Neonates were randomized to oral polio vaccine (OPV) and followed for mortality. Among those enrolled within the first 2 days, OPV was associated with a 42% (10%–62%) reduction in infant infectious disease mortality. OPV at birth may provide nonspecific protection against infections.
Keywords: oral polio vaccine, heterologous immunity, nonspecific effects, infant mortality, neonates
Abstract
Background. Routine vaccines may have nonspecific effects on mortality. An observational study found that OPV given at birth (OPV0) was associated with increased male infant mortality. We investigated the effect of OPV0 on infant mortality in a randomized trial in Guinea-Bissau.
Methods. A total of 7012 healthy normal-birth-weight neonates were randomized to BCG only (intervention group) or OPV0 with BCG (usual practice). All children were to receive OPV with pentavalent vaccine (diphtheria, tetanus, pertussis, Haemophilus influenzae type b, and hepatitis B) at 6, 10, and 14 weeks of age. Seven national OPV campaigns were also conducted during the trial period. Children were followed to age 12 months. We used Cox regression to calculate hazard ratios (HRs) for mortality.
Results. The trial contradicted the original hypothesis about OPV0 increasing male infant mortality. Within 12 months, 73 children in the BCG + OPV group and 87 children in the BCG-only group died, all from infectious diseases. Comparing BCG + OPV0 vs BCG only, the HR was 0.83 (95% confidence interval [CI], .61–1.13): 0.72 (95% CI, .47–1.10) in boys and 0.97 (95% CI, .61–1.54) in girls. For children enrolled within the first 2 days of life, the HR for BCG + OPV0 vs BCG only was 0.58 (95% CI, .38–.90). From enrollment until the time of OPV campaigns, the HR was 0.68 (95% CI, .45–1.00), the beneficial effect being separately significant for males (0.55 [95% CI, .32–.95]).
Conclusions. This is the only randomized trial of the effect of OPV0 on mortality. OPV0 may be associated with nonspecific protection against infectious disease mortality, particularly when given early in life. There are reasons to monitor mortality when OPV is being phased out.
Clinical Trials Registration. NCT00710983.
(See the Editorial Commentary by Frenkel on pages 1512–3.)
Vaccines may affect susceptibility to unrelated infections and have an impact on overall infectious disease mortality that is not explained by preventing the vaccine-targeted disease(s), termed “nonspecific effects” (NSEs) [1–8]. Live vaccines, including BCG and measles vaccine (MV), reduce mortality more than expected from preventing tuberculosis and measles [8–11]. In contrast, some inactivated vaccines may have negative NSEs; for example, girls vaccinated with the inactivated diphtheria-tetanus-pertussis (DTP) vaccine have higher mortality than DTP-unvaccinated girls in nearly all studies [7, 12–17]. The World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) recently reviewed the evidence for NSEs of BCG, MV, and DTP, and concluded that BCG and MV may have beneficial NSEs that warrant further research [18].
The effect of oral polio vaccine (OPV) on mortality has only been assessed in a few studies, and SAGE did not review the possible NSEs of OPV. OPV has been associated with lower infant mortality and morbidity, both in Guinea-Bissau [19, 20] and elsewhere [21, 22].
From 1985 to 2010, WHO recommended that OPV be given at birth or at first contact with the health system in low-income countries [23]. OPV given at birth (OPV0) remains the policy in countries at high risk for polio infection, including Guinea-Bissau. During a randomized trial of neonatal vitamin A supplementation conducted from 2002 to 2004 in Guinea-Bissau, the country experienced a period with a shortage of OPV; a total of 962 trial participants did not receive the recommended OPV0. Surprisingly, boys who missed OPV0 had significantly lower infant mortality compared with boys who received OPV0 (adjusted mortality rate ratio [MRR], 0.35 [95% confidence interval {CI}, .18–.71]) [24]. The tendency was opposite for girls (adjusted MRR, 1.14 [95% CI, .70–1.89]); thus, the effect of OPV0 differed significantly between boys and girls (P = .006). Receiving OPV0 was also associated with reduced immune responses to BCG in both sexes [25].
Based on these observations, we undertook a comprehensive evaluation of OPV0. Polio cases have not been reported in Guinea-Bissau for several years, and with the potential negative effect of OPV0, it was justified to assess the effect on infant mortality of OPV0 by randomizing neonates to OPV0 or no OPV0 with BCG at birth. We hypothesized that boys would benefit from not receiving OPV0, whereas girls would benefit from OPV0, and that the combined effect of a sex-differential policy would be at least a 30% reduction in infant mortality.
METHODS
Setting
The study was conducted at the Bandim Health Project (BHP) in Bissau, Guinea-Bissau. BHP runs a Health and Demographic Surveillance System (HDSS) in 6 suburban areas covering approximately 103 000 inhabitants. BHP assistants visit all houses in the study area monthly to register pregnancies and births. Children are followed every 3 months the first 3 years of life. The BHP has a team at the maternity ward of the National Hospital, where many women from the HDSS give birth. This team vaccinates all children before discharge. Furthermore, BHP assistants are placed at the 3 health centers in the HDSS area, where women who deliver at home typically come for the first vaccinations.
Participants
Neonate males and females with a birth weight of at least 2.5 kg, not older than 1 month at first vaccination contact, and living in the HDSS area, were eligible for enrollment. Sick children or children with malformations were not enrolled but referred for treatment. Children were invited to participate at discharge from the hospital, or, if delivered elsewhere, when they came to get their first vaccinations at the health centers in the area. All eligible children had access to free medical consultations and essential drugs if ill, regardless of participation in the trial.
Randomization
A trained BHP assistant gave the mother/guardian an oral and written explanation of the study. Mothers/guardians who provided oral and written consent drew a lot from an envelope containing 24 stapled lots; 12 lots were marked “BCG,” 12 “BCG OPV.” The study supervisor prepared the envelopes; there were separate envelopes for boys and girls. Same-sex twins were allocated the same treatment to avoid confusion.
Children randomized to OPV received 2 drops of the vaccine orally immediately after randomization. There was no placebo or blinding. Guinea-Bissau does not have a central vaccination registry, so it was important that the vaccination card contained the correct information if a child moved from the HDSS area.
All infants were vaccinated intradermally in the upper left deltoid region with 0.05 mL of BCG vaccine (Statens Serum Institut, Copenhagen, Denmark). All mothers were encouraged to take their children to receive recommended vaccinations with OPV and pentavalent vaccine (DTP, Haemophilus influenzae type b, and hepatitis B) at 6, 10, and 14 weeks of age.
At enrollment, anthropometric measures were obtained, and an interview was conducted. Children enrolled at the hospital were examined clinically. All enrollment data were double-entered and all data were checked for outliers. Information on socioeconomic factors was obtained from the HDSS.
Follow-up
All enrolled children were followed through the HDSS every 3 months and received a study-specific visit by a BHP assistant at age 12 months. Children who moved within the HDSS were visited at the new address.
During the study period, the Ministry of Health in Guinea-Bissau conducted 7 national OPV campaigns (6–9 March, 23–26 April, and 28–31 May 2010; 25–28 March, 29 April–3 May, and 25–29 November 2011; and 23–26 March 2012). These campaign vaccines were not always registered on the vaccination card. We did not have resources to follow all OPV campaign teams to register the vaccinations as done in some previous campaigns [26]. Hence, to estimate the effect of OPV0 in the absence of OPV campaigns, we censored all subsequent follow-up on the first day of the first campaign occurring after enrollment of a child. In 2 campaigns, we interviewed about participation; 92%–95% of infants had received OPV during the campaign.
Outcomes
The main outcome was overall mortality (excluding accidents) within the first 12 months of life. In addition, we analyzed the “pure” effect of OPV0 on survival from enrollment to age 6 weeks, before controls were likely to receive the first routine OPV. Furthermore, the protocol specified that if large campaigns were conducted during follow-up, analyses would also be conducted with censoring for such campaigns. Thus, we analyzed the impact on infant mortality with censoring for subsequent national OPV campaign, as OPV campaigns could potentially neutralize any differential effect of OPV0. During the conduct of the trial, there was also a national MV campaign (December 2009) and an H1N1 vaccine campaign (October 2010), both targeting children aged 6 months to 5 years. In additional analyses, we censored for these campaigns.
As described in the Supplementary Material, we also followed subgroups for possible adverse events until 31 days, and growth was assessed by anthropometry at 6 weeks, 6 months, and 12 months of age (Supplementary Figures 1 and 2; Supplementary Tables 1–3).
Outcomes related to antibody responses to OPV, thymus growth, and the response to BCG vaccine have been reported elsewhere [27–29].
Sample Size Considerations
Assuming a significance level of 95% and 80% power, and with an expected infant mortality rate of 50 per 1000 participants among normal-birth-weight infants (≥2.5 kg), we needed 2970 children in each group to detect a 30% reduction in infant mortality associated with boys not receiving OPV0 and girls receiving OPV0. With an expected loss to follow-up of 15% due to migration, we needed to recruit 7000 children.
Statistical Methods and Analyses
One child died in an accident and was censored. The remaining deaths were all considered due to infectious diseases. We used Cox regression to calculate hazard ratios (HRs) for mortality with 95% CIs. Age was used as the underlying time and was thus inherently controlled for. Tests for proportionality of hazard rates were computed using Schoenfeld residuals and by visual inspection of the cumulative risk curves, which were drawn using the Kaplan–Meier method.
The overall analysis was performed with follow-up to age 12 months. We furthermore conducted the analysis with censoring at age 6 weeks and with censoring for national campaigns.
After the first “natural experiment” where OPV was missing in 2004 [24], there was another small similar “natural experiment” in 2007. In this second natural experiment [30], we found a significant interaction between OPV0 and age at administration (≤2 days, ≥3 days). Hence, after the protocol was written, but before the enrollment for the present trial had ended, we decided to investigate this potential effect modifier. We used the cutoff used in the previous study; in a sensitivity analysis we tested other cutoffs from day 1 to day 7.
As prespecified, all analyses were done overall and stratified by sex. Statistical analysis was performed using Stata software version 11.2 (StataCorp, College Station, Texas).
Ethical Considerations
The Guinean Ministry of Health's Research Coordination Committee approved the protocol, and the Danish Central Ethics Committee gave its consultative approval (number 2008-7041-122). The trial was registered at ClinicalTrials.gov (NCT00710983). The trial was monitored by a data safety and monitoring board (DSMB).
RESULTS
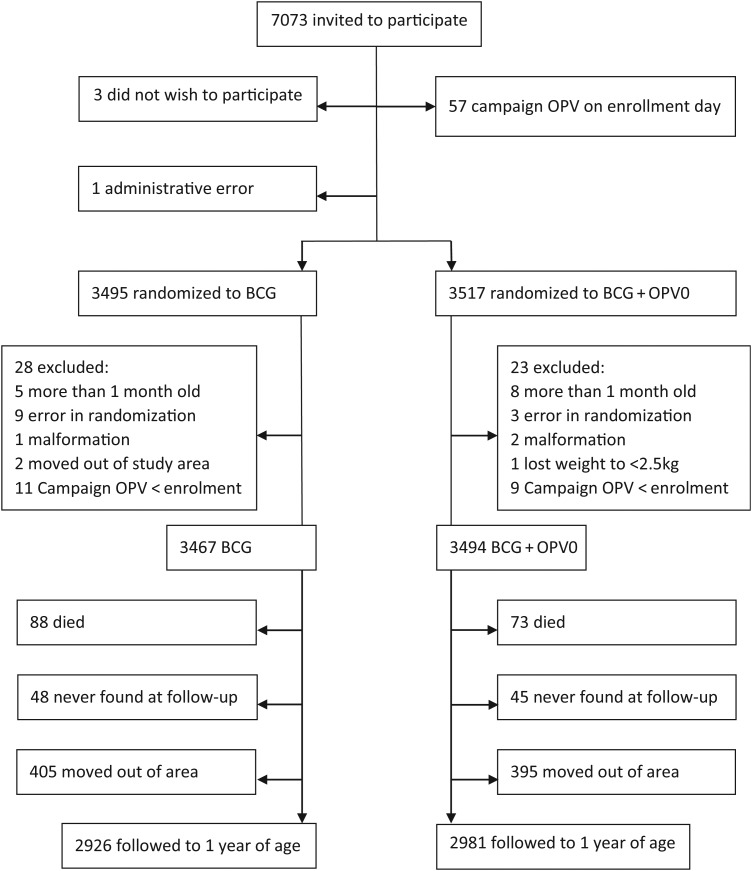
Enrollments started on 4 July 2008 and ended 23 October 2011. Among 7073 children invited to participate, 7012 children were enrolled, and 6991 children contributed follow-up time. The loss to follow-up was similar between the 2 randomization groups (Figure 1). The groups did not differ with regard to baseline characteristics (Table 1), and all analyses were done without adjustment.
Figure 1.
Flowchart of children randomized to BCG + oral polio vaccine at birth (OPV0) or BCG only, Guinea-Bissau, 2008–2011.
Table 1.
Baseline Characteristics of Participants Enrolled in the Trial, for Children Randomized to BCG Plus Oral Polio Vaccine at Birth or BCG Only, Guinea-Bissau, 2008–2011
| Characteristic | BCG + OPV0 (n = 3494) | BCG (n = 3467) |
|---|---|---|
| Male sex | 1847 (52.9) | 1823 (52.6) |
| Enrollment in rainy season | 1882 (53.9) | 1869 (53.9) |
| Enrollment at national hospital | 1729 (49.5) | 1724 (49.7) |
| Year of enrollment | ||
| 2008 | 472 (13.5) | 483 (13.9) |
| 2009 | 1065 (30.5) | 1050 (30.3) |
| 2010 | 1095 (31.3) | 1086 (31.3) |
| 2011 | 862 (24.7) | 848 (24.5) |
| Median age at enrollment, d (10%–90% range) | 2 (1–16) | 2 (1–16) |
| Anthropometrics at enrollment: | ||
| Mean (SD) weight, kg | 3.2 (0.45) | 3.2 (0.44) |
| Mean (SD) length, cm | 49.7 (2.2) | 49.7 (2.1) |
| Mean (SD) MUAC, mm | 99 (7.7) | 99 (7.8) |
| Mean (SD) head circumference, cm | 34.3 (1.7) | 34.3 (1.7) |
| Maternal schooling | ||
| Yes | 2232 (63.9) | 2253 (65.0) |
| No | 829 (23.7) | 793 (22.9) |
| Unknown | 433 (12.4) | 421 (12.1) |
| Mean (SD) maternal MUAC, mm | 257 (32) | 257 (31) |
Data are presented as No. (%) unless otherwise specified. Variables with 2 levels are presented by one of the levels.
Abbreviations: MUAC, mid-upper arm circumference; OPV0, oral polio vaccine given at birth; SD, standard deviation.
Based on previous observations [31], we anticipated an infant mortality rate of 50 per 1000 participants. However, during the study period, infant mortality declined to 26 per 1000 person-years. This reduced the power to detect a difference between groups. An interim report for the DSMB showed significantly higher mortality among children randomized to BCG only. As this was in favor of current policy, the DSMB recommended that the number of participants not be increased to compensate for the lower mortality. With follow-up of all children to age 12 months, the difference between the randomization groups was no longer significant.
Primary Outcome: Infant Mortality
Within 12 months (mean duration of follow-up, 328 days), 73 died in the BCG + OPV0 group and 87 died in the BCG-only group. Comparing BCG + OPV0 and BCG only overall, the HR was 0.83 (95% CI, .61–1.13; Table 2).
Table 2.
Infant Mortality Rates and Hazard Ratios for Children Randomized to BCG Plus Oral Polio Vaccine at Birth or BCG Only, Guinea-Bissau, 2008–2011
| Follow-up | MR per 1000 person-years (Death/Person-days) |
HR (95% CI) for BCG + OPV0 vs BCG | |
|---|---|---|---|
| BCG + OPV0 | BCG | ||
| Total | |||
| Boys | 22.1 (37/610 407) | 30.8 (50/593 164) | 0.72 (.47–1.10) |
| Girls | 24.3 (36/541 078) | 25.0 (37/540 814) | 0.97 (.61–1.54) |
| All | 23.2 (73/1 151 485) | 28.0 (87/1 133 978) | 0.83 (.61–1.13) |
| Censoring at 6 wk, the age of next routine OPV | |||
| Boys | 67.4 (11/59 590) | 124.1 (20/58 858) | 0.61 (.31–1.22) |
| Girls | 62.4 (9/52 655) | 62.8 (9/52 316) | 1.11 (.45–2.72) |
| All | 65.1 (20/112 245) | 95.3 (29/111 174) | 0.76 (.44–1.31) |
| Censoring at national OPV campaigna | |||
| Boys | 23.9 (20/305 639) | 43.6 (36/301 252) | 0.55 (.32–.95) |
| Girls | 28.8 (21/266 229) | 33.3 (24/263 415) | 0.87 (.48–1.56) |
| All | 26.2 (41/571 868) | 38.8 (60/564 667) | 0.68 (.45–1.00) |
Abbreviations: CI, confidence interval; HR, hazard ratio; MR, mortality rate; OPV0, oral polio vaccine given at birth.
a Follow-up was censored at the first day of the national OPV campaigns.
Age at Enrollment
The HR (with full follow-up and no censoring) for BCG +OPV0 vs BCG only among children enrolled during the first 2 days of life was 0.58 (95% CI, .38–.90), whereas for children enrolled at ≥3 days, the HR was 1.26 (95% CI, .79–2.00) (P for interaction = .02) (Table 3). In the sensitivity analysis, the pattern was the same for all cut-points during the first week; the strongest differential effect was found for children enrolled within the first 3 days of life (Supplementary Table 4)
Table 3.
Infant Mortality Rates and Hazard Ratios for Children Randomized to BCG Plus Oral Polio Vaccine at Birth or BCG Only, According to Age at Enrollment
| Age at Enrollment | MR per 1000 person-years (Death/Person-days) |
BCG + OPV0 vs BCG HR (95% CI) |
||
|---|---|---|---|---|
| BCG + OPV0 | BCG | All Follow-up Time | With Censoring for OPV Campaignsa |
|
| Overall | ||||
| 0–2 d | 19.5 (33/618 317) | 33.5 (55/599 148) | 0.58 (.38–.90) | 0.52 (.31–.88) |
| ≥3 d | 27.4 (40/533 168) | 21.9 (32/534 830) | 1.26 (.79–2.00) | 1.00 (.53–1.90) |
| P for interaction between OPV0 and age at enrollment | 0.02 | 0.12 | ||
| Boys | ||||
| 0–2 d | 19.8 (18/331 607) | 35.3 (31/321 204) | 0.57 (.32–1.01) | 0.41 (.20–.86) |
| ≥3 d | 24.9 (19/278 800) | 25.5 (19/271 960) | 0.98 (.52–1.85) | 0.83 (.36–1.92) |
| P for interaction between OPV0 and age at enrollment | 0.21 | 0.21 | ||
| Girls | ||||
| 0–2 d | 19.1 (15/286 710) | 31.5 (24/277 944) | 0.61 (.32–1.16) | 0.69 (.33–1.44) |
| ≥3 d | 30.2 (21/254 368) | 18.1 (13/262 870) | 1.67 (.84–3.33) | 1.30 (.48–3.49) |
| P for interaction between OPV0 and age at enrollment | 0.04 | 0.31 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; MR, mortality rate; OPV0, oral polio vaccine given at birth.
a Follow-up was censored at the first day of the national OPV campaigns.
Censoring at Age 6 Weeks
Within the first 6 weeks of life, before children were eligible for receiving routine OPV with pentavalent vaccine, males had much higher early mortality rates than females in the BCG-only group, and a nonsignificant 2-fold mortality reduction from OPV0, whereas the effect of OPV0 was limited for girls (Table 2).
Censoring for OPV Campaigns
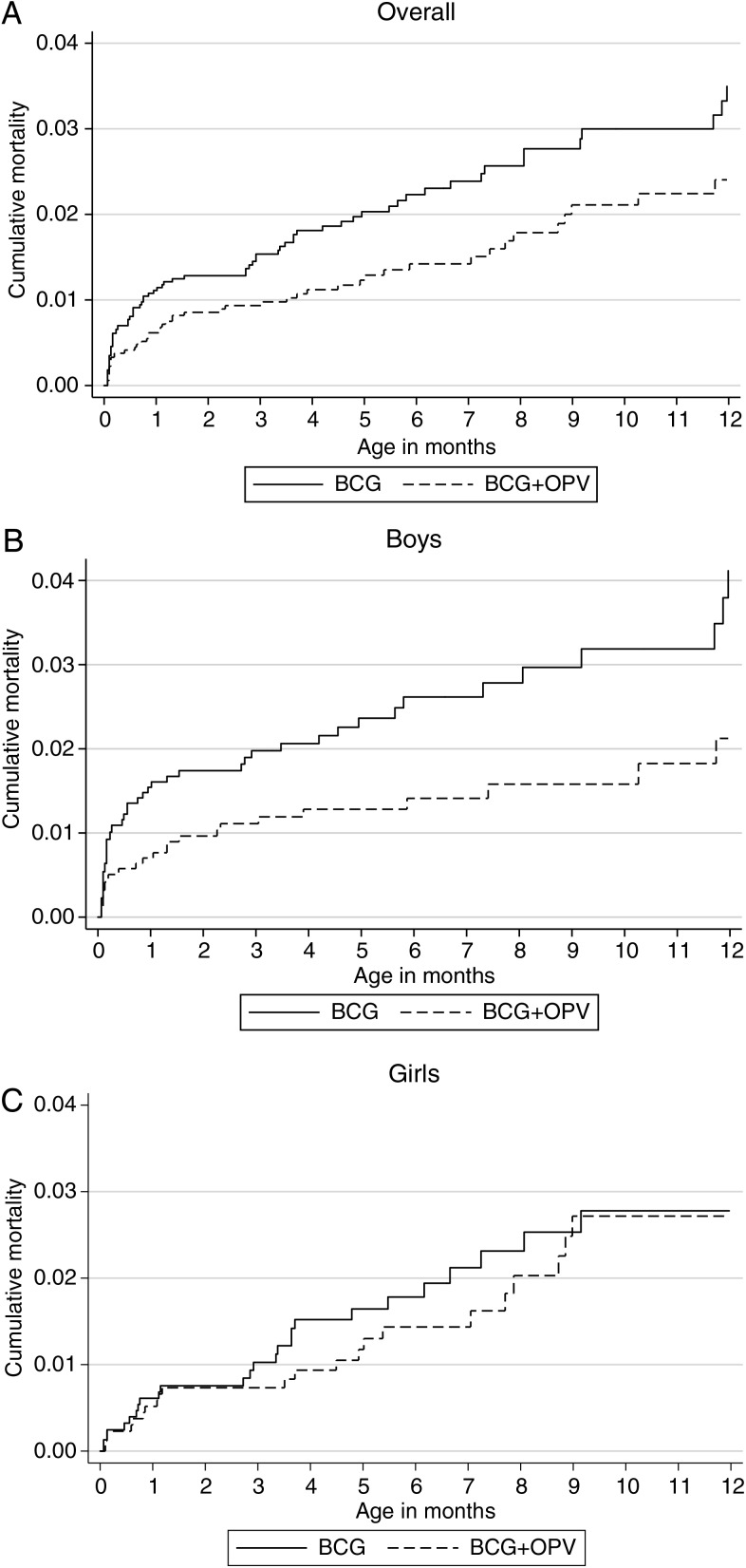
Within censoring for OPV campaigns (mean duration of follow-up = 163 days), 41 died in the BCG + OPV0 group and 60 died in the BCG-only group. Comparing BCG + OPV0 and BCG only, the HR was 0.68 (95% CI, .45–1.00): 0.55 (95% CI, .32–.95) for boys and 0.87 (95% CI, .48–1.56) for girls, with no evidence of interaction between OPV0 and sex (P for interaction = .26; Table 2). The cumulative mortality curves, overall and for boys and girls separately, are shown in Figures 2A–C.
Figure 2.
Cumulative mortality curves during infancy for children randomized to BCG + oral polio vaccine (OPV) at birth or BCG only, overall (A), for boys (B), and for girls (C). Follow-up was censored at the first day of the national OPV campaigns.
Censoring for Other Campaigns
Further censoring for the effect of the general MV and H1N1 campaigns did not affect the HR comparing BCG + OPV0 and BCG only; it was 0.75 (95% CI, .50–1.12): 0.56 (95% CI, .32–0.97) for boys and 1.10 (95% CI, .59–2.05) for girls.
Follow-up for Short-term Morbidity and Growth
During the first month postenrollment, the 2 randomization groups had similar incidence of reported illnesses, use of medication, health center consultations, and increased temperature (Supplementary Table 2). Adjusting for the anthropometric status at enrollment, most anthropometry indicators were insignificantly better in the BCG + OPV0 group at 6 weeks of age, particularly for males (Supplementary Table 3). There were no differences at age 6 months (Supplementary Table 3) and age 12 months (data not shown). In the anthropometry cohort, the BCG + OPV group tended to have better survival than the BCG-only group, the difference being statistically significant at 6 months (HR, 0.29 [95% CI, .11–.79]), also separately for boys (Supplementary Table 3).
DISCUSSION
Contrary to our initial hypothesis, both male and female children who received OPV0 with BCG tended to have better survival than those who received BCG only. As in a previous study, receiving OPV0 within the first days of life appeared to be associated with the strongest benefits. Many OPV campaigns occurred during the trial; when censoring for these campaigns, the beneficial effect of receiving OPV0 was borderline significant overall and significant separately in males. Although the power was reduced, the same tendency was seen when limiting the analysis to the period before 6 weeks of age, that is, before any children had received additional routine vaccinations.
One strength of our study was that the trial extended over 4 calendar years, making it less vulnerable to fluctuations in mortality rates that may have affected previous natural experiments [24, 30]. All analyses were prespecified before trial completion.
The trial setting necessitated a simple randomization procedure. The teams were carefully supervised, and every month we checked for imbalances between randomization groups with respect to age, place of inclusion, and anthropometric measures; we found no evidence of flaws.
A weakness of our study is that it was not blinded. However, health workers in the area were unaware of the study hypothesis and were unlikely to be influenced when treating the children. The outcome assessment was carried out by assistants unaware of the vaccination allocation. Hence, we do not believe that the lack of blinding affected the results.
In addition, national OPV campaigns could have led to environmental acquisition in children born in the period after a campaign. It is likely that, in a population-dense urban setting, indirect vaccination also occurs in the absence of OPV campaigns, through close contact with newly vaccinated children. This exposure, if anything, would be expected to dilute any differences between children who received OPV0 or no OPV0.
All background factors were evenly distributed between the 2 randomization groups and did not confound the results. OPV0 had the best effect among children enrolled early. Early enrollment could be a proxy for better socioeconomic status, as it is typically the more wealthy mothers who delivered at the maternity ward and were enrolled early. However, while it is difficult to imagine why OPV0 should be particularly beneficial for more privileged children, it seems plausible that OPV0 given early—into the almost sterile gut—would have more profound effects on the intestinal flora and thus the newborn immune system [32]. For instance, it was recently shown that children who received OPV0 and BCG within 48 hours of birth had higher excretion of the antimicrobial peptide human cathelicidin LL37 (P < .05) in stool at age 6 weeks than children vaccinated later [33].
We have previously reported 2 natural experiments with OPV0: one in 2004 [24], which was the reason for the present trial, and a smaller one in 2007 [30]. The results are not consistent. The first natural experiment showed that OPV0 was associated with increased male infant mortality; this was contradicted by the subsequent natural experiment, and by the present trial. Natural experiments with vaccines provide stronger evidence than observational studies based on self-selection to vaccines. Nonetheless, the divergent results suggest that the increased male mortality after OPV0 observed in 2004 was a chance finding. It should be noted, though, that one factor which has varied across the 3 studies is the intensity of OPV campaigns. In 2004 there were several campaigns, and none in 2007, but many during the present trial. Censoring for these campaigns made the results more comparable, as OPV0 tended to be most beneficial before additional OPV was given in campaigns.
In the 2007 study [30], comparing OPV0 vs no OPV0 in children who were aged 0–2 days at the time of BCG vaccination was associated with an HR of 0.20 (95% CI, .07–.58). The present randomized trial supported the idea that OPV0 is beneficial when administered within 2 days of life. The first natural experiment in 2004 did not show the same tendency (C. S. Benn, unpublished data), and thus again seems to be an outlier.
Several previous studies have suggested that OPV might have a beneficial effect on child survival; for example, when DTP was missing in Guinea-Bissau, children who received only OPV had significantly lower hospital case fatality than children who received the recommended combination of OPV and DTP [19]. In a small randomized trial of OPV0 vs vitamin A to low-birth-weight boys who did not receive BCG at birth, there was a nonsignificant 32% lower MRR for boys randomized to OPV0 [34].
The present trial contradicted our initial hypothesis that OPV0 was associated with higher male mortality [24]. This hypothesis has also been contradicted in another natural experiment [30]. The present randomized trial is clearly the strongest evidence so far. When the effect of subsequent campaign OPV was excluded by censoring to estimate the “true” effect, OPV0 was associated with a borderline significant 32% reduction in infant mortality. A cautious interpretation, backed by the fact that other routine live attenuated vaccines have also been shown in randomized trials to harness beneficial NSEs [9–11], seems to be that OPV0 administered with BCG in the first days of life lowers infant mortality. As there were no polio cases in Guinea-Bissau during the conduct of the trial, this effect is evidently nonspecific, possibly related to some form of innate immune training, as has previously been shown for BCG [35].
CONCLUSIONS
The trial is the only randomized trial evaluating the possible impact of OPV0 on survival. Administration of OPV0, particularly in the first days of life and in the absence of OPV campaigns, may have a marked beneficial impact on infant survival. The global health community intends to replace OPV with inactivated polio vaccine in the endgame for polio because the attenuated virus in OPV can, in rare cases, cause vaccine-associated polio paralysis. If OPV0 has beneficial nonspecific effects, this strategy may have negative effects on overall survival. Hence, there are reasons to monitor early mortality when OPV is phased out or replaced with inactivated polio vaccine.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Author contributions. N. L., P. A., C. S. B., A. R., and H. R. conceptualized and designed the study. N. L., A. S. K. H., F. S. J., A. B., S. B.-S., and A. R. took part in the primary data collection. N. L., A. A., and H. R. performed the statistical analyses. N. L. drafted the initial manuscript. All other authors critically revised the manuscript for important intellectual content. N. L. is the guarantor of the paper. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Financial support. This work was supported by the European Research Council (ERC-2009-StG, grant agreement no. 243149 to C. S. B.). Additional support was obtained from the Lundbeck Foundation, the Novo Nordisk Foundation, the Aase and Ejnar Danielsen Foundation, the Foundation for Medical Students at Copenhagen University, and Arvid Nilsson's Foundation. N. L. was funded by a grant from the Danish Council for Independent Research (to C. S. B.). The Department of Pediatrics, Kolding Hospital, also provided support for N. L.'s salary. P. A. holds a research professorship grant from the Novo Nordisk Foundation. The Research Center for Vitamins and Vaccines (CVIVA) is funded by the Danish National Research Foundation (DNRF108).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Roth A, Jensen H, Garly ML et al. Low birth weight infants and Calmette-Guérin bacillus vaccination at birth: community study from Guinea-Bissau. Pediatr Infect Dis J 2004; 23:544. [DOI] [PubMed] [Google Scholar]
- 2.Roth A, Garly ML, Jensen H, Nielsen J, Aaby P. Bacillus Calmette-Guerin vaccination and infant mortality. Expert Rev Vaccines 2006; 5:277–93. [DOI] [PubMed] [Google Scholar]
- 3.Koenig MA, Khan MA, Wojtyniak B et al. Impact of measles vaccination on childhood mortality in rural Bangladesh. Bull World Health Organ 1990; 68:441. [PMC free article] [PubMed] [Google Scholar]
- 4.Aaby P, Samb B, Simondon F et al. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ 1995; 311:481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aaby P, Bhuiya A, Nahar L et al. The survival benefit of measles immunization may not be explained entirely by the prevention of measles disease: a community study from rural Bangladesh. Int J Epidemiol 2003; 32:106–15. [DOI] [PubMed] [Google Scholar]
- 6.Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ 2000; 321:1435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aaby P, Benn C, Nielsen J et al. Testing the hypothesis that diphtheria-tetanus-pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open 2012; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benn CS, Netea MG, Selin LK, Aaby P. A small jab—a big effect: nonspecific immunomodulation by vaccines. Trends Immunol 2013; 34:431–9. [DOI] [PubMed] [Google Scholar]
- 9.Aaby P, Roth A, Ravn H et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis 2011; 204:245–52. [DOI] [PubMed] [Google Scholar]
- 10.Biering-Sorensen S, Aaby P, Napirna BM et al. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guerin vaccination at first health center contact. Pediatr Infect Dis J 2012; 31:306–8. [DOI] [PubMed] [Google Scholar]
- 11.Aaby P, Martins CL, Garly ML et al. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ 2010; 341:c6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aaby P, Jensen H, Gomes J, Fernandes M, Lisse IM. The introduction of diphtheria-tetanus-pertussis vaccine and child mortality in rural Guinea-Bissau: an observational study. Int J Epidemiol 2004; 33:374. [DOI] [PubMed] [Google Scholar]
- 13.Aaby P, Jensen H, Walraven G. Age-specific changes in the female-male mortality ratio related to the pattern of vaccinations: an observational study from rural Gambia. Vaccine 2006; 24:4701–8. [DOI] [PubMed] [Google Scholar]
- 14.Aaby P, Biai S, Veirum JE et al. DTP with or after measles vaccination is associated with increased in-hospital mortality in Guinea-Bissau. Vaccine 2007; 25:1265–9. [DOI] [PubMed] [Google Scholar]
- 15.Aaby P, Benn CS, Nielsen J et al. DTP vaccination and child survival in observational studies with incomplete vaccination data. Trop Med Int Health 2007; 12:15–24. [DOI] [PubMed] [Google Scholar]
- 16.Biai S, Rodrigues A, Nielsen J, Sodemann M, Aaby P. Vaccination status and sequence of vaccinations as risk factors for hospitalisation among outpatients in a high mortality country. Vaccine 2011; 29:3662–9. [DOI] [PubMed] [Google Scholar]
- 17.Veirum JE, Sodemann M, Biai S et al. Routine vaccinations associated with divergent effects on female and male mortality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine 2005; 23:1197–204. [DOI] [PubMed] [Google Scholar]
- 18.Meeting of the Strategic Advisory Group of Experts on immunization, April 2014 — conclusions and recommendations. Wkly Epidemiol Rec 2014; 89:221–36. [PubMed] [Google Scholar]
- 19.Aaby P, Rodrigues A, Biai S et al. Oral polio vaccination and low case fatality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine 2004; 22:3014–7. [DOI] [PubMed] [Google Scholar]
- 20.Aaby P, Hedegaard K, Sodemann M et al. Childhood mortality after oral polio immunisation campaign in Guinea-Bissau. Vaccine 2005; 23:1746–51. [DOI] [PubMed] [Google Scholar]
- 21.Contreras G. Sabin's vaccine used for nonspecific prevention of infant diarrhea of viral etiology. Bull Pan Am Health Organ 1974; 8:123–32. [PubMed] [Google Scholar]
- 22.Contreras G. Effect of the administration of oral poliovirus vaccine on infantile diarrhoea mortality. Vaccine 1989; 7:211–2. [DOI] [PubMed] [Google Scholar]
- 23.Global advisory group. Expanded programme on immunization. Wkly Epidemiol Rec 1985; 60:13–20. [Google Scholar]
- 24.Benn CS, Fisker AB, Rodrigues A et al. Sex-differential effect on infant mortality of oral polio vaccine administered with BCG at birth in Guinea-Bissau. A natural experiment. PLoS One 2008; 3:e4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sartono E, Lisse IM, Terveer EM et al. Oral polio vaccine influences the immune response to BCG vaccination. A natural experiment. PLoS One 2010; 5:e10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benn CS, Martins C, Rodrigues A, Lisse IM, Aaby P. Randomised study of the impact of different doses of vitamin A on childhood morbidity and mortality. BMJ 2005; 331:1428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen AS, Lund N, Flanagan KL et al. Randomized trial: the effect of oral polio vaccine at birth on polio antibody titers at 6 weeks and 6 months of age. Trials Vaccinol 2014; 3:33–9. [Google Scholar]
- 28.Eriksen HB, Lund N, Biering-Sørensen S et al. Does oral polio vaccine at birth affect the size of the thymus? Observations within a randomized trial. Vaccine 2014; 32:3293–9. [DOI] [PubMed] [Google Scholar]
- 29.Jensen KJ, Karkov HS, Lund N et al. The immunological effects of oral polio vaccine provided with BCG vaccine at birth: a randomised trial. Vaccine 2014; 32:5949–56. [DOI] [PubMed] [Google Scholar]
- 30.Lund N, Andersen A, Monteiro I, Aaby P, Benn CS. No Effect of oral polio vaccine administered at birth on mortality and immune response to BCG. A natural experiment. Vaccine 2012; 20:6694–9. [DOI] [PubMed] [Google Scholar]
- 31.Benn CS, Diness BR, Roth A et al. Effect of 50,000 IU vitamin A given with BCG vaccine on mortality in infants in Guinea-Bissau: randomised placebo controlled trial. BMJ 2008; 336:1416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tourneur E, Chassin C. Neonatal immune adaptation of the gut and its role during infections. Clin Dev Immunol 2013; doi:10.1155/2013/270301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alam J, Rashid M, Kabir Y, Raqib R, Ahmad SM. On birth single dose live attenuated OPV and BCG vaccination induces gut cathelicidin LL37 responses at 6 week of age: a natural experiment. Vaccine 2015; 33:18–21. [DOI] [PubMed] [Google Scholar]
- 34.Lund N, Biering-Sørensen S, Andersen A et al. Neonatal vitamin A supplementation associated with a cluster of deaths and poor early growth in a randomised trial among low-birth-weight boys of vitamin A versus oral polio vaccine at birth. BMC Pediatr 2014; 14:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleinnijenhuis J, Quintin J, Preijers F et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012; 109:17537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.