Abstract
Humans share with non-human animals perceptual biases that might form the basis of complex cognitive abilities. One example comes from the principles described by the iambic–trochaic law (ITL). According to the ITL, sequences of sounds varying in duration are grouped as iambs, whereas sequences varying in intensity are grouped as trochees. These grouping biases have gained much attention because they might help pre-lexical infants bootstrap syntactic parameters (such as word order) in their language. Here, we explore how experience triggers the emergence of perceptual grouping biases in a non-human species. We familiarized rats with either long–short or short–long tone pairs. We then trained the animals to discriminate between sequences of alternating and randomly ordered tones. Results showed animals developed a grouping bias coherent with the exposure they had. Together with results observed in human adults and infants, these results suggest that experience modulates perceptual organizing principles that are present across species.
Keywords: iambic–trochaic law, perceptual bias, experience, rats
1. Introduction
To make sense of the world, human as well as non-human animals use a series of perceptual organizing principles that help them interact with the environment in an organized manner. For example, recently hatched chicks already display a behaviour compatible with the Kanizsa illusion: if reared in the presence of a partially occluded object, they will later prefer a complete version of the object even if they have never seen it before [1]. In the auditory modality, cotton-top tamarin monkeys complete information lacking in conspecific calls, in a phenomenon similar to phoneme restoration in humans. They respond to artificially modified calls just as they respond to intact calls [2]. A key question in comparative cognitive research is to understand how these organizing principles emerge. In this study, we focus on the grouping biases described by the iambic–trochaic law (ITL), and explore how the appropriate experience might help to develop them.
The ITL was first proposed in the music domain [3] and described how sequences are grouped depending on specific cues. According to the ITL, sequences varying in duration are grouped as iambs, with the strong element at the end of the sequence. Sequences varying in intensity are grouped as trochees, with the strong element at the beginning of the sequence. Recently, research on the ITL has gained much attention, because the general grouping principles it describes have been found to apply to speech at the phrasal level [4], where pitch in addition to intensity has been shown to characterize trochaic prominence, and might be at the root of how human infants bootstrap complex syntactic regularities in speech. At the phonological phrase level, in fact, trochaic rhythm characterizes object–verb languages, and iambic rhythm characterizes verb–object languages [5]. Extensive experimental work has demonstrated that human adults and infants [6–10] group sequences according to the ITL.
However, there seem to be different timelines and conditions for the iambic and the trochaic grouping principles to emerge. Trochaic grouping has been observed from very early ages and across different linguistic backgrounds [7,8]. Iambic grouping on the contrary appears only after seven months of age [9,11], and it might be modulated by the listener's native language [11–13]. This suggests that experience likely plays a central role in the development of the iambic grouping principle. More central to this study, there is no evidence that the iambic bias is present in non-human animals. It has been shown that when rats are presented with sequences varying in pitch, they group them as trochees. However, when rats are presented with sequences varying in duration, there is no evidence for any grouping [14]. This raises the question of whether a non-human animal might develop this grouping bias if given the appropriate experience. Here, we tackle this issue by exploring how the iambic grouping bias develops depending on experience.
2. Material and methods
(a). Subjects
Subjects were 22 Long–Evans rats (four males) of four months of age. They were food-deprived until they reached 85% of their free-feeding weight. They had access to water ad libitum. Food was administered after each training session. Half of the animals were assigned to the long–short condition (LS, n = 11) and the other half to the short–long condition (SL, n = 11).
(b). Stimuli
Stimuli were 16 alternating (AS) and 16 random sequences (RS). AS were composed by the concatenation of 16 pure tones with a fundamental frequency of 440 Hz each. AS alternated short (200 ms) and longer tones (350, 400, 450 or 500 ms; figure 1), resulting in sequences such as (in ms) 200–350–200–500–200–400–200–450–200–500–200–400–200–450–200–350. Half of the AS started with a short tone, and half with a long tone. The same tones were combined at random to form the RS. In RS, there was no systematic alternation of short and long tones (e.g. 450–500–200–350–200–200–200–200–450–400–400–200–500–350–200–200). Tones in all sequences were separated by 200 ms. Every sequence lasted 8 s and was faded 1 s both at its onset and at its offset. This made individual tones at the beginning and at the end of the sequences gradually more and less notable, respectively. We also created eight tone pairs. There were four short–long pairs (420–525, 420–630, 420–735, 420–840 Hz), and four long–short pairs (525–420, 630–420, 735–420, 840–420 Hz). Four of these pairs were used during familiarization (short–long 420–525, 420–735; long–short 525–420, 735–420). The remaining four were used during the test. All tones were synthesized with Amadeus II software at a sampling rate of 44.1 kHz, and a sampling size of 16 bit. Fundamental frequency and duration of all tones are within the range that rats can easily perceive [15].
Figure 1.
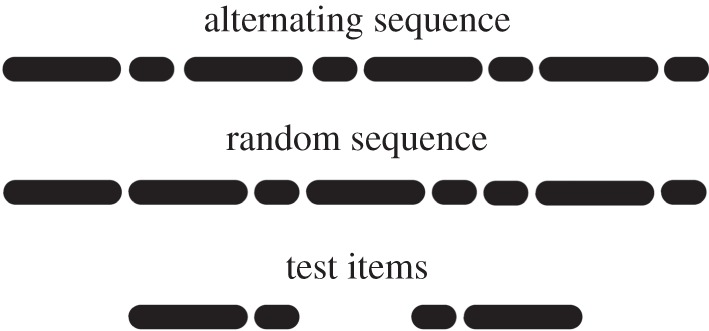
Schematic organization of short and long tones on alternating, random and test sequences.
(c). Apparatus
For each experimental session, rats were placed in response boxes. A computer using a custom-made program presented the stimuli, recorded the lever-press responses and provided reinforcement. A Pioneer stereo amplifier A-445 and an EV (s-40) speaker, located beside each box, were used to present the stimuli.
(d). Procedure
Before the experiment began, rats were trained to press a lever to obtain food pellets. When the rats reached stable response rates, we started the discrimination training. The discrimination training consisted of 30 sessions, one session per day. During each training session, rats were placed individually in a response box. At the beginning of each session, they were familiarized with two pairs of tones (two short–long tone pairs for the animals in the SL condition, and two long–short tone pairs for the animals in the LS condition). Each pair was repeated 10 times, for a total of 20 presentations balanced across pairs. There was an interpair interval of 30 s. No food was delivered during familiarization with the pairs of tones. The purpose of this familiarization phase was to provide the animals with the experience of a regular group of tones (either short–long or long–short, depending on the condition). After familiarization, 32 sequences (16 AS and 16 RS) were presented with an intersequence interval of 60 s. The sequence presentation was balanced within each session, so that no more than three sequences of the same type would be presented in a row. Every time an AS was presented, food was delivered after lever-pressing responses. No food was delivered, no matter how often the rat pressed the lever, after the presentation of RS.
After 30 training sessions a test session was run. Instead of sequences, four new pairs of tones were presented (two short–long and two long–short pairs). These pairs were different from those used during familiarization. Each pair was presented twice, so the test session consisted of eight test trials. Pair presentation was randomized with the only restriction that no more than two pairs of the same type were presented in a row. As in the training phase, there were 60 s between the presentations of each pair. Food was delivered after both short–long and long–short pairs. All experimental procedures were conducted in accordance with Catalan, Spanish and European guidelines and received the necessary ethical approval by the ethical committee.
3. Results
Animals learnt to discriminate between AS and RS during training. An analysis of variance with the factors familiarization condition (long–short, short–long) and sequence (AS, RS), showed differences between sequences (F1,116 = 72.82, p < 0.005), but no differences between either familiarization conditions (F1,116 = 0.159, p = 0.69), or interaction between them (F1,116 = 0.221, p = 0.639). A different pattern emerged during the test. An analysis of variance with the factors familiarization condition (long–short, short–long) and type of test (long–short, short–long) revealed no main effects (familiarization condition: F1,20 = 0.036, p = 0.85; type of test: F1,20 = 0.24, p = 0.62). However, we observed a significant interaction between the factors (F1,20 = 11.23, p < 0.005; figure 2). For rats familiarized with the long–short pairs of tones, the mean number of responses to long–short test pairs was higher (M = 40.6, s.d. = 6.88) than the mean number of responses to short–long test pairs (M = 35.66, s.d. = 9.19; t10 = 2.35, p < 0.05). Conversely, for rats familiarized with the short–long pairs of tones, the mean number of responses to short–long test pairs was higher (M = 39.25, s.d. = 10.47) than the mean number of responses to long–short test pairs (M = 35.57, s.d. = 10.05; t10 = 2.47, p < 0.05). That is, the animals seemed to develop an experience-dependent grouping bias based on the regularities presented during familiarization.
Figure 2.
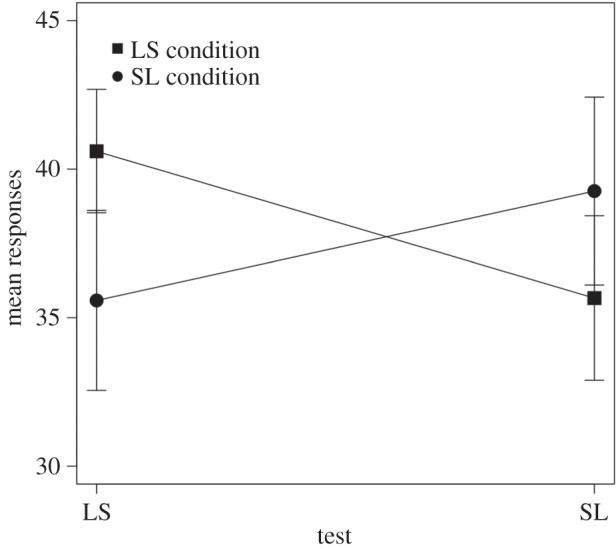
Mean number of lever-pressing responses and standard error bars for long–short (LS) and short–long (SL) test pairs depending on familiarization condition.
One could argue that the differences observed during the test are not related to the development of a grouping strategy by the animals, but instead reflect a simple effect of familiarity with the set of tone pairs (either long–short or short–long) that were presented before training. However, this is unlikely to be the case, because the specific tones used during the test were different from those used during training. But it is possible that the animals learnt the relation between the tones (strong–weak or weak–strong) that were presented during familiarization, and that they were just responding more to more familiar relations. In this case, reinforcement during AS would not have been relevant for the pattern of responses observed during the test. To explore this possibility, we ran a control experiment. This was identical to the previous experiment, except that RS were reinforced rather than AS. This was done, so that it was not possible to establish a link during training between coherent groups (either long–short or short–long) and food delivery. New animals (n = 20, half for each condition) were assigned to the LS and to the SL familiarization conditions. The animals learnt to discriminate between AS and RS (F1,116 = 52.47, p < 0.005) during training, with no differences across familiarization conditions (F1,116 = 0.118, p = 0.732). However, during the test, there were also no differences in responses across familiarization conditions (F1,18 = 0.046, p = 0.833), types of test (F1,18 = 1.228, p = 0.282) or interactions among them (F1,18 = 0.085, p = 0.774). The animals did not show any differences in responding to long–short or short–long test pairs in this control experiment, independently of the condition (LS condition: t9 = 0.492, p = 0.634; SL condition: t9 = 1.25, p = 0.242). When compared with the results from the previous experiment, we observed a difference between them (F1,38 = 7.678, p < 0.01). More importantly, there was an interaction between experiment and familiarization condition (F1,38 = 4.139, p < 0.05); while test responses were modulated by familiarization condition in the first experiment, no modulation was observed in the control experiment. Thus, our results demonstrate that familiarization by itself does not lead to differential responding to test pairs. It is only when animals are reinforced for responding to specific alternating sequences that familiarized tone pairs become relevant.
4. Discussion
We explored how experience might modulate the emergence of a grouping bias in a non-human animal. The results suggest that passive exposure to a regular pattern (either strong–weak or weak–strong sound pairs) leads animals to consistently group sequences varying in duration as either strong–weak or weak–strong, depending on familiarization. Research on the ITL in humans suggests that linguistic experience modulates how sequences varying in duration are grouped. While speakers of languages such as English or Spanish show a consistent weak–strong grouping bias, speakers of languages such as Japanese or Basque do not [11–13]. To the best of our knowledge, no strong–weak grouping bias has been reported in humans for sequences varying in duration (although there is a trend in this direction for Japanese speakers in the study by Iversen et al. [12]). Thus, speakers of verb–object languages might be directed to iambic grouping biases by experience with their language of exposure. Rats are very good subjects to address questions about the role of experience in the development of acoustic biases. They are non-human animals that do not use complex interspecific vocalizations as a mean of communication. They thus do not have extensive experience in producing or processing biologically relevant sounds that might give rise to the iambic grouping bias. In fact, when presented with sequences varying in duration, they do not tend to group them consistently without the kind of pre-exposure presented here [14].
Human and non-human animals share a wide variety of perceptual abilities that have been shown to lay the foundations for highly complex tasks. These include the ability to detect both affixation-like patterns [16] and long-distance dependencies [17]. A key issue is thus to understand the relevant variables that allow the development of such seemingly universal perceptual abilities. In this context, this work highlights how experience might allow the emergence of grouping biases that are shared across distant species.
Supplementary Material
Supplementary Material
Ethics
All experimental procedures were approved by the ethical committee from the Universitat Pompeu Fabra and the Generalitat de Catalunya (protocol number 7872).
Data accessibility
The datasets supporting this article have been uploaded as electronic supplementary material.
Authors' contributions
J.M.T. and M.N. designed the study. J.M.T. ran the experiments. J.M.T. and M.N. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Research supported by the European Research Council, ERC grant agreement no. 269502 (PASCAL) to M.N. and ERC grant agreement no. 312519 to J.M.T.
References
- 1.Regolin L, Vallortigara G. 1995. Perception of partially occluded objects by young chicks. Percept. Psychophys. 57, 971–976. ( 10.3758/BF03205456) [DOI] [PubMed] [Google Scholar]
- 2.Miller C, Dibble E, Hauser M. 2001. Amodal completion of acoustic signals by a nonhuman primate. Nat. Neurosci. 4, 783–784. ( 10.1038/90481) [DOI] [PubMed] [Google Scholar]
- 3.Bolton T. 1894. Rhythm. Am. J. Psychol. 6, 145–238. ( 10.2307/1410948) [DOI] [Google Scholar]
- 4.Nespor M, Vogel I. 2008. Prosodic phonology. Dordrecht, The Netherlands: Foris. [Google Scholar]
- 5.Nespor M, Shukla M, van de Vijver R, Avesani C, Schraudolf H, Donati C. 2008. Different phrasal prominence realizations in VO and OV languages. Lingue e Linguaggio 2, 1–29. [Google Scholar]
- 6.Bhatara A, Boll-Avetisvan N, Unger A, Nazzi T, Höhle B. 2013. Native language affects rhythmic grouping of speech. J. Acoust. Soc. Am. 134, 3828–3843. ( 10.1121/1.4823848) [DOI] [PubMed] [Google Scholar]
- 7.Bion R, Benavides-Varela S, Nespor M. 2011. Acoustic markers of prominence influence infants’ and adults’ segmentation of speech sequences. Lang. Speech 54, 123–140. ( 10.1177/0023830910388018) [DOI] [PubMed] [Google Scholar]
- 8.Hay JF, Diehl R. 2007. Perception of rhythmic grouping: testing the iambic/trochaic law. Percept. Psychophys. 69, 113–122. ( 10.3758/BF03194458) [DOI] [PubMed] [Google Scholar]
- 9.Hay JF, Saffran JR. 2012. Rhythmic grouping biases constrain infant statistical learning. Infancy 17, 610–641. ( 10.1111/j.1532-7078.2011.00110.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peña M, Bion R, Nespor M. 2011. How modality specific is the iambic–trochaic law? Evidence from vision. J. Exp. Psychol. Learn. Mem. Cogn. 37, 1199–1208. ( 10.1037/a0023944) [DOI] [PubMed] [Google Scholar]
- 11.Yoshida KA, Iversen JR, Patel AD, Mazuka R, Nito H, Gervain J, Werker JF. 2010. The development of perceptual grouping biases in infancy: a Japanese–English cross-linguistic study. Cognition 115, 356–361. ( 10.1016/j.cognition.2010.01.005) [DOI] [PubMed] [Google Scholar]
- 12.Iversen JR, Patel AD, Ohgushi K. 2008. Perception of rhythmic grouping depends on auditory experience. J. Acoust. Soc. Am. 124, 2263–2271. ( 10.1121/1.2973189) [DOI] [PubMed] [Google Scholar]
- 13.Molnar M, Lallier M, Carreiras M. 2014. The amount of language exposure determines nonlinguistic tone grouping biases in infants from a bilingual environment. Lang. Learn. 64, 45–64. ( 10.1111/lang.12069) [DOI] [Google Scholar]
- 14.de la Mora D, Nespor M, Toro JM. 2013. Do humans and non-human animals share the grouping principles of the iambic–trochaic law? Attent. Percept. Psychophys. 75, 92–100. ( 10.3758/s13414-012-0371-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly JB, Cooke JE, Gilbride PC, Mitchell C, Zhang H. 2006. Behavioral limits of auditory temporal resolution in the rat: amplitude modulation and duration discrimination. J. Comp. Psychol. 120, 98–105. ( 10.1037/0735-7036.120.2.98) [DOI] [PubMed] [Google Scholar]
- 16.Endress A, Cahill D, Block S, Watumull J, Hauser M. 2009. Evidence of an evolutionary precursor to human language affixation in a non-human primate. Biol. Lett. 5, 749–751. ( 10.1098/rsbl.2009.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravignani A, Sonnweber R, Stobbe N, Fitch T. 2013. Action at a distance: dependency sensitivity in a New World primate. Biol. Lett. 9, 20130852 ( 10.1098/rsbl.2013.0852) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as electronic supplementary material.


