Abstract
In a consistently urbanizing world, anthropogenic noise has become almost omnipresent, and there are increasing evidence that high noise levels can have major impacts on wildlife. While the effects of anthropogenic noise exposure on adult animals have been widely studied, surprisingly, there has been little consideration of the effects of noise pollution on developing organisms. Yet, environmental conditions experienced in early life can have dramatic lifelong consequences for fitness. Here, we experimentally manipulated the acoustic environment of free-living house sparrows (Passer domesticus) breeding in nest boxes. We focused on the impact of such disturbance on nestlings’ telomere length and fledging success, as telomeres (the protective ends of chromosomes) appear to be a promising predictor of longevity. We showed that despite the absence of any obvious immediate consequences (growth and fledging success), nestlings reared under traffic noise exposure exhibited reduced telomere lengths compared with their unexposed neighbours. Although the mechanisms responsible for this effect remain to be determined, our results provide the first experimental evidence that noise alone can affect a wild vertebrate's early-life telomere length. This suggests that noise exposure may entail important costs for developing organisms.
Keywords: anthropogenic noise, development, house sparrow, telomere length
1. Introduction
In many vertebrate species, it is widely established that environmental conditions experienced in early life can shape individual life histories, particularly due to influence on phenotypic development [1]. Over the last decade, there has been growing interest in understanding the mechanisms underlying the long-term consequences of developmental conditions on fitness [1]. Telomere length has recently been suggested as a relevant molecular tool to investigate this question because it appears to be a promising predictor of survival in wild vertebrates [2–4]. Made of repetitive non-coding sequences of DNA, telomeres protect chromosomes during cell division [2]. Telomeres shorten throughout the life of an organism, and this rate of shortening can be accelerated by environmental stressors [5,6]. Previous studies have shown that most telomere loss occurs in early life (e.g. [7]). Environmental conditions experienced during development are thus likely to be a particularly important driver of telomere shortening [8,9], and consequently, might entail important costs (e.g. reduced longevity [3]).
To date, most research addressing the impact of environmental conditions on phenotypic development has only considered changes in various aspects of the natural environment (e.g. nutritional conditions, sibling competition [1,8–10]). However, in a rapidly urbanizing world, organisms are exposed to novel environmental challenges [11], and in particular, to large increases in the level of background noise. Anthropogenic noise has become nearly omnipresent and can have major impacts on wildlife [12], making noise pollution a research priority. The effects of anthropogenic noise exposure on adult animals have been widely studied, mainly in the context of acoustic communication (reviewed in [12]). But surprisingly, there has been very little consideration of the likely effects of noise pollution on developing organisms (but see [13]). Anthropogenic noise could alter phenotypic development through direct impact on the developing organism (e.g. noise-induced developmental stress [13]), or indirect impact through altered parental behaviour [14,15]. Accordingly, organisms that develop in a noisy environment are overall likely to be of poor phenotypic quality [14]. However, the potential influence of early-life noise exposure on phenotypic development has yet to be explored in wild populations.
Here, we experimentally investigate the impact of chronic anthropogenic noise exposure on telomere length and fledging success of developing wild birds, by manipulating the acoustic environment (traffic noise versus control) of free-living house sparrows (Passer domesticus) breeding in nest boxes. To explore the causes of potential difference in telomere length between sound treatments, we also examine the effect of noise exposure on morphological (i.e. body size and condition) and physiological (i.e. baseline corticosterone level) parameters. We predicted that nestlings reared under traffic noise exposure should have shorter telomeres, be of poorer phenotypic quality (reduced size and condition), have increased physiological stress (i.e. increased corticosterone levels) and have a lower fledging success relative to controls.
2. Material and methods
The field experiment was conducted on a population of house sparrows breeding in nest boxes at the Centre d'Etudes Biologiques de Chizé (46°08′52″ N, 0°25′34″ W), France. During the 2013 breeding period, nest boxes were exposed to either a playback of traffic noise (‘disturbed treatment’, noise levels at the entrance hole: 63.3 ± 1.7 dB(A), 21 nest boxes) or the rural background noise of the study site (‘control treatment’, noise levels: 43.0 ± 0.5 dB(A), 46 nest boxes). Treatment began several weeks before egg-laying and ended at the end of the chick-rearing period, and consisted of a traffic noise recording that was played for 6 h a day, 7 days a week, using Logitech LS11 stereo speakers and iPod shuffles. Speakers were hidden 3–4 m from the nest boxes and volume was adjusted to produce noise levels similar to those experienced by birds breeding in urban environments [15]. We visited the nest boxes every 2 days to determine occupancy rates, laying dates, clutch sizes and hatching dates. None of these variables were affected by sound treatment (see [15] and the electronic supplementary material for details).
When nestlings were 9-days old, we collected morphological (body size and condition), physiological (baseline corticosterone level) and molecular (telomere length) data. Specifically, 37 nestlings (21 ‘disturbed nestlings’ from nine broods and 16 ‘control nestlings’ from seven broods) were measured (tarsus, bill and wing lengths) and weighed. Body size and body condition indices were calculated using these morphological measurements (see the electronic supplementary material). In addition, nestlings were blood sampled (50–100 µl) within 3 min of capture and ringed with a numbered metal ring. Finally, we checked the nest boxes 17 days after hatching to record fledging success.
Blood samples were centrifuged (4500 r.p.m., 7 min), and plasma and red blood cells were separated and stored at −20°C until analysed. Plasma concentrations of corticosterone were measured by radio-immunoassay. Genomic DNA was extracted from frozen red blood cells using DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer's protocol. The sex of nestlings was then determined by molecular sexing. Telomeres were finally measured using real-time quantitative PCR, following a protocol previously validated for birds (see [16] and the electronic supplementary material for details on assays).
Statistical analyses were performed in R. v. 3.1.0. We used linear mixed models (LMMs) with ‘body size’, ‘body condition’, log-transformed ‘baseline corticosterone’ or log-transformed ‘telomere length’ as our dependent variable, ‘sound treatment’ (disturbed versus control), ‘sex’, ‘brood size’ and two-way interactions as independent variables/factors, and ‘nest identity’ as a random factor. For telomere and corticosterone analyses, we also included ‘body condition’ as an independent variable. Fledging success was analysed on a per brood basis. We used generalized linear models (GLMs, binomial error distribution, logit link function) with ‘fledging success’ (proportion of nestlings that fledged) as our dependent variable, and ‘sound treatment’, brood size and their interaction as independent variables/factors.
3. Results
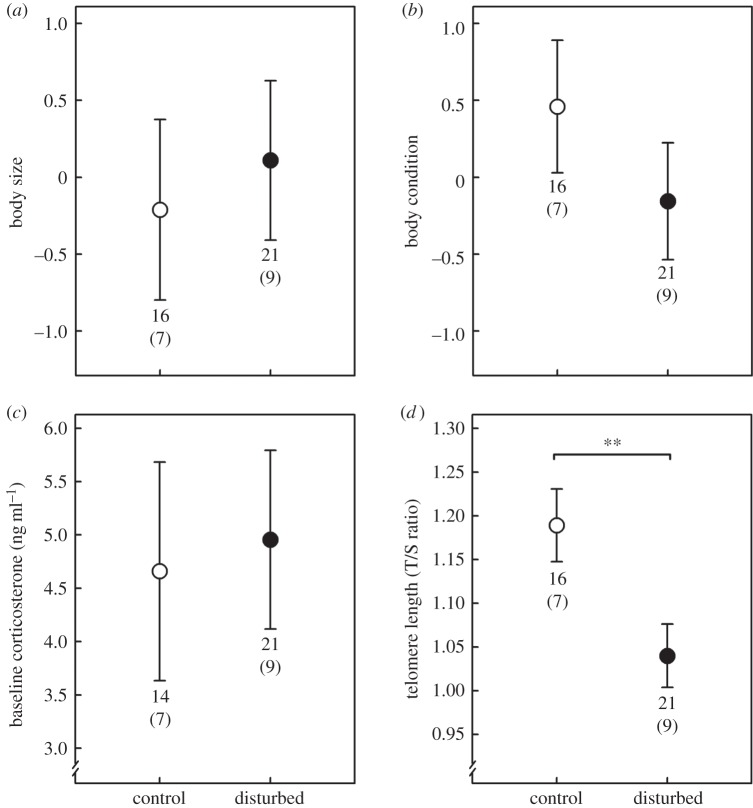
Noise exposure did not affect nestlings' growth, condition and fledging success: disturbed nestlings had similar body size (LMM: sound treatment effect: F1,14 = 0.13, p = 0.720; figure 1a and table 1a), body condition (LMM: F1,14 = 1.08, p = 0.318; figure 1b and table 1b) and fledging success (GLM: parameter estimates [logits]—disturbed versus control: −2.01 ± 1.29, 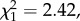 p = 0.120) as controls. Moreover, baseline corticosterone levels did not differ between disturbed and control nestlings (LMM: F1,13 = 0.12, p = 0.740; figure 1c and table 1c). However, the sound treatment affected nestlings' telomere length (LMM: F1,13 = 9.77, p = 0.008; figure 1d and table 1d), with nestlings reared under chronic noise exposure having significantly shorter telomeres than controls. There were no significant effects of sex, body condition or brood size on telomere length (all p > 0.394; table 1d).
p = 0.120) as controls. Moreover, baseline corticosterone levels did not differ between disturbed and control nestlings (LMM: F1,13 = 0.12, p = 0.740; figure 1c and table 1c). However, the sound treatment affected nestlings' telomere length (LMM: F1,13 = 9.77, p = 0.008; figure 1d and table 1d), with nestlings reared under chronic noise exposure having significantly shorter telomeres than controls. There were no significant effects of sex, body condition or brood size on telomere length (all p > 0.394; table 1d).
Figure 1.
Effect of noise exposure on nestlings' (a) body size, (b) body condition, (c) baseline corticosterone level and (d) telomere length. Filled circles represent disturbed nestlings and open circles represent controls (means ± s.e. from LMMs including nest identity as a random factor). A significant effect of sound treatment is symbolized: **p < 0.010. Numbers below bars indicate sample size (nestlings(broods)).
Table 1.
Effect of noise exposure on nestlings' (a) body size, (b) body condition, (c) baseline corticosterone level (log-transformed) and (d) telomere length (log-transformed). Fitted models include sound treatment, sex, brood size and body condition (for (c,d) only), with nest as a random factor. All two-ways interactions were non-significant (p > 0.221) and were removed from the models. Parameter estimates ± s.e. are reported and significant variables are shown in italic.
| dependent variable | independent variable/factor | β ± s.e. | F | p |
|---|---|---|---|---|
| (a) body size | intercept | 2.28 ± 1.87 | — | — |
| sound treatmenta | 0.28 ± 0.76 | 0.13 | 0.720 | |
| sexb | 0.18 ± 0.40 | 0.20 | 0.660 | |
| brood size | −0.57 ± 0.40 | 2.03 | 0.178 | |
| (b) body condition | intercept | 2.01 ± 1.35 | — | — |
| sound treatmenta | −0.57 ± 0.55 | 1.08 | 0.318 | |
| sexb | −0.33 ± 0.44 | 0.59 | 0.453 | |
| brood size | −0.33 ± 0.29 | 1.28 | 0.279 | |
| (c) baseline corticosterone | intercept | 1.92 ± 0.64 | — | — |
| sound treatmenta | −0.09 ± 0.25 | 0.12 | 0.740 | |
| sexb | −0.17 ± 0.23 | 0.55 | 0.467 | |
| brood size | −0.05 ± 0.14 | 0.14 | 0.719 | |
| body condition | −0.14 ± 0.09 | 2.62 | 0.124 | |
| (d) telomere length | intercept | 0.23 ± 0.13 | — | — |
| sound treatmenta | −0.15 ± 0.05 | 9.77 | 0.008** | |
| sexb | 0.03 ± 0.05 | 0.36 | 0.555 | |
| brood size | −0.01 ± 0.03 | 0.27 | 0.607 | |
| body condition | −0.02 ± 0.02 | 0.76 | 0.394 |
aEstimate is for noise treatment compared to control.
bEstimate is for male compared to female.
4. Discussion
Only a few studies have investigated the effects of noise pollution on nestlings directly (e.g. [13,17,18]). Overall, they report that noise exposure has subtle effects on physiology and behaviour of nestlings (stress physiology [13]; begging calls [17,18]) without obvious effects on growth, condition or fledging success. Strengthening these results, we found a strong and significant effect of noise exposure on nestling telomere length, but no effect on body size, body condition and fledging success. Recent studies have shown that early-life telomere length can be a reliable predictor of future life expectancy and fitness [2–4]. Reduced telomere length of disturbed nestlings may therefore suggest a detrimental effect of noisy environments on developing sparrows that may carry over later in life (i.e. reduced fitness). Future investigations should usefully assess these potential fitness consequences of reduced telomere length in nestlings.
The proximate causes of the effect of noise on telomere length remain to be determined. Genetic (e.g. inheritance of short telomeres by parents of poor quality), parental (e.g. reduced parental investment) and/or environmental (e.g. noise-induced stress) factors could all be involved [2,4]. The similar occupancy rates (disturbed: 52.4%, control: 54.4%) and high availability of unoccupied control nest boxes (21 nest boxes) suggest that low-quality individuals were not excluded from undisturbed nest boxes by high-quality sparrows [15]. Moreover, there were no differences in clutch size or body size and condition of parents—proxies for individual quality—between sound treatments (see the electronic supplementary material). Overall, parent quality is thus unlikely to have differed across the two sound treatments. Shorter telomeres are also unlikely to result from altered parental behaviour and nutritional restriction because of similar growth and fledging success between disturbed and control nestlings [14]. Accelerated telomere attrition of disturbed nestlings could result from oxidative stress, via noise-induced physiological stress (e.g. elevated stress hormones [4]). Indeed, recent studies have suggested that exposure to stress can accelerate telomere loss [4,10,19,20]. Here, we did not detect any effect of noise exposure on baseline corticosterone levels, suggesting that reduced telomere length did not result from an increased secretion of stress hormones. Importantly, we measured immediate corticosterone levels (when the chicks were 9-days old). It is plausible that an integrative measure of corticosterone levels (for instance, in the feathers) may provide a more accurate assessment of the stress levels experienced by the chicks throughout their development [21]. In addition, other factors could have accounted for the difference in telomere length between experimental chicks and controls. For instance, noise exposure may have increased the activity level of nestlings, or disrupted their normal sleep–wake cycle. Overall, these modifications may have increased oxidative stress and DNA damage, potentially explaining the results we found [4,20]. Future mechanistic studies should more deeply investigate the proximate mechanisms that mediate the effect of noise on telomere length in nestlings. Since early exposure to corticosterone and oxidative stress can affect telomere dynamics [10,20], specific attention should be paid to oxidative stress and integrative measures of corticosterone levels.
Our experiment demonstrates, for the first time, that anthropogenic noise can affect nestlings' telomere length without any obvious morphological effects. This finding raises fascinating questions regarding the impact of anthropogenic noise on life-history trajectories in wild populations. Furthermore, our results highlight the importance of investigating the impact of human-induced changes on cryptic aspects of phenotypic development to fully understand the influence of anthropogenic environments on populations.
Supplementary Material
Acknowledgements
We thank G. Gouchet, S. Ruault, C. Parenteau, C. Trouvé, L. Sourisseau, J.K. Grace for their kind assistance. We also thank three anonymous reviewers for constructive comments.
Ethics
All applicable institutional and/or national guidelines for the care and use of animals were followed and all experimental procedures were approved by the ‘Comité d'Ethique en Expérimentation Animale Poitou-Charentes’, France (permit no.: CE2013-3).
Data accessibility
Raw data are provided in the electronic supplementary material.
Authors' contributions
A.M., F.B. and F.A. conceived and designed the field experiment. A.M. collected and analysed the data. F.A. and C.R. performed telomere assays. A.M., F.B. and F.A. drafted the manuscript with intellectual input from C.R. All authors contributed to and approved the final version of the manuscript.
Competing interests
The authors have no competing interests.
Funding
This work was supported by the Fyssen Foundation and by the CNRS. A.M. was supported by the ‘Région Poitou-Charentes’ and the ‘Conseil Général des Deux-Sèvres’.
References
- 1.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645. ( 10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monaghan P, Haussmann MF. 2006. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53. ( 10.1016/j.tree.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 3.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monaghan P. 2014. Organismal stress, telomeres and life histories. J. Exp. Biol. 217, 57–66. ( 10.1242/jeb.090043) [DOI] [PubMed] [Google Scholar]
- 5.Angelier F, Vleck CM, Holberton RL, Marra PP. 2013. Telomere length, non-breeding habitat and return rate in male American redstarts. Funct. Ecol. 27, 342–350. ( 10.1111/1365-2435.12041) [DOI] [Google Scholar]
- 6.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 7.Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MH, Verhulst S. 2009. Telomere shortening and survival in free-living corvids. Proc. R. Soc. B 276, 3157–3165. ( 10.1098/rspb.2009.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nettle D, Monaghan P, Gillespie R, Brilot B, Bedford T, Bateson M. 2015. An experimental demonstration that early-life competitive disadvantage accelerates telomere loss. Proc. R. Soc. B 282, 20141610 ( 10.1098/rspb.2014.1610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichert S, Criscuolo F, Zahn S, Arrivé M, Bize P, Massemin S. 2015. Immediate and delayed effects of growth conditions on ageing parameters in nestling zebra finches. J. Exp. Biol. 218, 491–499. ( 10.1242/jeb.109942) [DOI] [PubMed] [Google Scholar]
- 10.Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P. 2014. Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc. R. Soc. B 281, 20133151 ( 10.1098/rspb.2013.3151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinney ML. 2002. Urbanization, biodiversity, and conservation. Bioscience 52, 883–890. ( 10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2) [DOI] [Google Scholar]
- 12.Francis CD, Barber JR. 2013. A framework for understanding noise impacts on wildlife: an urgent conservation priority. Front. Ecol. Environ. 11, 305–313. ( 10.1890/120183) [DOI] [Google Scholar]
- 13.Crino OL, Johnson EE, Blickley JL, Patricelli GL, Breuner CW. 2013. Effects of experimentally elevated traffic noise on nestling white-crowned sparrow stress physiology, immune function and life history. J. Exp. Biol. 216, 2055–2062. ( 10.1242/jeb.081109) [DOI] [PubMed] [Google Scholar]
- 14.Schroeder J, Nakagawa S, Cleasby IR, Burke T. 2012. Passerine birds breeding under chronic noise experience reduced fitness. PLoS ONE 7, e39200 ( 10.1371/journal.pone.0039200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meillère A, Brischoux F, Angelier F. 2015. Impact of chronic noise exposure on antipredator behavior: an experiment in breeding house sparrows. Behav. Ecol. 26, 569–577. ( 10.1093/beheco/aru232) [DOI] [Google Scholar]
- 16.Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG, Griffiths K, Gault EA, Monaghan P. 2009. Real-time quantitative PCR assay for measurement of avian telomeres. J. Avian Biol. 40, 342–347. ( 10.1111/j.1600-048X.2008.04623.x) [DOI] [Google Scholar]
- 17.Leonard ML, Horn AG. 2008. Does ambient noise affect growth and begging call structure in nestling birds? Behav. Ecol. 19, 502–507. ( 10.1093/beheco/arm161) [DOI] [Google Scholar]
- 18.Swaddle JP, Kight CR, Perera S, Davila-Reyes E, Sikora S. 2012. Constraints on acoustic signaling among birds breeding in secondary cavities: the effects of weather, cavity material, and noise on sound propagation. Ornithol. Monogr. 74, 63–77. ( 10.1525/om.2012.74.1.63) [DOI] [Google Scholar]
- 19.Hau M, Haussmann MF, Greives TJ, Matlack C, Costantini D, Quetting M, Adelman JS, Miranda AC, Partecke J. 2015. Repeated stressors in adulthood increase the rate of biological ageing. Front. Zool. 12, 1–10. ( 10.1186/s12983-014-0093-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. 2012. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. R. Soc. B 279, 1447–1456. ( 10.1098/rspb.2011.1913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenni-Eiermann S, Helfenstein F, Vallat A, Glauser G, Jenni L. 2015. Corticosterone: effects on feather quality and deposition into feathers. Methods Ecol. Evol. 6, 237–246. ( 10.1111/2041-210X.12314) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are provided in the electronic supplementary material.