Abstract
When exotic animal species invade new environments they also bring an often unknown microbial diversity, including pathogens. We describe a novel and widely distributed virus in one of the most globally widespread, abundant and damaging invasive ants (Argentine ants, Linepithema humile). The Linepithema humile virus 1 is a dicistrovirus, a viral family including species known to cause widespread arthropod disease. It was detected in samples from Argentina, Australia and New Zealand. Argentine ants in New Zealand were also infected with a strain of Deformed wing virus common to local hymenopteran species, which is a major pathogen widely associated with honeybee mortality. Evidence for active replication of viral RNA was apparent for both viruses. Our results suggest co-introduction and exchange of pathogens within local hymenopteran communities. These viral species may contribute to the collapse of Argentine ant populations and offer new options for the control of a globally widespread invader.
Keywords: pathogen, virus, invasive ant, honeybee (Apis mellifera), spillover
1. Introduction
Invasive species are recognized as a direct and major cause of biodiversity loss [1]. A less recognized issue with invasive species is the concomitant introduction of microorganisms that they host, including pathogens. Novel pathogens can have devastating effects on evolutionary and immunologically naive communities. These pathogens may even facilitate their host's invasion and contribute to biodiversity loss through spillover and differential effects on new hosts [2]. However, the potential threat of co-introduced pathogens is difficult to quantify given our poor knowledge of viruses in arthropods [3].
While invasive species can arrive with pathogens that may facilitate their establishment [2], new environments also expose exotic species to novel microbial communities. Established invasive species may experience population declines in their introduced range, which are often attributed to pathogens [4]. They have been hypothesized to have a low immuno-genetic diversity due to founder effects making introduced species less resilient to infection [5].
Argentine ants (Linepithema humile) are one of the six most widespread, abundant and damaging invasive ants [6]. They frequently form large colonies with the interchange of workers over a wide area. Such behaviours could increase the probability of disease transmission and facilitate epidemics [5]. Population collapse or dramatic range reductions have been previously observed with Argentine ants [7,8]. Cooling et al. [8] recorded the absence of these ants from 60 out of 150 (40%) previously known infestations, and hypothesized that pathogens were a potential mechanism for this collapse. No viruses, however, have been described from Argentine ants or other invasive ants, with few exceptions (e.g. Solenopsis invicta [9,10]). Here, we discovered a novel virus in Argentine ants. We also show that Argentine ants are a common host for Deformed wing virus (DWV): a widespread bee pathogen associated with honeybee mortality [11].
2. Methods and material
(a). Metagenomic discovery of viruses
Argentine ants were collected in 2013 from two nests in Wellington, New Zealand (41.2218° S, 174.8724° E). RNA was extracted from a pool of 30 ants from each nest using an iPrep PureLink Virus kit (Life Technologies; see the electronic supplementary material). The two extractions were combined into a single sample to increase nucleic acid amount. To detect RNA viruses, DNA was removed using DNase treatment with Ambion DNA-free (Life Technologies). Eight microlitres of DNA-free RNA was incorporated into first-strand cDNA synthesis (Life Technologies) including RNase H digestion. To ensure more than 1 µg of DNA was available for library preparation, the cDNA was amplified by multiple displacement amplification using a Whole Transcriptome Amplification kit (Qiagen). Sequencing libraries were prepared with the Illumina TruSeq DNA library preparation kit (Qiagen) followed by sequencing on an Illumina MiSeq producing 250 bp paired-end reads.
The quality of the sequence data was examined using Fast QC. Reads were trimmed when the average Phred score was less than 30. Duplicate reads were collapsed using FASTX-Toolkit 0.10.1. Velvet 1.2.07 [12] was used for de novo assembly of the trimmed sequence data with a k-mer of 75. Contigs were searched against the NCBI GenBank non-redundant nucleotide sequence database using BLASTN and BLASTX (BLAST+ 2.2.27 [13]) with an e-value threshold of 0.001. MEGAN 4.7 [14] was used for taxonomic assignment with a threshold criteria for inclusion requiring a bit score of more than 50 and contig sequence complexity more than 0.44.
(b). Virus prevalence and distribution
To confirm the viral sequences and to detect their presence in natural populations, Argentine ants were collected between 2012 and 2015 in either 100% ethanol or Ambion RNAlater (Life Technologies). Samples were from 30 sites throughout the ant's invaded range in New Zealand, two sites in Australia, and three sites from the Argentinian native range. RNA was extracted for each site from a pool of 30 ants using either an iPrep PureLink Virus kit (Life Technologies), as described above, or a GeneJET Viral DNA & RNA Purification Kit (Thermo Scientific). We also analysed for viruses previously observed in ants or other insects [9–11,15] using one-step RT-PCRs: Israeli acute paralysis virus (IAPV), Kashmir bee virus (KBV), Acute paralysis bee virus (APBV), DWV and Solenopsis invicta virus-1 and -2 (SINV-1 and SINV-2). Four contigs from the RNA metagenome (n1000, n1905 and n1050, n6409) were also assessed using RT-PCR (see the electronic supplementary material).
Phylogenetic analysis was conducted in MEGA 6.06 [16] after ClustalW alignment [17]. An evolutionary model was chosen using Bayesian information criterion scores (BIC) derived in MEGA 6.06. Maximum-likelihood trees with 1000 bootstrap replicates were generated using the LG model with a gamma parameter of 4.65 (LG + G [18]) for the Linepithema humile virus 1 (LHUV-1) amino acid sequences and the Tamura 3-parameter model with uniform rates (T92 [19]) for the DWV nucleotide sequences.
To investigate the active replication of DWV and LHUV-1, a modified RT-PCR was used to detect the RNA negative strands of both viruses. This assay was first undertaken on a pooled sample of 30 workers. We additionally assayed for both viral presence, and the active replication, of DWV and LHUV-1 in 15 individual Argentine ant workers and 14 queens from one New Zealand location (Paraparaumu; see the electronic supplementary material).
3. Results
(a). Metagenomic discovery of viruses
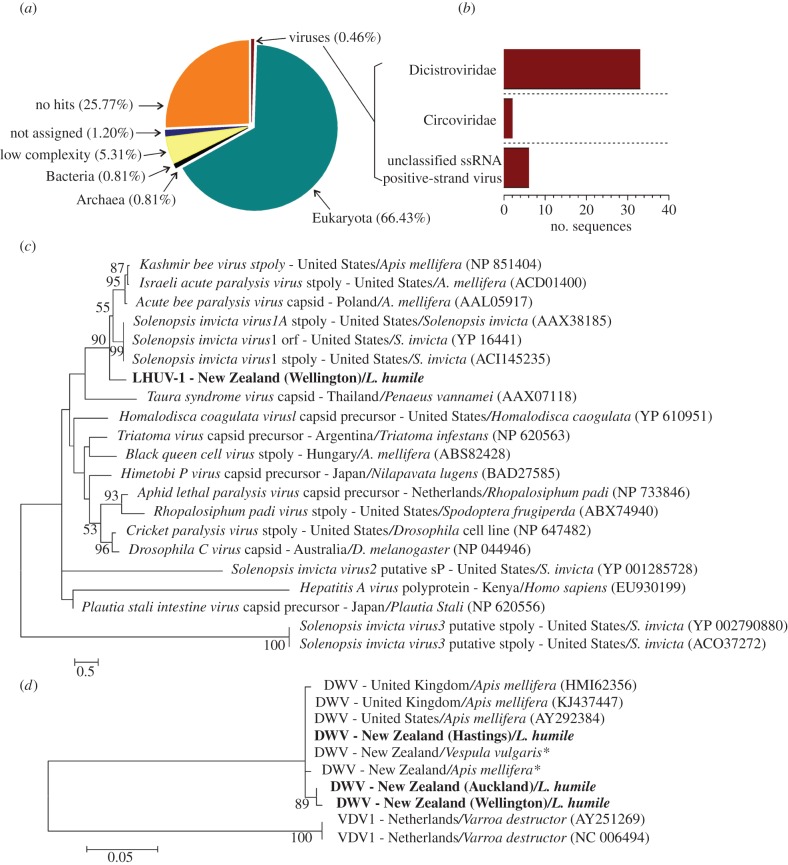
A total of 139 943 contigs were assembled for the RNA metagenome. A BLASTN search revealed 33 contigs similar to viral sequences in the Dicistroviridae family (figure 1a,b). PCR assays using published primers failed to detect viral sequences from IAPV, KBV, ABPV, SINV-1 and SINV-2 (see the electronic supplementary material).
Figure 1.
(a) Taxonomic proportions in the RNA metagenome of Argentine ants in New Zealand. (b) Viral families detected in the RNA metagenome. (c) Maximum-likelihood phylogenetic analysis of Dicistroviridae including the novel LHUV-1 virus: sP, partial structural protein; stpoly, partial structural polyprotein; capsid, partial capsid protein. (d) A maximum-likelihood phylogenetic analysis of DWV, based on a DWV helicase protein gene. DWV sequences amplified from Argentine ant (Linepithema humile) samples are shown in bold. Asterisk (*) denotes sequences from [20].
RNA metagenome contig n6409 matched the structural polyprotein (capsid region) of dicistroviruses and therefore was suitable for a phylogenetic analysis for provisional taxonomic assignment of the putative virus. The presence of contig n6409 sequence was confirmed using Sanger sequencing, and phylogenetic analysis positioned the contig with other dicistroviruses (figure 1c). This sequence appears to be from a novel virus we have provisionally named LHUV-1.
(b). Virus prevalence and distribution
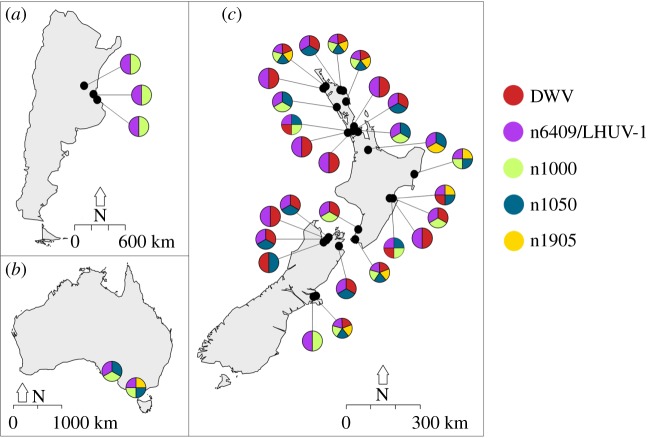
LHUV-1 was detected in all sampling sites, with one exception in New Zealand (figure 2). The contig n1000 sequence was present in all sites in Argentina and Australia but in less than half the sites in New Zealand (13/27). The n1905 and n1050 contigs were only detected in Australia and New Zealand (figure 2). All Sanger sequencing data were identical to the RNA metagenome contig, with the exception of a few nucleotide differences (less than 1%; see the electronic supplementary material).
Figure 2.
Distribution of the honeybee DWV, the novel RNA dicistrovirus LHUV-1, and the three viral contigs n1000, n1905 and n1050, in (a) Argentina, (b) Australia, and (c) New Zealand.
We used these additional ant samples from around New Zealand to examine for viruses previously observed in bees, ants or other insects [8–10,14]: IAPV, KBV, ABPV, DWV, SINV-1 and SINV-2. Only DWV was detected, and it was only found in New Zealand (22 of 27 sites; figure 2). The DWV sequences grouped with other known DWV found in honeybees (Apis mellifera) and common wasps (Vespula vulgaris; figure 1c). Both DWV and LHUV-1 negative strands were detected indicating that the two viruses actively replicate in Argentine ants.
We further assayed for both viral presence, and the active replication, of DWV and LHUV-1 in individual Argentine ant workers and queens from one location in New Zealand (Paraparaumu; see the electronic supplementary material). DWV and LHUV-1 presence were confirmed by RT-PCR and Sanger sequencing, and all positive samples showed active viral replication after confirmation by a modified one-step RT-PCR. In worker ants, DWV was observed with replication in 7% (infection 1/15; replication 1/1) and LHUV-1 in 13% (infection 2/15; replication 2/2). Additional sampling in winter showed a single LHUV-1-positive individual queen (infection 1/14; replication 1/1) with viral replication, but no DWV in queens at this time. We did not observe any evidence of viral co-infections in any of these individually assayed ants.
4. Discussion
This is the first study to describe viruses in one of the world's most widespread, abundant and damaging invasive ants. We describe the presence of the novel LHUV-1, of the Dicistroviridae family, in three countries including the native range of Argentine ants. Viruses related to LHUV-1 can devastate insect populations, including those associated with colony collapse in honeybees [11]. Six dicistroviruses have been described from red imported fire ants (Solenopsis invicta), with some being considered as biocontrol agents [9,10]. LHUV-1 and the DWV were observed to be actively replicating in Argentine ants. This replication indicates that the viruses were likely parasitizing the ants, rather than merely being vectored as particles. Therefore, these viruses may be candidates for the population declines observed in Argentine ants [7,8] and may have potential for biocontrol programmes.
We also found evidence for other potential viral species in Argentine ants (i.e. contigs n1000, n1905 and n1050). We did not obtain sequence from the novel viruses for homologous genes encoding structural proteins or conserved replication function (i.e. capsid, RNA-dependent RNA polymerase). Such genetic data would be necessary in order to develop informative phylogenetic trees and confirm taxonomic relationships. Our BLASTN and BLASTX analyses were indicative that these contigs are closely related to the dicistroviruses found in bees including IAPV and KBV. IAPV and DWV have been observed in ants previously [15]. However, our PCR analysis using established primers for IAPV or KBV and other related bee viruses, with the exception of DWV, failed to find evidence for the presence of these viruses. Thus, whether these contigs represent new viruses or are strains of known species remains an open question. Our study also raises additional questions for further work. For example, it seems likely that LHUV-1 was introduced by Argentine ants into New Zealand and Australia. While this scenario seems likely, it may also be the case that the LHUV-1 is present in other species around the world. Additional work is needed to assess the virus specificity and seasonal population dynamics in Argentine ants and other insects. It is possible that LHUV-1 may even infect honeybees, as our and other work is indicative of pathogen sharing between insect hosts [15,20,21].
Our results indicate that Argentine ants host, and likely act as a reservoir of the DWV, an aetiological agent implicated in honeybee deaths [11]. Transmission of viruses between foraging hymenopteran host species has been demonstrated [20]. A more direct interaction between honeybees and Argentine ants occurs when these ants raid beehives. The New Zealand DWV sequences were similar to strains from local bee and wasp populations [21], suggesting that ants, wasps and bees share pathogen strains. Such effects clearly represent a new dimension to a poorly considered effect of biological invasions. When exotic species invade new environments, become widespread and abundant, they may have a major impact by becoming hosts and reservoirs for important pathogens.
Supplementary Material
Acknowledgements
We thank Meghan Cooling, Antoine Felden, Ben Hoffmann, Carolina Paris, and Andy Suarez for providing samples. Wlodek Stanislawek provided helpful advice. Two referees provided immensely valuable comments. We also wish to acknowledge New Zealand Genomics Limited (NZGL) and the Massey Genome Service.
Data accessibility
Metagenomic data are deposited in the European Nucleotide Archive (http://www.ebi.ac.uk/ena, accession no. PRJE10079). Sequences for LHUV-1, DWV, and contigs n1000, n1905 and n1050 can be accessed from Genbank (Accession numbers KT713624, KT695629, KT713625, KT713626, and KT713627, respectively).
Authors' contributions
A.S., R.J.H., M.A.M.G. and P.J.L. conceived the study. A.S. and N.E.M. performed the laboratory work. All authors participated in the analysis and interpretation of the data. P.J.L. and A.S. wrote the article with input from all authors.
Competing interests
The authors declare no competing interests.
Funding
Victoria University funded A.S., P.J.L. and M.A.M.G. The Core Research Fund provided by MBIE funded R.J.H., J.W. and N.E.M. P.J.L. was supported by an RSNZ James Cook Fellowship.
References
- 1.Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689–710. ( 10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2) [DOI] [Google Scholar]
- 2.Vilcinskas A, Stoecker K, Schmidtberg H, Rohrich CR, Vogel H. 2013. Invasive harlequin ladybird carries biological weapons against native competitors. Science 340, 862–863. ( 10.1126/science.1234032) [DOI] [PubMed] [Google Scholar]
- 3.Bichaud L, de Lamballerie X, Alkan C, Izri A, Gould EA, Charrel RN. 2014. Arthropods as a source of new RNA viruses. Microb. Pathog. 77, 136–141. ( 10.1016/j.micpath.2014.09.002) [DOI] [PubMed] [Google Scholar]
- 4.Simberloff D, Gibbons L. 2004. Now you see them, now you don't—population crashes of established introduced species. Biol. Invasions 6, 161–172. ( 10.1023/B:BINV.0000022133.49752.46) [DOI] [Google Scholar]
- 5.Ugelvig LV, Cremer S. 2012. Effects of social immunity and unicoloniality on host–parasite interactions in invasive insect societies. Func. Ecol. 26, 1300–1312. ( 10.1111/1365-2435.12013) [DOI] [Google Scholar]
- 6.Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ. 2002. The causes and consequences of ant invasions. Ann. Rev. Ecol. Syst. 33, 181–233. ( 10.1146/annurev.ecolsys.33.010802.150444) [DOI] [Google Scholar]
- 7.Wetterer JK, Espadaler X, Wetterer AL, Aguin-Pombo D, Franquinho-Aguiar AM. 2006. Long-term impact of exotic ants on the native ants of Madeira. Ecol. Entomol. 31, 358–368. ( 10.1111/j.1365-2311.2006.00790.x) [DOI] [Google Scholar]
- 8.Cooling M, Hartley S, Sim D, Lester PJ. 2012. The widespread collapse of an invasive species: Argentine ants (Linepithema humile) in New Zealand. Biol. Lett. 8, 430–433. ( 10.1098/rsbl.2011.1014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valles SM, Strong CA, Hashimoto Y. 2007. A new positive-strand RNA virus with unique genome characteristics from the red imported fire ant, Solenopsis invicta. Virology 365, 457–463. ( 10.1016/j.virol.2007.03.043) [DOI] [PubMed] [Google Scholar]
- 10.Valles SM, Porter SD, Firth AE. 2014. Solenopsis invicta virus 3: pathogenesis and stage specificity in red imported fire ants. Virology 460, 66–71. ( 10.1016/j.virol.2014.04.026) [DOI] [PubMed] [Google Scholar]
- 11.Schroeder DC, Martin SJ. 2012. Deformed wing virus: the main suspect in unexplained honeybee deaths worldwide. Virulence 3, 589–591. ( 10.4161/viru.22219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18, 821–829. ( 10.1101/gr.074492.107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403–410. ( 10.1016/S0022-2836(05)80360-2) [DOI] [PubMed] [Google Scholar]
- 14.Huson DH, Auch AF, Qi J, Schuster SC. 2007. MEGAN analysis of metagenomic data. Genome Res. 17, 377–386. ( 10.1101/gr.5969107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levitt AL, Singh R, Cox-Foster DL, Rajotte E, Hoover K, Ostiguy N, Holmes EC. 2013. Cross-species transmission of honey bee viruses in associated arthropods. Virus Res. 176, 232–240. ( 10.1016/j.virusres.2013.06.013) [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 30, 2725–2729. ( 10.1093/molbev/mst197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larkin MA, et al. 2007. ClustalW and ClustalX version 2. Bioinformatics 23, 2947–2948. ( 10.1093/bioinformatics/btm404) [DOI] [PubMed] [Google Scholar]
- 18.Le SQ, Gascuel O. 2008. LG: an improved, general amino-acid replacement matrix. Mol. Biol. Evol. 25, 1307–1320. ( 10.1093/molbev/msn067) [DOI] [PubMed] [Google Scholar]
- 19.Tamura K. 2002. Evolutionary distance estimation under heterogeneous substitution pattern among lineages. Mol. Biol. Evol. 19, 1727–1736. ( 10.1093/oxfordjournals.molbev.a003995) [DOI] [PubMed] [Google Scholar]
- 20.Singh R, et al. 2010. RNA viruses in hymenopteran pollinators: evidence of inter-taxa virus transmission via pollen and potential impact on non-Apis hymenopteran species. PLoS ONE 5, e14357 ( 10.1371/journal.pone.0014357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lester PJ, et al. 2015. No evidence of enemy release in pathogen and microbial communities of common wasps (Vespula vulgaris) in their native and introduced range. PLoS ONE 10, e0121358 ( 10.1371/journal.pone.0121358) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Metagenomic data are deposited in the European Nucleotide Archive (http://www.ebi.ac.uk/ena, accession no. PRJE10079). Sequences for LHUV-1, DWV, and contigs n1000, n1905 and n1050 can be accessed from Genbank (Accession numbers KT713624, KT695629, KT713625, KT713626, and KT713627, respectively).