of any oncHPV, HPV16, HPV18, and non-16/18 oncHPV at baseline, after adjusting for other established cervical risk factors. These covariates were a priori parameterized as in prior WIHS studies [9, 10, 21], except for age, which was treated as a continuous variable to preserve degrees of freedom. Cox models of women with LSIL at baseline included prevalent cases of CIN-2+ and CIN-3+, as WIHS participants with LSIL were by protocol referred for immediate colposcopy; that is, baseline CIN-2+ or CIN-3+ is an important component of the overall cumulative cervical precancer risk in women with LSIL in this study [22, 23]. The lowest detectable HIV RNA level changed over calendar time as the assay sensitivity improved. To simplify modeling, we therefore used the HIV RNA detection threshold from early in the WIHS, ≤4000 copies/mL, as this could be used as the referent across all women at all visits [22, 23]. In secondary analysis, we included nonparametric splines in our Cox models to assess whether incorporating time-varying effects meaningfully impacted the results [24, 25]. Statistical significance was defined as P < .05 determined using 2-sided tests.
RESULTS
There were 1727 HIV-infected and 806 HIV-uninfected women with a normal cervical Pap at enrollment. Women were excluded from analysis if (1) their baseline HPV or CD4 cell count data were unavailable (n = 198 HIV-infected and n = 66 HIV-uninfected women); (2) hysterectomy was performed prior to enrollment (n = 129 and n = 37); (3) follow-up data were unavailable (n = 115 and n = 71); or (4) HIV seroconversion occurred during follow-up (n = 9). Among the remaining 1285 HIV-infected and 623 HIV-uninfected women, 290 were not compliant with colposcopy (n = 206 and n = 84), and 79 were excluded due to self-reported cervical treatment prior to baseline (n = 58 and n = 21).
Overall, 1021 HIV-infected and 518 HIV-uninfected women were included in the analysis of CIN-2+ and CIN-3+ cumulative risk. Table 1 shows selected baseline characteristics of these women. Although HIV-infected women reported less recent sexual activity, they were more likely than HIV-uninfected women to test positive for any HPV DNA (37% vs 19%; P < .0001). OncHPV was detected in 154 (15%) HIV-infected and 27 (5%) HIV-uninfected women (P < .0001) with normal Pap tests. For HPV16 and HPV18 the prevalence was 2% and 1%, respectively, among the HIV-infected women, whereas it was 1% each among the HIV-uninfected women. The women with HIV were older and less likely than HIV-uninfected women to be current smokers. Sixty-seven percent of the HIV-infected women had a CD4 count ≥350 cells/μL, and 16% (ie, half of those recruited during 2001–2002) reported highly active antiretroviral therapy (HAART) use at baseline. The length of follow-up averaged 14 person-visits, including a median of 13 person-visits in HIV-infected women and 14 person-visits in HIV-uninfected women—a total of 14 415 and 7382 person-visits of data, respectively.
Table 1.
Baseline Characteristics of Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Women Who Had a Normal Pap Result at Enrollment in the Women's Interagency HIV Study
| Baseline Characteristic | HIV-Infected (n = 1021) | HIV-Uninfected (n = 518) | P Valuea |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 35 (8) | 32 (8) | <.0001 |
| Race/ethnicity, No. (%) | .59 | ||
| Black | 535 (52) | 271 (52) | |
| Hispanic | 291 (29) | 146 (28) | |
| White | 165 (16) | 79 (15) | |
| Other | 30 (3) | 22 (4) | |
| Enrollment period, No. (%) | <.0001 | ||
| 1994–1995 | 683 (67) | 277 (53) | |
| 2001–2002 | 338 (33) | 241 (47) | |
| Smoking, No. (%) | .001 | ||
| Never | 382 (38) | 158 (31) | |
| Former | 166 (16) | 68 (13) | |
| Current | 470 (46) | 292 (56) | |
| Sexually active in the last 6 mo, No. (%) | <.0001 | ||
| Yes | 714 (70) | 422 (82) | |
| No | 300 (30) | 95 (18) | |
| Lifetime No. of sexual partners, No. (%) | .003 | ||
| <5 | 261 (26) | 97 (19) | |
| 5–9 | 205 (20) | 118 (23) | |
| 10–49 | 319 (32) | 199 (39) | |
| ≥50 | 219 (22) | 100 (19) | |
| HPV DNA test results, No. (%) | <.0001 | ||
| Negative | 646 (63) | 416 (80) | |
| Nononcogenic | 221 (22) | 75 (14) | |
| Any oncogenic | 154 (15) | 27 (5) | |
| HPV16b | 24 (2) | 3 (1) | |
| HPV18b | 14 (1) | 4 (1) | |
| CD4 count, cells/µL, No. (%) | |||
| <200 | 151 (15) | ||
| 200–349 | 188 (18) | ||
| 350–499 | 245 (24) | ||
| ≥500 | 437 (43) | ||
| HIV RNA, copies/mL, No. (%) | |||
| ≤4000c | 442 (44) | ||
| 4001–20 000 | 228 (23) | ||
| 20 001–100 000 | 214 (21) | ||
| >100 000 | 121 (12) | ||
| HAART use in past 6 mo, No. (%) | |||
| Yes | 161 (16) | ||
| No | 860 (84) | ||
Abbreviations: HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; HPV, human papillomavirus; SD, standard deviation.
a Based on 2-sided t test (means), Wilcoxon test (medians), or Pearson χ2 test (proportions) comparing HIV-infected and HIV-uninfected women.
b Two HIV-infected women were positive for both HPV16 and HPV18 at baseline.
c The lowest detectable HIV RNA level changed over time as assay sensitivity improved. To simplify modeling, we used the detection threshold from early in the study (ie, ≤4000 copies/mL) as this could be used as referent for all women at all visits.
Cumulative Risk of CIN-2+ and CIN-3+
Any Oncogenic HPV
CIN-2+
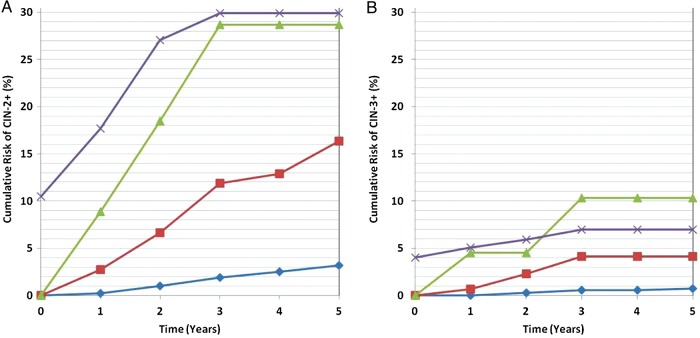
HIV-infected women who had a normal Pap and were oncHPV negative (n = 867) had a total of 22 CIN-2+ cases during 5 years of follow-up. More specifically, the cumulative risk was 2% (95% CI, 0%–5%) in HIV-infected women with a CD4 count <350 cells/µL, 4% (95% CI, 1%–6%) with a CD4 count of 350–499 cells/µL, 3% (95% CI, 1%–5%) with a CD4 count ≥500 cells/µL, and 2% (95% CI, 1%–3%) in HIV-uninfected women (n = 491). Among women with a normal Pap result who were oncHPV positive (n = 154 HIV-infected and n = 27 HIV-uninfected), the 5-year cumulative risk of CIN-2+ was 22% (95% CI, 9%–34%) in HIV-infected individuals with a CD4 count <350 cells/µL, 12% (95% CI, 0%–22%) with a CD4 count 350–499 cells/µL, and 14% (95% CI, 2%–25%) with a CD4 count ≥500 cells/µL—an overall cumulative risk of 16% (95% CI, 9%–23%) among oncHPV-positive, HIV-infected women (Figure 1A). The CIN-2+ risk was 10% (95% CI, 0%–21%) in oncHPV-positive, HIV-uninfected women.
Figure 1.
Cumulative risk of cervical intraepithelial neoplasia grade 2 or greater (CIN-2+; A) and grade 3 or greater (CIN-3+; B), among human immunodeficiency virus-infected women who at baseline had a normal Pap result with no oncogenic human papillomavirus (HPV) (blue diamonds), any oncogenic HPV (red squares), HPV type 16 (green triangles), or had a baseline Pap diagnosed as low-grade squamous intraepithelial lesion (LSIL) (purple crosses). The cumulative risk for women with LSIL includes both prevalent and incident cases, as LSIL is an indication for immediate colposcopy, whereas for all other groups the data reflect cumulative incidence.
CIN-3+
No cases of CIN-3+ occurred in HIV-uninfected women during 5 years of follow-up. In HIV-infected women with a normal Pap result who were oncHPV negative, 9 were CIN-3+ cases, a cumulative risk of ≤1% in each CD4 stratum. Among the oncHPV-positive women, there was a total of 5 CIN-3+ cases, with a 5-year cumulative risk of 7% (95% CI, 0%–15%) in HIV-infected women with a CD4 count <350 cells/µL, 2% (95% CI, 0%–7%) with a CD4 count 350–499 cells/µL, and 2% (95% CI, 0%–7%) with a CD4 count ≥500 cells/µL—an overall cumulative risk of 4% (95% CI, 0%–8%) among oncHPV-positive, HIV-infected women (Figure 1B).
HPV16/18 and LSIL
As shown in Figure 1A, HIV-infected women with a normal Pap result who were HPV16 positive had a 3-year cumulative risk of CIN-2+ of 29% (95% CI, 6%–46%) with no additional cases detected between 3–5 years, whereas those with non-16 oncHPV (not shown) had a 3-year and 5-year cumulative incidence of 9% (95% CI, 3%–14%) and 14% (95% CI, 7%–21%), respectively. The CIN-3+ cumulative risk (Figure 1B) in HPV16-positive, HIV-infected women was 10% (95% CI, 0%–23%) after 3 years, which remained unchanged (with no additional cases) through 5 years of follow-up, whereas the risk over 5 years in those with non-16 oncHPV was 3% (95% CI, 0%–6%) (not shown).
No cases of HPV18-positive CIN-2+ or CIN-3+ were detected in the HIV-infected women studied. However, among HIV-infected women with LSIL, the 3-year cumulative risk of CIN-3+ was 7% (95% CI, 3%–11%), with no additional cases detected between 3 and 5 years of follow-up.
Multivariate Cox Models
Our initial models assessed the associations of oncHPV detection (yes/no) and host immune status (ie, HIV infection and CD4 count) with subsequent risk of CIN-2+ and CIN-3+ after 3 years or 5 years of follow-up, controlling for multiple cofactors. For CIN-2+ (Table 2), the detection of any oncHPV vs no oncHPV was associated with a 3-year hazard ratio (HR) of 6.4 (95% CI, 3.1–12.9; P < .0001) and a 5-year HR of 5.3 (95% CI, 2.9–9.4; P < .0001). Although HIV status was associated with a significant increase in CIN-2+ risk, there was no clear biologic gradient between CIN-2+ and decreasing CD4 cell count (Ptrend > .5) in these multivariate analyses. Similar results were also obtained using CIN-3+ as the endpoint (Table 3). In additional models (Table 4), we found strong associations of HPV16 with CIN-3+ risk during 3 years (HR, 18.7 [95% CI, 3.6–96.9]; P < .001) and 5 years (HR, 13.3 [95% CI, 2.7–64.3]; P < .001) of follow-up, as well as between detection of LSIL and CIN-3+ risk during the same 3-year (HR, 12.2 [95% CI, 4.0–37.0]; P < .0001) and 5-year (HR, 9.4 [95% CI, 3.5–25.6]; P < .0001) periods. These results were unaltered by limiting analysis to only HIV-infected women, controlling for HIV RNA level (as parameterized in Table 1) and use of HAART, and incorporating “number of sex partners in the prior 6 months” instead of “lifetime number of sexual partners” in the model; that is, the 5-year relative risk of CIN-3+ related to HPV16 detection was an HR of 14.5 (95% CI, 2.7–78.7; P = .001), whereas for LSIL it was an HR of 11.9 (95% CI, 3.8–37.0). Moreover, no statistically significant differences in CIN-3+ risk were found between HIV-infected women with a normal Pap who were HPV16 positive vs those who had LSIL (all P > .50). Additional details regarding each model are reported in the footnotes to Tables 2–4. Year of enrollment had no relation with CIN-2+ or CIN-3+ risk in any multivariate models.
Table 2.
Risk of Cervical Intraepithelial Neoplasia Grade 2 or Greater (CIN-2+) After 3 or 5 Years of Follow-up, and Its Relationship With Oncogenic Human Papillomavirus Detection at Baseline in Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Women With a Normal Baseline Pap Resulta
| Characteristic | 3 y HR (95% CI) | P Value | 5 y HR (95% CI) | P Value |
|---|---|---|---|---|
| HPV status | ||||
| OncHPV negative | Reference | Reference | ||
| OncHPV positive | 6.4 (3.1–12.9) | <.0001 | 5.3 (2.9–9.4) | <.0001 |
| Year of enrollment | ||||
| 1994–1995 | Reference | Reference | ||
| 2001–2002 | 1.1 (.5–2.3) | .8 | 1.0 (.5–1.8) | .9 |
| CD4 count (HIV+) | ||||
| HIV− | Reference | Reference | ||
| >500 cells/µL | 3.2 (1.1–9.4) | .04 | 2.3 (1.0–5.1) | .05 |
| 350–500 cells/µL | 2.2 (.6–7.4) | .2 | 1.8 (.7–4.5) | .2 |
| <350 cells/µL | 4.9 (1.6–15.4) | .01 | 3.0 (1.3–7.1) | .01 |
| Age at baseline (continuous) | 0.9 (.9–1.0) | .005 | 0.9 (.9–1.0) | .003 |
| Race | ||||
| Black | Reference | Reference | ||
| Hispanic | 1.6 (.7–3.4) | .3 | 1.1 (.6–2.2) | .7 |
| White | 0.2 (.0–1.6) | .1 | 0.3 (.1–1.0) | .1 |
| Other | 2.3 (.6–8.2) | .2 | 2.1 (.7–6.1) | .2 |
| Smoking | ||||
| Never | Reference | Reference | ||
| Past | 1.7 (.6–4.4) | .3 | 1.2 (.5–3.0) | .7 |
| Current | 1.1 (.5–2.5) | .8 | 1.5 (.8–2.9) | .2 |
| Lifetime No. of male sexual partners | ||||
| <5 | Reference | Reference | ||
| 5–9 | 1.5 (.5–4.4) | .4 | 1.2 (.5–2.8) | .7 |
| 10–49 | 2.0 (.7–5.4) | .2 | 1.6 (.7–3.6) | .2 |
| ≥50 | 1.4 (.4–4.6) | .6 | 0.9 (.3–2.3) | .8 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HPV, human papillomavirus; HR, hazard ratio; OncHPV; oncogenic human papillomavirus.
a In secondary data analysis, we observed similar results when incorporating nonparametric splines in the Cox model; that is, the average HRs after 3 or 5 years of follow-up, as measured using the area under the curve, were similar to those presented in the tables, and no statistically significant departures from proportionality were detected.
Table 3.
Risk of Cervical Intraepithelial Neoplasia Grade 3 or Greater (CIN-3+) After 3 or 5 Years of Follow-up, and Its Relationship With Oncogenic Human Papillomavirus Detection at Baseline in Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Women With a Normal Baseline Pap Resulta
| Characteristic | 3 y HR (95% CI) | P Value | 5 y HR (95% CI) | P Value |
|---|---|---|---|---|
| HPV status | ||||
| OncHPV negative | Reference | Reference | ||
| OncHPV positive | 5.3 (1.6–18.3) | .01 | 4.1 (1.3–12.8) | .02 |
| CD4 count (HIV+) | ||||
| HIV− | Reference | Reference | ||
| >500 cells/µL | 1.3 (.2–9.4) | .8 | 1.0 (.2–4.5) | 1.0 |
| 350–500 cells/µL | 2.7 (.4–17.1) | .3 | 1.4 (.3–6.4) | .7 |
| <350 cells/µL | 3.7 (.6–23.1) | .2 | 1.8 (.4–7.8) | .5 |
| Age at baseline (continuous) | 0.9 (.9–1.0) | .1 | 1.0 (.9–1.0) | .2 |
| Smoking | ||||
| Never | Reference | Reference | ||
| Past | 1.5 (.3–8.3) | .7 | 1.4 (.2–7.7) | .7 |
| Current | 1.1 (.3–4.2) | .9 | 1.7 (.5–5.8) | .4 |
| Lifetime No. of male sexual partners | ||||
| <5 | Reference | Reference | ||
| 5–9 | 1.1 (.2–5.4) | 1.0 | 1.3 (.3–6.0) | .7 |
| ≥10 | 1.0 (.2–4.1) | 1.0 | 1.1 (.3–4.4) | 1.0 |
Abbreviations: CI, confidence interval; CIN, cervical intraepithelial neoplasia; HIV, human immunodeficiency virus; HPV, human papillomavirus; HR, hazard ratio; OncHPV; oncogenic human papillomavirus.
a The number of covariates were a priori reduced from the model shown in Table 2 to decrease the degrees of freedom used in this model, given the smaller number of CIN-3+ than CIN-2+ events. In secondary data analysis, we observed similar results when incorporating nonparametric splines in the Cox model; that is, the average HRs after 3 or 5 years of follow-up, as measured using the area under the curve, were similar to those presented in the tables, and no statistically significant departures from proportionality were detected.
Table 4.
Multivariate Cox Model of Cervical Intraepithelial Neoplasia Grade 3 or Greater (CIN-3+) Risk Among Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Women With a Normal Pap Result After 3 or 5 Years of Follow-upa, Women's Interagency HIV Study
| Characteristic | 3 y HR (95% CI) | P Value | 5 y HR (95% CI) | P Value |
|---|---|---|---|---|
| HPV and LSIL status | ||||
| OncHPV negative | Reference | Reference | ||
| Non-16 OncHPV positive | 4.2 (1.0–17.6) | .05 | 2.9 (.7–11.3) | .13 |
| HPV16 positive | 18.7 (3.6–96.9) | .001 | 13.3 (2.7–64.3) | <.001 |
| LSIL | 12.2 (4.0–37.0) | <.0001 | 9.4 (3.5–25.6) | <.0001 |
| Enrollment period | ||||
| 1994–1995 | Reference | Reference | ||
| 2001–2002 | 0.9 (.3–2.5) | .83 | 0.9 (.4–2.3) | .81 |
| CD4 count (HIV+) | ||||
| HIV− | Reference | Reference | ||
| >500 cells/µL | 2.6 (.5–13.9) | .27 | 1.7 (.5–6.3) | .44 |
| 350–500 cells/µL | 1.9 (.3–12.2) | .50 | 1.1 (.2–5.1) | .95 |
| <350 cells/µL | 3.4 (.7–17.7) | .15 | 2.1 (.6–7.5) | .28 |
| Age at baseline | 0.97 (.9–1.0) | .37 | 0.97 (.9–1.0) | .40 |
| Race | ||||
| Black | Reference | Reference | ||
| Hispanic | 1.3 (.5–3.5) | .60 | 1.1 (.5–2.9) | .79 |
| White | 0.6 (.1–2.7) | .48 | 0.5 (.1–2.3) | .38 |
| Other | 1.3 (.2–10.3) | .82 | 2.2 (.5–10.2) | .31 |
| Smoking | ||||
| Never | Reference | Reference | ||
| Past | 2.3 (.6–9.0) | .23 | 2.3 (.6–8.9) | .23 |
| Current | 2.6 (.9–7.7) | .08 | 3.0 (1.1–8.5) | .04 |
| Lifetime No. of male sexual partners | ||||
| <5 | Reference | Reference | ||
| 5–9 | 0.5 (.1–2.0) | .31 | 0.6 (.2–2.1) | .40 |
| 10–49 | 1.4 (.5–4.1) | .51 | 1.2 (.4–3.5) | .69 |
| ≥50 | 0.1 (.0–1.1) | .06 | 0.4 (.1–1.5) | .15 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HPV, human papillomavirus; HR, hazard ratio; LSIL, low-grade squamous intraepithelial lesion; OncHPV; oncogenic human papillomavirus.
a In secondary data analysis, we observed similar results when incorporating nonparametric splines in the Cox model; that is, the average HRs after 3 or 5 years of follow-up, as measured using the area under the curve, were similar to those presented in the table, and no statistically significant departures from proportionality were detected.
DISCUSSION
We determined the cumulative risk of CIN-2+ and CIN-3+ among HIV-infected women who, despite having a normal baseline Pap, co-tested positive for HPV16 or other oncHPV types. Whereas prior studies reported that HIV-infected women with a normal Pap who additionally tested oncHPV negative had a similar low incidence of CIN-2+ and CIN-3+ as HIV-uninfected individuals [10, 26], the risk of high-grade cervical lesions (ie, precancer) in HIV-infected women with a normal Pap result who instead tested oncHPV positive has not to our knowledge been reported.
The most striking finding was the very high risk of CIN-2+ and CIN-3+ among HIV-infected women with a normal Pap result who tested HPV16 positive; that is, a 5-year cumulative incidence of CIN-2+ of 29% and CIN-3+ of 10%. No HPV18-positive CIN-2+ and CIN-3+ were detected during the same follow-up period. In multivariable Cox models, testing positive for HPV16 vs oncHPV negative at baseline was associated with a 13-fold greater risk of CIN-3+ among HIV-infected women with a normal Pap result.
To benchmark these results against an accepted indication for immediate colposcopy, we concomitantly measured the risk of CIN-3+ in HIV-infected women with a Pap diagnosis of LSIL [12, 13]. In both Kaplan–Meier and multivariate Cox models, HIV-infected women with a normal Pap result who tested positive for HPV16 had nonsignificantly higher risk of CIN-3+ than did HIV-infected women with LSIL. Based on the principle of “equal management of equal risks,” the follow-up in both groups should be similar because they have similar risk of CIN-3+ [12, 13]. If correct, the collective data therefore suggest that immediate colposcopy might be appropriate in HIV-infected women who test HPV16 positive despite a normal Pap result, as in HIV-infected women with LSIL.
Furthermore, current US guidelines recommend follow-up screening in 1 year for women in the general population who are oncHPV positive but lack additional HPV genotype data, or who test positive only for oncHPV types other than HPV16 or HPV18 [1, 14]. Consistent with this, our data showed that although the risk of CIN-2+ and CIN-3+ was elevated in HIV-infected women with a normal Pap and any oncHPV (or non-16 oncHPV types), relative to those who tested oncHPV-negative, this risk was lower than in those with LSIL. Thus, the current findings suggest that repeat screening in 1 year might be appropriate in HIV-infected women with a normal Pap and non-16 oncHPV types or who lack type-specific results.
The current findings are also interesting for another reason: They provide new information regarding the special character of HPV16, the most carcinogenic HPV type. Our group and others have previously shown that the prevalence of HPV16 is the least affected of any oncHPV type by changes in host immune status among HIV-infected women (as measured by CD4 cell count) [27–29]. This relative independence of HPV16 infection from host immune status has been interpreted as evidence that HPV16 may have a greater innate ability to avoid the effects of immune surveillance than other oncHPV types. Consistent with this, we have recently reported that HPV16 is present in a significantly lower percentage of CIN-3+ in HIV-infected (25%) than in HIV-uninfected (>60%) women in the WIHS cohort (L. S. Massad et al, submitted). Taken together, the current study and prior data collectively suggest that although the prevalence of HPV16 may be less affected by low CD4 cell count than other oncHPV types, when cervical HPV16 infection does occur, it is strongly associated with risk of CIN-2+ and CIN-3+ in HIV-infected women.
Several limitations to this study should be considered in interpreting the findings. The first and most important is that the cumulative risk estimates for CIN-2+ and CIN-3 had wide CIs, indicating that statistical power in this study was somewhat limited. This concern may also explain the failure to detect HPV18 in any CIN-2+ or CIN-3+ cases. Thus, a multicohort analysis to expand upon the current results may be warranted, as there are few other similarly large cohorts with appropriate follow-up and data. It would be beneficial for such an analysis to focus more extensively on the current HAART era and to more carefully address the effects of HAART than was possible in the current study. Additionally, the results in WIHS women may not be generalizable beyond HIV-infected individuals who are likewise in long-term care; albeit, this includes a growing fraction of US women with HIV. Last, the extent to which these data are applicable to populations outside the United States is uncertain, particularly those in resource-limited settings.
Overall, the current data suggest that HIV-infected women may benefit from cervical cancer co-testing using Pap and oncHPV assays, as is recommended in the general population [1]. Repeat co-testing at 1 year may be a reasonable approach in HIV-infected women with a normal Pap who are oncHPV positive [1]. HPV genotyping may also be appropriate in HIV-infected women, as the high risk of CIN-2+ and CIN-3+ in individuals with a normal Pap and HPV16 suggests that immediate colposcopy may be appropriate in these individuals. Were these practices adopted, the current data suggest that approximately 15% of HIV-infected women with a normal Pap would be referred to follow-up screening in 1 year and 2% to immediate colposcopy; that is, 15% of women with a normal Pap were oncHPV positive and 2% were HPV16 positive. However, HIV-infected women who are unable to obtain oncHPV testing can also be followed with annual Pap tests, and for those with 3 serial negative Pap tests, longer intervals until repeat screening may be appropriate [30]. In future studies, it will be important to focus more extensively on HIV-infected women with abnormal Pap tests and to incorporate additional molecular assays to more accurately identify the subset of individuals with high risk of cervical precancer and cancer, either at the time of screening or within a few years of gynecologic assessment. Cost effectiveness should be carefully considered prior to implementation of any new cervical cancer screening guidelines in HIV-infected women.
Notes
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Cancer Institute (NCI) (grant numbers R01-CA-085178 and R01-CA-174634 to H. D. S. and P30-CA-013330); the National Institute of Allergy and Infectious Diseases (NIAID) (grant number P30-AI-051519); and the Women's Interagency HIV Study (WIHS). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen), U01-AI-034993; Metropolitan Washington WIHS (Mary Young), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Alexandra Levine and Marek Nowicki), U01-HD-032632 (WIHS I–WIHS IV). The WIHS is funded primarily by the NIAID, with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the NCI, the National Institute on Drug Abuse, and the National Institute of Mental Health. Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and other Communication Disorders, and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
Potential conflicts of interest. P. E. C. has received commercial HPV tests for research at a reduced or no cost from Roche, Qiagen, BD, and MTM; has been compensated financially as a member of a Merck data and safety monitoring board for HPV vaccines; has been a paid consultant for BD, Gen-Probe/Hologic, Roche, Cepheid, ClearPath, Guided Therapeutics, Inovio, and GE Healthcare; and has received honoraria as a speaker for Roche and Cepheid. K. A. has received honoraria from Bristol-Myers Squibb (BMS). J. M. P. reports grants and nonfinancial support from Merck and Hologic, nonfinancial support from Vaxgenetics, and has been compensated financially by BMS. A study of H. D. S. involves free blinded testing using HPV E6/E7 protein assays by Arbor Vita, p16/Ki67 cytology by MTM Laboratories/Ventura–Roche, and MCM-2/TOP2A cytology by BD Diagnostics; no financial payments to H. D. S. or his home institution were received. All other authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Saslow D, Solomon D, Lawson HW et al. . American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis 2012; 16:175–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katki HA, Schiffman M, Castle PE et al. . Five-year risks of CIN 3+ and cervical cancer among women who test Pap-negative but are HPV-positive. J Low Genit Tract Dis 2013; 17(5 suppl 1):S56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394773.htm. Accessed 26 January 2015.
- 4.Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015; 136:189–97. [DOI] [PubMed] [Google Scholar]
- 5.Abraham AG, D'Souza G, Jing Y et al. . Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study. J Acquir Immune Defic Syndr 2013; 62:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guiguet M, Boue F, Cadranel J et al. . Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol 2009; 10:1152–9. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst 2009; 101:1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strickler HD, Burk RD, Fazzari M et al. . Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst 2005; 97:577–86. [DOI] [PubMed] [Google Scholar]
- 9.Massad LS, Xie X, D'Souza G et al. . Incidence of cervical precancers among HIV-seropositive women. Am J Obstet Gynecol 2014; 212:606.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller MJ, Burk RD, Xie X et al. . Risk of cervical precancer and cancer among HIV-infected women with normal cervical cytology and no evidence of oncogenic HPV infection. JAMA 2012; 308:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. Am J Obstet Gynecol 2007; 197:356.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katki HA, Schiffman M, Castle PE et al. . Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis 2013; 17(5 suppl 1):S28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiffman M, Burk RD, Boyle S et al. . A study of genotyping for management of human papillomavirus-positive, cytology-negative cervical screening results. J Clin Microbiol 2015; 53:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massad LS, Einstein MH, Huh WK et al. . 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2013; 17(5 suppl 1):S1–27. [DOI] [PubMed] [Google Scholar]
- 15.Bacon MC, von Wyl V, Alden C et al. . The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurman RJ, Solomon D. The Bethesda system for reporting cervical/vaginal cytologic diagnoses. New York: Springer-Verlag, 1994. [Google Scholar]
- 17.Solomon D, Davey D, Kurman R et al. . The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–9. [DOI] [PubMed] [Google Scholar]
- 18.Burk RD, Ho GY, Beardsley L, Lempa M, Peters M, Bierman R. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. J Infect Dis 1996; 174:679–89. [DOI] [PubMed] [Google Scholar]
- 19.Munoz N, Bosch FX, de Sanjose S et al. . Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–27. [DOI] [PubMed] [Google Scholar]
- 20.Bouvard V, Baan R, Straif K et al. . A review of human carcinogens—part B: biological agents. Lancet Oncol 2009; 10:321–2. [DOI] [PubMed] [Google Scholar]
- 21.Massad LS, Xie X, D'Souza G et al. . Incidence of cervical precancers among HIV-seropositive women. Am J Obstet Gynecol 2015; 212:606.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huh WK, Ault KA, Chelmow D et al. . Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125:330–7. [DOI] [PubMed] [Google Scholar]
- 23.Kitchener HC, Canfell K, Gilham C et al. . The clinical effectiveness and cost-effectiveness of primary human papillomavirus cervical screening in England: extended follow-up of the ARTISTIC randomised trial cohort through three screening rounds. Health Technol Assess 2014; 18:1–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15:361–87. [DOI] [PubMed] [Google Scholar]
- 25.Spleeper LA, Harrington DP. Regression splines in the Cox model with application to covariate effects in liver disease. J Am Stat Assoc 1990; 85:941–9. [Google Scholar]
- 26.Harris TG, Burk RD, Palefsky JM et al. . Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA 2005; 293:1471–6. [DOI] [PubMed] [Google Scholar]
- 27.Strickler HD, Palefsky JM, Shah KV et al. . Human papillomavirus type 16 and immune status in human immunodeficiency virus-seropositive women. J Natl Cancer Inst 2003; 95:1062–71. [DOI] [PubMed] [Google Scholar]
- 28.Clifford GM, Goncalves MA, Franceschi S; HPV and HIV Study Group. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS 2006; 20:2337–44. [DOI] [PubMed] [Google Scholar]
- 29.Xue X, Gange SJ, Zhong Y et al. . Marginal and mixed-effects models in the analysis of human papillomavirus natural history data. Cancer Epidemiol Biomarkers Prev 2010; 19:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massad LS, D'Souza G, Tian F et al. . Negative predictive value of pap testing: implications for screening intervals for women with human immunodeficiency virus. Obstet Gynecol 2012; 120:791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]