Abstract
Domesticated animals tend to develop a coherent set of phenotypic traits. Tameness could be a central underlying factor driving this, and we therefore selected red junglefowl, ancestors of all domestic chickens, for high or low fear of humans during six generations. We measured basal metabolic rate (BMR), feed efficiency, boldness in a novel object (NO) test, corticosterone reactivity and basal serotonin levels (related to fearfulness) in birds from the fifth and sixth generation of the high- and low-fear lines, respectively (44–48 individuals). Corticosterone response to physical restraint did not differ between selection lines. However, BMR was higher in low-fear birds, as was feed efficiency. Low-fear males had higher plasma levels of serotonin and both low-fear males and females were bolder in an NO test. The results show that many aspects of the domesticated phenotype may have developed as correlated responses to reduced fear of humans, an essential trait for successful domestication.
Keywords: genetics, domestication, stress
1. Background
Animal domestication changed human history and was used by Darwin as a proof of principle for evolution [1]. During domestication, animals adapt through genetic and learning mechanisms to living with humans, and simultaneously develop a suite of traits, the ‘domesticated phenotype’ [2]. This may include increased size and reproduction. Mechanisms underlying this phenotypic complex recurring in many species are largely unknown, but it has been suggested that they may partly be side effects of reduced fear of humans [3,4]. However, it is not clear if tameness is a unique trait or reflects generally increased boldness and reduced stress susceptibility.
Chickens were domesticated 8000 years ago and are now the most abundant food-producing animals [5]. In a previous experiment, ancestral red junglefowl selected only for reduced fear of humans developed correlated traits, e.g. higher food competitiveness, larger eggs and bigger offspring [6]. Hence, less fear of humans could be associated with higher basal metabolic rate (BMR) and more efficient food conversion [7], for example through linkage effects [8]. Changes in levels of serotonin (5-HT) may be involved, since high peripheral 5-HT levels are associated with less fear and anxiety in chickens [9].
We studied BMR, feed conversion and 5-HT levels, as well as boldness and stress responses in red junglefowl, selected during five or six generations for high or low fear of humans, respectively.
2. Material and methods
The complete data set is available as electronic supplementary material (table S1). We studied red junglefowl from the fifth (S5) and sixth (S6) generation of populations, divergently selected for low (L-birds) versus high (H-birds) fear of humans. The breeding scheme and housing conditions have been described elsewhere [6,10]. Briefly, birds bred and maintained with identical experiences of humans were selected in each generation based on divergent scores in a fear-of-human test. Each generation was maintained at about 50 birds per selection line, from 5 to 10 families per generation and selection line. The birds were hatched and reared under standardized conditions in mixed groups (see below).
In S5, we measured BMR using open flow respirometry as previously described [11] in 48 birds (six females and nine males from the low-fear line; 15 females and 18 males from the high-fear line) at five to six weeks of age. Four birds were individually placed in 3.6 l opaque tight plastic containers (CurTec) in a thermostatically controlled chamber at 24°C (Rubarth GmbH type 3001) for overnight measurements in darkness (18.00–06.00 h, similar to their normal diurnal light pattern). Airflow through the chambers was set at 1000 ml min−1. Flow was measured continuously in all chambers using a multi-channel flow meter (FlowBar8, Sable Systems Inc.). A computer controlled multiplexer (Sable Systems Inc.) allowed the cycling of oxygen measurements between all chambers and a baseline channel. Oxygen concentration in the sample channel was measured with a FoxBox oxygen meter (Sable Instruments Inc.). BMR was assessed from the lowest 90 min average oxygen consumption during the night, which corresponds to three consecutive measurements per chamber
When 19 weeks old (135 days), birds were placed in individual battery cages (60 × 40, height 40 cm) with water, perch, nest box and dust bath, and we measured the feed intake during 7 days in eight females and 10 males from the low-fear line, and 14 females and 12 males from the high-fear line. Feed was offered ad libitum in a spill-safe food-container, and consumption was assessed by weighing feed remains daily. As a proxy for feed conversion (‘feed efficiency’), we divided the average weekly growth of each bird between days 112 and 200 by its total feed intake during these 7 days.
At 21 weeks of age, when the birds were still in the cages, we performed a novel object (NO) test in the same birds, as a measure of boldness. An egg was cracked in the feed trough (known to be attractive food), and when the bird started eating from it, an NO (33 cl Coca Cola can) was placed 10 cm from the egg. During the test, the observer was hidden behind a screen. We recorded latency to start feeding after the NO had been placed in the trough, with a max time of 180 s.
In S6 at 28 weeks of age, we assessed plasma corticosterone levels before and after a short period of physical restraint, using methods described in detail earlier [12]. Briefly, each bird (eight males and seven females from the low line; six males and eight females from the high line) was removed from its individual cage, and a blood sample was obtained. It was then stressed by being placed in a hanging fish net for 10 min, followed by a second blood sample, after which it was returned to its home cage. A third blood sample was collected 30 min after the first one. When the S6 birds were 47 weeks old, blood samples were obtained in undisturbed conditions during mid-day, from 19 males and 10 females from the low-fear line, and six males and 10 females from the high-fear line, for serotonin analysis. Blood was centrifuged at 900g at room temperature for 20 min and the plasma was stored at –18°C until analysis.
Corticosterone was measured with an enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Sciences, NY, USA) following the manufacturer's instructions. All samples were tested in duplicate and the analytic range of the assay was 32–20 000 pg ml−1. The concentration of serotonin in the plasma samples was determined in duplicates using a commercial serotonin ELISA kit (analytic range 6.25–200 ng ml−1, MyBiosource, San Diego, CA, USA).
Data were analysed with generalized linear models (linear mixed models for repeated measures on corticosterone), using SPSS v. 22.0, with ‘selection’ (high versus low) and ‘sex’ (male versus female) as well as their interaction as predictors. We used the scale response ‘linear’ and the link function ‘identity’, except for the truncated variable ‘latency to eat’ in the NO test, where we used gamma distribution with log link. The Omnibus test was significant in all cases (p < 0.05). Significance was determined by the Wald χ2-statistics for generalized linear models, and F-statistics for mixed models, and estimated marginal means were calculated where applicable.
3. Results
BMR was significantly higher in L-birds (figure 1a). There was also a significant sex effect, where females had lower BMR than males, but no interaction was found between sex and selection.
Figure 1.
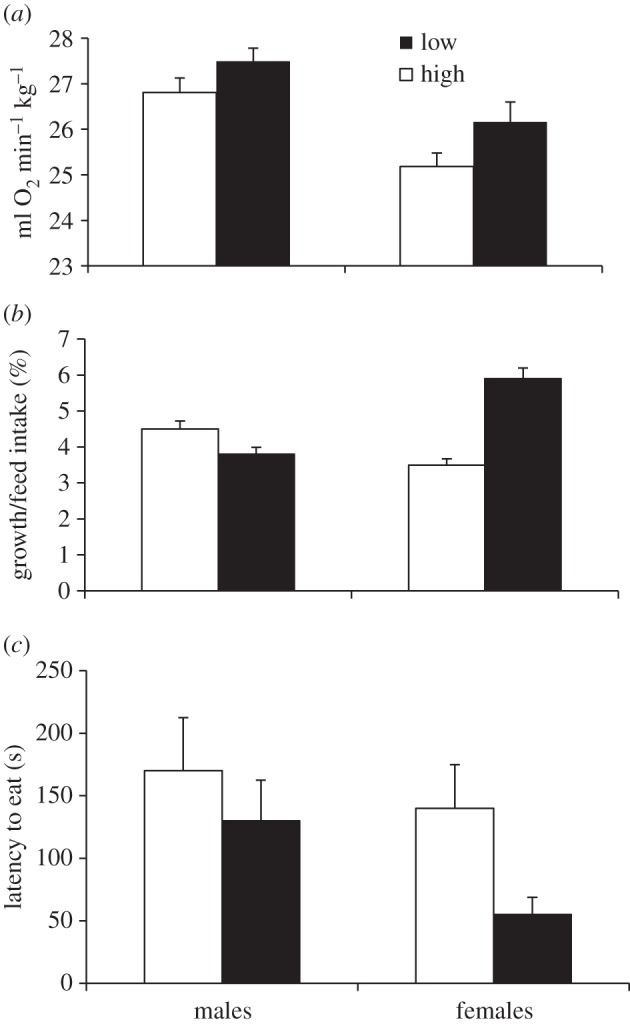
Metabolic rates, feed efficiency and boldness in the two selection lines (high and low fear of humans; estimated marginal means with SEM). (a) BMR (millilitre oxygen per minute and kilogram bodyweight) in males and females. Effects of selection: 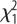 = 5.4, p = 0.02; sex:
= 5.4, p = 0.02; sex: 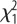 = 17.1, p < 0.001; selection × sex:
= 17.1, p < 0.001; selection × sex: 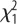 = 0.16, p = 0.68. (b) Feed efficiency, in males and females. Effects of selection:
= 0.16, p = 0.68. (b) Feed efficiency, in males and females. Effects of selection: 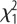 = 3.5, p = 0.061; Sex:
= 3.5, p = 0.061; Sex: 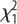 = 1.52, p = 0.21; selection × sex:
= 1.52, p = 0.21; selection × sex:  = 10.5, p = 0.001. (c) Latency to eat in the NO test for males and females of both selection lines. Selection:
= 10.5, p = 0.001. (c) Latency to eat in the NO test for males and females of both selection lines. Selection: 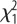 = 4.1, p = 0.044; sex:
= 4.1, p = 0.044; sex: 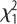 = 6.37, p = 0.012; selection × sex:
= 6.37, p = 0.012; selection × sex: 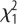 = 1.67, p = 0.20.
= 1.67, p = 0.20.
There was a tendency for higher feed efficiency in L-birds, but no sex effects (figure 1b). However, the interaction was significant, showing that the females principally drove the effect. L-birds weighed significantly more than H-birds at 112 (825 ± 10.9 g versus 691 ± 12.7 g; 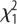 = 64.2, p < 0.001) and 200 days (1054 ± 16.0 g versus 845 ± 13.2 g;
= 64.2, p < 0.001) and 200 days (1054 ± 16.0 g versus 845 ± 13.2 g; 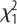 = 101.4, p < 0.001).
= 101.4, p < 0.001).
In the NO test, L-birds (particularly females) were significantly bolder (figure 1c). There was a significant effect of time after restraint on corticosterone levels (F1,65 = 94.7, p < 0.001) but no effects of selection or sex or its interactions (figure 2a). Serotonin levels were significantly higher in L-males, as shown by a significant selection × sex interaction (figure 2b).
Figure 2.
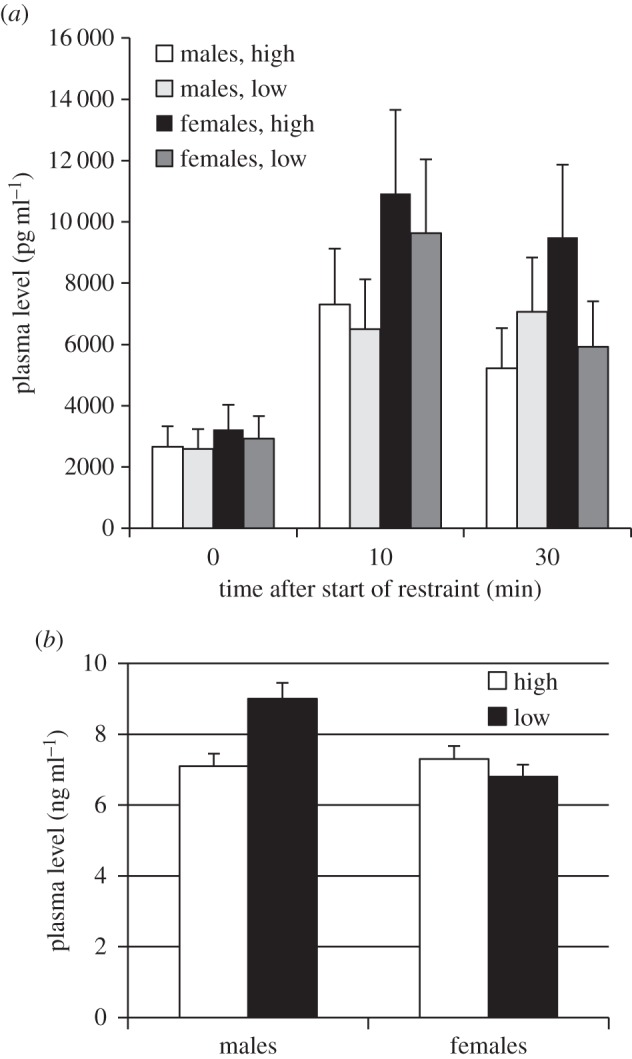
Physiological responses to selection in the two lines (high and low fear of humans; estimated marginal means with SEM). (a) Plasma levels of corticosterone in males and females at baseline, after 10 min of physical restraint, and 30 min after first blood sample. Effects of selection: F1,41 = 0.17, p = 0.68; sex: F1,41 = 1.02, p = 0.32: selection × sex: F1,41 = 0.27, p = 0.61. (b) Plasma levels of serotonin (5-HT) in males and females. Effects of selection:  = 1.1, p = 0.29; sex:
= 1.1, p = 0.29; sex: 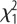 = 6.0, p = 0.014; selection × sex:
= 6.0, p = 0.014; selection × sex: 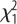 = 5.7, p = 0.017.
= 5.7, p = 0.017.
4. Discussion
Red junglefowl, intentionally selected during few generations for reduced fear of humans only, had higher BMR and tended to have higher feed efficiency, traits normally associated with the domesticated phenotype. The effects may be mediated by increased levels of serotonin.
Tameness is a product of both genetics and experience [2]. We maintained all birds under identical rearing conditions and selected them only based on fear of humans in a standardized test, so the differences between the selection lines should be mainly a consequence of genetic differences [2].
It is debated whether domestication phenotypes evolve due to independent selection of separate traits, or because they are genetically linked to some selected key trait. For example, Trut et al. [3] argued that morphological, physiological and behavioural traits evolved as a complex in artificially selected foxes because of linkage or pleiotropy in genes controlling tameness, whereas Rubin et al. [13] believed that the most likely explanation for domesticated colour phenotypes is independent selection specifically for each desired trait. Our results suggest that tameness may be a driving factor for the domesticated phenotype. They also indicate that increased secretion of 5-HT may be a mediating factor, at least in males, supporting earlier research showing a relationship between high 5-HT in blood and less fear in chickens, perhaps also affecting gastro-intestinal function [9]. Serotonin in plasma is correlated with platelet levels, but results should be interpreted with care [14]. The profound differences between the selection responses of the two sexes corroborate previous results on sex-specific genetic and behavioural responses to domestication [15].
We expected that low fear of humans in the selected red junglefowl would cause a general reduced stress and fear response. However, even though the selected birds differed in boldness (approaching an NO), they did not differ in corticosterone response to restraint.
In conclusion, our results support the hypothesis that reduced fear of humans during the early phases of chicken domestication may have caused birds to evolve a phenotypic complex involving increased boldness and a more growth-promoting metabolism. This could be driven by an enhanced serotonergic response.
Supplementary Material
Ethics
The experiments were approved by the Linköping Animal Ethics Committee, license no. 122-10.
Authors' contributions
B.A. and R.K. collected and analysed data; J.A. supervised the metabolic measurements and analyses; P.J. conceived and coordinated the study, and wrote the paper with input from all others. All authors gave final approval for publication.
Competing interests
Authors declare no competing interests.
Funding
The project was financed by the research council Formas, and ERC (grant no. 322206 GENEWELL).
References
- 1.Larson G, et al. 2014. Current perspectives and the future of domestication studies. Proc. Natl Acad. Sci. USA 111, 6139–6146. ( 10.1073/pnas.1323964111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price EO. 2003. Animal domestication and behavior. New York, NY: CABI Publishing. [Google Scholar]
- 3.Trut L, Oskina I, Kharlamova A. 2009. Animal evolution during domestication: the domesticated fox as a model. Bioessays 31, 349–360. ( 10.1002/bies.200800070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert FW, et al. 2008. Phenotypic differences in behavior, physiology and neurochemistry between rats selected for tameness and for defensive aggression towards humans. Horm. Behav. 53, 413–421. ( 10.1016/j.yhbeh.2007.11.010) [DOI] [PubMed] [Google Scholar]
- 5.Appleby MC, Mench JA, Hughes BO. 2004. Poultry behaviour and welfare. Wallingford, CT: Cabi Publishing. [Google Scholar]
- 6.Agnvall B, Ali A, Olby S, Jensen P. 2014. Red junglefowl (Gallus gallus) selected for low fear of humans are larger, more dominant and produce larger offspring. Animal 8, 1498–1505. ( 10.1017/S1751731114001426) [DOI] [PubMed] [Google Scholar]
- 7.Jackson S, Diamond J. 1996. Metabolic and digestive responses to artificial selection in chickens. Evolution 50, 1638–1650. ( 10.2307/2410900) [DOI] [PubMed] [Google Scholar]
- 8.Schütz KE, Kerje S, Jacobsson L, Forkman B, Carlborg O, Andersson L, Jensen P. 2004. Major growth QTLs in fowl are related to fearful behavior: possible genetic links between fear responses and production traits in a red junglefowl × white leghorn intercross. Behav. Genet. 34, 121–130. ( 10.1023/B:BEGE.0000009481.98336.fc) [DOI] [PubMed] [Google Scholar]
- 9.Bolhuis JE, et al. 2009. Effects of genetic group selection against mortality on behavior and peripheral serotonin in domestic laying hens with trimmed and intact beaks. Physiol. Behav. 97, 470–475. ( 10.1016/j.physbeh.2009.03.021) [DOI] [PubMed] [Google Scholar]
- 10.Agnvall B, Jöngren M, Strandberg E, Jensen P. 2012. Heritability and genetic correlations of fear-related behaviour in red junglefowl—possible implications for early domestication. PLoS ONE 7, e35162 ( 10.1371/journal.pone.0035162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindgren I, Altimiras J. 2013. Prenatal hypoxia programs changes in β-adrenergic signaling and postnatal cardiac contractile dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R1093–R1101. ( 10.1152/ajpregu.00320.2013) [DOI] [PubMed] [Google Scholar]
- 12.Ericsson M, Fallahsharoudi A, Bergquist J, Kushnir MM, Jensen P. 2014. Domestication effects on behavioural and hormonal responses to acute stress in chickens. Physiol. Behav. 133, 161–169. ( 10.1016/j.physbeh.2014.05.024) [DOI] [PubMed] [Google Scholar]
- 13.Rubin CJ, et al. 2012. Strong signatures of selection in the domestic pig genome. Proc. Natl Acad. Sci. USA 109, 19 529–19 536. ( 10.1073/pnas.1217149109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Audhya T, Adams JB, Johansen L. 2012. Correlation of serotonin levels in CSF, platelets, plasma, and urine. Biochim. Biophys. Acta 1820, 1496–1501. ( 10.1016/j.bbagen.2012.05.012) [DOI] [PubMed] [Google Scholar]
- 15.Nätt D, Agnvall B, Jensen P. 2014. Large sex differences in chicken behavior and brain gene expression coincide with few differences in promoter DNA-methylation. PLoS ONE 9, e96376 ( 10.1371/journal.pone.0096376) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


